Abstract
Osteoporosis (OP), a common systemic metabolic bone disease, is characterized by low bone mass, increasing bone fragility and a high risk of fracture. At present, the clinical treatment of OP mainly involves anti-bone resorption drugs and anabolic agents for bone, but their long-term use can cause serious side effects. The development of stem cell therapy and regenerative medicine has provided a new approach to the clinical treatment of various diseases, even with a hope for cure. Recently, the therapeutic advantages of the therapy have been shown for a variety of orthopedic diseases. However, these stem cell-based researches are currently limited to animal models; the uncertainty regarding the post-transplantation fate of stem cells and their safety in recipients has largely restricted the development of human clinical trials. Nevertheless, the feasibility of mesenchymal stem cells to treat osteoporotic mice has drawn a growing amount of intriguing attention from clinicians to its potential of applying the stem cell-based therapy as a new therapeutic approach to OP in the future clinic. In the current review, therefore, we explored the potential use of mesenchymal stem cells in human OP treatment.
Similar content being viewed by others
Introduction
The concept of osteoporosis (OP) can be traced back to 1885 when Gustav Pommer, German pathologist, defined the distinction between OP and osteomalacia [1]. Subsequently, of OP the predisposing factors, general aetiology and pathogenesis, clinical presentation, prevention, treatment, and prognosis were deeply studied in the twentieth century [2, 3]. In the 1990s, a clear definition of OP was put forward to be recognized worldwide [4, 5]. Currently, it is believed that OP is a multifactorial, systemic metabolic bone disease characterized by the deterioration of bone mass and microstructure destruction, which lead to decreased bone mineral density (BMD), increased bone fragility and the risk of bone fracture [6]. The occurrence and progression of OP are believed to be closely related to aging, with its incidence being the highest in elderly men and postmenopausal women. As previously reported, an estimated 54 million American women and men aged over 50 suffered from OP and osteopenia [7], and in China the OP-stricken population would reach over 120 million by 2050 [8].
In the treatment of OP, the primary goal is to maintain the balance between bone metabolism and reduction of bone loss. At present, the clinically administered medications to treat OP are divided into three categories according to pathogenesis: basic supplements, antiresorptive agents and bone formation-accelerating agents [9]. Vitamin D, as a basic supplement and was demonstrated not to have a therapeutic effect on fracture or BMD [10]. The antiresorptive agents to treat OP include bisphosphonate, which has a variety of side effects [11]. In recent years, the stem cell therapy for OP has been reported to reduce bone loss and decrease the patient’s vulnerability to fractures [12]. Stem cells, derived from embryos, fetuses or adults with unlimited self-renewal, proliferation and differentiation ability under specific conditions, can be classified into totipotent SCs (TSCs), pluripotent SCs (PSCs) and unipotent SCs (USCs) according to their differentiating potential [13]. As pluripotent stem cells, mesenchymal stem cells (MSCs) show relatively high versatility and differentiate into multipotent bone, cartilage and adipose tissue cells; these cells have become the most appropriate source for stem cell-based therapy [14].
The goal of our research was to have a comprehensive review of the recent advances in treating OP with mesenchymal stem cell therapy. In particular, our review focused on the preclinical and clinical researches on the use of bone marrow-derived mesenchymal stem cells (BM-MSCs), adipose tissue-derived MSCs (AD-MSCs), and umbilical cord-derived MSCs (UC-MSCs) for OP treatment.
Physiopathology of osteoporosis
OP is a complex metabolic disease that is associated with risk factors such as high BMI, history of smoking and drinking, age at menopause, and postmenopausal status [15, 16]. High BMI caused by obesity was previously considered to be conducive to maintaining bone health and reducing the risk of fracture [17]. Recent studies have shown that obesity can cause a harmful effect on bone metabolism, and the weakened bone strength may be related to the location of fat accumulation. In obesity, although the overall fat mass increases significantly, the part of lean body weight gain has been proved to have a beneficial effect on BMD [18]. We believe that this phenomenon is related to the increase of bone mechanical load. However, high body fat rate and high waist circumference are considered to be associated with low BMD and osteoporotic fractures [19, 20]. Peptide hormones produced by adipose tissue are involved in bone metabolism [21]. Leptin plays a two-way regulatory role in bone metabolism. On the one hand, leptin can stimulate the differentiation of osteoblasts and inhibit the differentiation of pluripotent stem cells into adipocytes to promote osteogenesis; on the other hand, bone formation can be decreased by reducing insulin resistance and inhibiting bone anabolism [21, 22]. Additionally, the increase of fat content is related to the increase of androgen to estrogen, which has a positive effect on bone metabolism. Therefore, when discussing the contradictory relationship between obesity and OP, the protective and harmful effects of fat on bone must be considered simultaneously [23].
With pain being the predominant symptom, OP facilitates compression fractures of the vertebrae and traumatic fractures of the femoral neck in its victims, thus leading to a significant decline in the quality of life. In fact, the pathophysiological mechanism of OP is sophisticated and multifactorial. As a consequence of aging and/or oestrogen deficiency, the disorders of bone metabolism act as the principal pathophysiologic mechanism of primary OP. Under normal physiological conditions, the homeostasis of bone metabolism is mainly regulated by osteoblasts, osteoclasts, and osteocytes. Osteocytes are the most abundant cells in bone tissues, accounting for more than 90% there, and seeming to play an indispensable role in signal transduction in bone metabolism [24]. Osteoclasts, which are multinucleated cells that originate in haematopoietic myeloid cells in bone marrow (BM), can absorb mineralized bone matrix, as the primary cells involved in bone destruction in OP. Osteoblasts, which are responsible for the formation of new bone have three main processes involved; firstly, extracellular matrix proteins are synthesized by osteoblasts, and subsequently, extracellular matrix proteins are covered by a layer of calcium hydroxyapatite crystals within the next few months, thereby mineralizing the matrix, and ultimately, bone remodelling occurs.
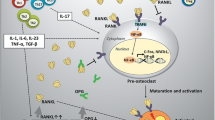
Actually, numerous signalling pathways are involved in the regulation of bone metabolism; the key ones mainly refer to the receptor activator of nuclear factor-kappa B (RANK)-RANK ligand (RANKL) and Wnt/β-catenin signalling pathway. In the 1990s, it was discovered that the RANKL/RANK/Osteoprotegrin (OPG) signalling pathway is the key to the regulation of osteoclast generation, providing a theoretical basis for the research on and development of new anti-reabsorption drugs such as denosumab [25]. RANKL combines with RANK on the cell membrane surface to regulate the recruitment and differentiation of osteoclasts, thus playing a role in inducing the differentiation and maturation of osteoclasts and promoting bone absorption. OPG, a soluble trap receptor secreted by osteoblasts, plays a competitive role in binding RANKL along this pathway. OPG shows a stronger affinity with RANKL than does RANK, thereby inhibiting osteoclast differentiation, activation, and maturation by blocking binding between RANKL and RANK, which significantly reduces the ability of RANK to promote osteoclast formation. Furthermore, OPG can promote bone formation by activating osteoblasts, thus exerting an inhibitory effect on bone resorption [26].
As the major regulator of osteoblast-mediated bone formation, the canonical Wnt/β-catenin signalling pathway, once activated, promotes dishevelled protein expression and activation and mediates GSK-3β phosphorylation, facilitating the stable maintenance and continuous aggregation of β-catenin in target cells. β-catenin is known to enter the nucleus from the cytoplasm; when its concentration reaches a certain level in the cytoplasm, it combines with LEF/TCF family members to recruit bcl9 and other related factors to positively regulate the expression of target genes, promoting the proliferation and differentiation of bone marrow mesenchymal stem cells into osteoblasts. Simultaneously, activated Wnt signalling can promote the secretion of OPG in the RANK/RANKL/OPG signalling pathway, thus inhibiting the differentiation, activation, maturation of osteoclasts, and promoting bone formation [27].
The current treatment of OP
The treatment involving nonpharmacological interventions is the primary approach to those who are at the early stage of OP. This approach is mainly taken in the form of ensuring adequate daily calcium, vitamin D, and protein intake; administering an appropriate amount of weight-bearing physical exercise to maintain or improve physical quality; and facilitating appropriate lifestyle changes such as smoking cessation and alcohol consumption moderation. As the disease progresses, pharmacological interventions become inevitable; calcium and vitamin D are routinely recommended for all patients as OP treatment. Both substances are essential for the stabilization of the normal bone internal environment so as to reduce the rate of bone loss and fracture risk.
Previous study has shown that additional intake of vitamin D and calcium supplements each day can significantly improve the calcium balance in postmenopausal women, resulting in a significant increase in BMD at the femoral neck exerting favourable effects on glucose and lipid level [28]. At present, a variety of medications are clinically prescribed to treat OP, which mainly refer to bisphosphonates, parathyroid hormone (PTH) and its analogues, a selective oestrogen-receptor modulator (SERM, raloxifene), a human monoclonal antibody against RANKL (denosumab), a humanized monoclonal antibody against sclerostin (romosozumab) and a cathepsin K inhibitor (odanacatib). As the most widely administered bone resorption inhibitor, bisphosphonates can induce osteoblasts to secrete inhibitors, exerting cytotoxic effects on osteoclasts, inhibiting the activation of osteoclasts, and restraining bone resorption. Moreover, bisphosphonates which combine with calcium phosphate are adsorbed on the surface of bone hydroxyapatite crystals to prevent the loss of calcium in bone, thus reducing the incidence of lumbar and hip fractures in patients with OP [29]. Bisphosphonates, widely used in the clinic, definitely have a curative effect, but with some side effects, as indicated in the previously reported evidence that they may cause typical osteonecrosis of femoral fracture and jaw [30, 31]. Teriparatide (recombinant human PTH [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34]), the first bone anabolic agent approved by the FDA, can effectively promote bone formation on a short-term course [32], but increase the occurrence of osteosarcoma in the preclinical rat models on a long-term and high-dose course [33]; therefore, the treating course of teriparatide has been limited to 24 months by the FDA. Selective oestrogen-receptor modulators, similar to oestrogen replacement therapy (ERT), are mainly taken to prevent bone loss in postmenopausal women; however, the use of these drugs increases the risk of breast cancer, cardiovascular events, stroke, and pulmonary embolism [34]. Denosumab, a human monoclonal antibody against RANKL, is capable of competitively binding to the RANKL protein, as in the case of OPG, inhibiting the differentiation and activation of osteoclasts mediated by the RANKL/RANK/OPG signalling pathway, reducing osteoclastic activity and improving bone mass and bone density [35].
As indicated in a clinical study, OP patients receiving denosumab could experience a 68% reduction in vertebral fracture and a 40% decrease in hip fracture [36]. The most common complication of the denosumab treatment is hypocalcaemia, and another serious one, osteonecrosis of the jaw, which was the first to be reported in the patients on bisphosphate [37]. In the FREEDOM experiment, of 4550 patients who received denosumab 5 developed severe complications of osteonecrosis in the jaw [38]. Romosozumab, a humanized monoclonal antibody against sclerostin, can bind to osteosclerotin in vivo, inhibiting its binding with LRP5/6 and other proteins of the low-density lipoprotein-related receptor family, enhancing the Wnt signalling pathway, significantly increasing bone mass formation, and reducing bone resorption, which exerts a dual regulatory effect [39]. When compared with other monoclonal antibody medications including denosumab, romosozumab does not increase the risk of fracture after drug withdrawal and have a significant effect on the incidence of tumours in a rat model [40], but it may be more prone to causing local reactions at the injection site such as discomfort, pain, erythema, rash, haematoma or bleeding [41]. Cathepsin K, a lysosomal protease that is highly expressed in osteoclasts, plays a key role in the degradation of bone matrix proteins, and cathepsin K inhibitors show a similar efficiency as bisphosphonates in increasing BMD and decreasing the risk of fragility fractures [42]. Odanacatib inhibits bone resorption without reducing the numbers of osteoclasts or inhibiting bone formation, and is well tolerated without significant drug-related side effects [43].
In the past few decades, an extraordinary understanding has been achieved on the biology of bones, so have remarkable progresses been made in the development of OP medications. However, further researches are still needed on the development of new drugs and therapies with fewer side effects. Since stem cell therapy is considered a promising new therapeutic strategy for restoring normal tissue structure and delaying disease progression. OP therapy based on mesenchymal stem cells can be of great interest.
The characteristics of MSCs
For the first time in 1968, Friedenstein et al. isolated MSCs from bone marrow [44]. As indicated in the subsequent studies, MSCs exhibit immune and nutritional activity and high in vitro self-renewal and multilineage differentiation capabilities [45], and they also express and secrete numerous bioactive factors, including cytokines [46], growth factors and chemokines, which are involved in the paracrine activity of MSCs [47]. Adult MSCs have been found to exist in almost all tissues, such as bone marrow, umbilical cord and placenta, adipose tissue, peripheral blood, and endometrial tissue. Not only can they differentiate into a variety of connective tissues, such as bone, muscle, adipose tissue, and cartilage, but they can also differentiate into nonmesodermal lineage cells, such as hepatocytes, neuron-like cells and pancreatic cells; moreover, they can differentiate into other types of cells under suitable conditions [48].
As indicated in the previously reported studies, many potential MSC surface markers are related to stem cell characteristics, including CD73, CD90, and CD105, but from this list are missing CD19, CD45, CD34, CD14, CD79α and HLA-DR. There are significant differences in the expression of these markers on MSCs from different sources [49]. Human bone marrow, umbilical cord blood, and adipose tissue have been the most used sources of adult MSCs [50]. Among these cell sources, BM-MSCs have been extensively studied in the context of tissue regeneration and repair due to their efficient differentiation ability. Adipose tissue is especially abundant and readily available in our body, which makes it the safest and most reliable site for stem cell isolation [51]. Considered to be the most promising stem cells for articular cartilage repair, hUC-MSCs show a higher ability of proliferation and cloning than BM-MSCs and AD-MSCs [52].
MSCs-based treatment of OP
The incidence of the fractures caused indirectly by OP still accounts for the highest in the elderly population, even though remarkable progresses have been made in the development of OP medications. A present, certain drug and nondrug therapies are mainly performed to treat OP by improving bone strength and preventing fracture [53]. Although they are administered on the patient with OP, these medications have some limitations and even cause adverse reactions. As in the case of MSC transplantation which has been proven to be a feasible and novel therapeutic approach in regenerative medicine and stem cell therapy. Stem cells can differentiate into target tissues by self-differentiation, and promote tissue progenitor cells to differentiate into target tissues by secreting various proteins, enzymes and factors. Through the direct interaction between cells to promote the differentiation of other cells into target tissues, these factors enable stem cells to repair damaged tissues [54]. A growing number of researchers have begun to explore the potential of MSCs in the treatment of chronic diseases [55]. In the current review, therefore, we illustrated the mechanisms of osteogenic and adipogenic differentiation of MSC (Fig. 1) and evaluated potential of bone marrow, umbilical cord, and adipose-derived mesenchymal stem cells in the treatment of OP (Table 1).
Bone marrow-derived mesenchymal stem cells
BM-MSCs have various biological advantages in terms of tissue repair, and their high osteogenic differentiation, the means of which are widely applied to the repair of bone and cartilage injuries [56]. Previous studies have mainly focused on clarifying the positive role of BM-MSCs in promoting osteogenesis [57, 58]. The efficacy of BM-MSCs in the treatment of OP has been demonstrated in quite a few preclinical animal models, as indicated in the previously reported two studies where both the haemopoietic system and the bone marrow microenvironment were normalized after the direct infusion of allogeneic bone marrow cells into the bone marrow cavity of irradiated SAMP6 mice, and the levels of IL-6, RANKL and IL-11, involved in regulating bone reconstruction, returned to normal, similar to those of B6 mice, thereby ameliorating the imbalance between bone formation and resorption [59, 60]. Moreover, the intraosseous injection of allograft BM-MSCs into the femur in a postmenopausal rat model of OP definitely caused the femoral trabecula of the treated rats to increase significantly within two months, appearing similar to the femurs of the controls. GFP labelling results also confirmed the presence of transplanted BM-MSCs on the trabecular surface of the treated rats [61]. In the Sprague–Dawley adult rat model, of closed transverse fracture with internal fixation, a systemic MSC injection and local MSC injection were performed at the fracture site on the 4th day after fracture; the results of weekly X-ray, micro-CT, mechanical tests, etc., showed that the callus size grew significantly larger in the rats after treatment, but no difference in fracture healing was found between the two regimens administered [62].
With the research furthering on BM-MSC-mediated bone tissue repair, bone marrow aspirate concentrate (BMAC), which is rich in cytokines, has been widely used in the treatment of knee osteoarthritis and other diseases [63]. According to the review of the relevant studies, researchers have come to believe that the body can regulate the function of BM-MSCs through multiple signalling pathways, including the TGF β/Smad pathway [64, 65], BMP-2/Smad pathway [66, 67], MAPK pathway [68, 69], Wnt/β-catenin pathway [70], PPAR-γ pathway [71], etc., and that the body can either promote their osteogenic differentiation or inhibit adipogenic differentiation to regulate OP. As indicated in a previously reported study where the expression of Foxf1 was detected in various tissues of OVX mice and its expression increased significantly in bone extracts and BMSCs, followed by a gradual decrease during the osteogenic differentiation of BM-MSCs, the results of in vitro loss-of-function approach showed that Foxf1 could regulate the osteoblast differentiation of BM-MSCs through the Wnt/β-catenin signalling pathway [72].
Recent researches indicate that each type of factors mainly mediates the cell signal transduction of the corresponding signalling pathways to regulate the function of BM-MSCs. The in vivo and in vitro studies have shown that p38α deficiency can affect the synthesis of RANKL, M-CSF, or other molecules involved in the regulation of osteoclast formation, and that p38α expressed by BM-MSCs can effectively regulate osteogenesis through the TAK1-NF-κB signalling pathway and promote the production of OPG by stem cells to regulate the generation of osteoclasts [73]. Furthermore, the influence of RNA in regulating the osteogenic differentiation of BM-MSCs, in which microRNAs [74] and lncRNAs [75] were aberrantly expressed and combined with their respective target proteins in the patients with OP, thus playing a role in the regulation of osteogenic differentiation of BMSCs.
Adipose tissue-derived mesenchymal stem cells
Known as pluripotent stem cells, adipose-derived mesenchymal stem cells are one of the common types of MSCs in cell therapy and regenerative medicine. AD-MSCs, mainly isolated from adipose tissues, are more abundant in brown adipose tissues than in white adipose ones. Mostly AD-MSCs which emerge from the mesoderm can be directed to differentiate into other mesoderm-derived tissues such as fat, bone, muscle, tendon, and blood vessel [76]. When the inflammation or injury occurs in vivo, the body may reduce the activity of AD-MSCs, affecting their immune function by regulating paracrine function and differentiation potential [77]. However, the inflammatory cytokines and chemokines released at the injury site can enable AD-MSCs to be recruited there, showing strong local functions in immunoregulation, tissue repair or other processes [51].
Similar to BM-MSCs, AD-MSCs have a high amplification capacity and multilineage differentiation potential [78]; moreover, they are present in adipose tissue, thus producing the advantages of being relatively easy and safe to obtain and low immunogenicity, and their proliferation and differentiation potential are less likely to be affected by the age of the donor and the number of passages [79, 80]. Therefore, the human adult adipose tissue may be the most suitable source of MSCs. As confirmed in the previously reported studies on ovariectomized mouse models [81], rabbit models [82], and in vitro osteogenic experiments involving AD-MSCs [79, 83], AD-MSC-based cell transplant therapy can effectively promote the recovery of OP in the short term and enhance bone regeneration in vivo. Further studies have revealed that osteogenic inducible factors in humans can regulate human adipose-derived stem cells through the ERK pathway [84], PKA pathway [85], and Wnt/β-catenin pathway [86], which significantly improves the osteogenic differentiation potential of AD-MSCs.
All this indicates that AD-MSCs have the promising potential to become a major source for cell transplantation therapy of OP in the future. Nevertheless, the attempts clinically made to inject AD-MSCs into where OP is present in the patient, are prohibitively difficult and of little clinical utility. Therefore, few research teams have been registered to conduct clinical trials of AD-MSCs, locally or systemically, to treat OP patients.
In recent years, however, it has been gratifying that AD-MSCs have been reported to show good results in the clinical treatment of joint microfractures [87], bone defects [88], osteoarthritis [89]. In the prospective randomized trial, autologous AD-MSCs were injected into the joints of patients with knee osteoarthritis for treatment, the therapeutic results showing a significant improvement in the patients’ joint mobility, pain, and bone and cartilage regeneration within 12 months, and indicating the adverse events to be comparable between the experimental group and control in the subsequent follow-ups [89]. From the preclinical study on AD-MSCs and their clinical applications to some diseases, a clear demonstration has been shown on their safety and reliability in vivo [90].
In view of which, we also believe that AD-MSC-based cell transplant therapy can be performed as a novel therapeutic approach to various orthopaedic diseases, including fractures and cartilage destruction. As for the application to OP and to other systemic bone diseases, however, quite a number of preclinical studies are still needed to explore the dosage, mode of administration and adverse reactions of the drug in the clinical applications.
Human umbilical cord-derived mesenchymal stem cells
Human umbilical cord mesenchymal stem cells are mainly derived from umbilical cord blood, Wharton’s jelly, blood vessels and their surrounding tissues [91]. An early study showed that many pluripotent MSCs could be isolated from UC samples, of which the cells obtained from umbilical cord blood were the most abundant, and UC-MSCs were more abundant in preterm cord blood than in term blood [92]. Since then, UC-MSCs have become another important source for the isolation of mesenchymal stem cells thanks to their nonharmful acquisition mode and few related ethical issues [93].
Biologically, UC-MSCs show advantages over bone marrow and adipose-derived stem cells, in terms of low immunogenicity, high proliferation and differentiation, and excellent anti-inflammatory and other immunomodulatory functions [94,95,96]. Since 2009, when researchers began to commit themselves to exploring the therapeutic potential of the allogeneic transplantation of UC-MSCs, the successful potential of UC-MSCs in the animal models and of their clinical application has been promoted [97]. UC-MSCs, as a potential cell source for cartilage and bone regeneration, are mainly regulated by Wnt [98], Notch [99], and AKT signalling [100], among other signalling pathways, as well as by cytokines such as TNF-α [101] that promote the differentiation of MSCs towards the osteogenic lineage.
Recent studies have suggested that the efficacy of UC-SMCs in disease treatment mainly depends on cell secretion [102], and the concentration of active factors secreted by UC-MSCs is 10–100 times that of factors secreted by BM-MSCs under the same culture conditions [103]. From these cells the secreted active molecules facilitate the restoring of viable cell proliferation and differentiation, and contribute to bone remodelling in the bone metabolism system. It has been confirmed that the secretory group of hUC-MSCs, producing TGF-β, FGF, VEGF, EGF, etc., has the ability to restore stem cell potential and delay local bone loss in age-related OP [104]. Nowadays, the application of UC-MSCs to orthopaedic diseases is mainly related to the repair of articular cartilage, as indicated in a clinical randomized controlled trial which demonstrated that the patients who had received a local injection of allogeneic UCMSCs showed an improvement in pain and function, without serious adverse events [105]. Also, UC-MSCs have achieved a good curative effect in treating rheumatoid arthritis and a good therapeutic effect in repairing bone tissue defects, as indicated in a recent retrospective study on the clinical efficacy of hUC‑MSCs in osteonecrosis of the femoral head (ONFH), which suggested that arterially infused hUC‑MSCs could migrate via the blood to the area of femoral head necrosis and differentiate into osteoblasts; 24 months later, these cells could significantly reduce the necrotic volume of the femoral head [106]. Moreover, in a study on the application of bone particles combined with hUC-MSCs to repair rabbit lumbar bone defects, hUC-MSCs, when transplanted into the defect area of the vertebral lamina and foot arch, promoted effectively bone regeneration there [107]. It was suggested that the loss of stem cell function was closely related to the occurrence of OP, and that UC-MSCs could present a higher therapeutic potential than MSCs derived from other tissues [108].
Additionally, the secretion patterns of MSCs from different tissues may be different, the differences indicating specificity for different diseases [109]. In the perimenopausal rat models, hUC-MSC therapy could significantly delay ovarian aging, thus improving the ovarian reserve function [110], and the osteogenic effect of hUC-MSC therapy could significantly increase the bone density of the osteoporotic mandible [111]. In the case of AD-MSCs, we still believe that UC-MSCs have a promising potential of treating OP clinically, although they have not yet been registered for the clinical trials of OP.
Current challenges and safety issues
Currently, MSCs are generally considered safe and effective in the animal models, and in the clinical trials, no major adverse events have been reported. Admittedly, the stem cells have the potential of self-renewal and multilineage differentiation as a unique biological characteristic; however, they can give rise to such safety problems as the potential for vascular injury [112], vascular embolism [113], infection, immune responses and abnormal accumulation of amyloid-β [114], of which the most worrisome is the development of tumours induced by chromosomal abnormalities. In a rat stroke model, cerebral blood flow decreased while the embolic events and associated lesion size increased in the rats after the intra-arterial cell infusion of MSCs; the occurrence of these phenomena seemed to be related to the cell dose and the velocity of cell infusion [115]. In a randomized multicentre clinical trial where 53 patients with refractory rheumatoid arthritis were treated with allogeneic AD-MSCs, a total of 141 side effects were observed, including 1 severe lacunar infarction [116]. Due to the characteristics of MSCs and the interference of external conditions, the occurrence of tumours cannot be completely avoided. In an early study in which mouse cancer models were used, the immune cells including neutrophils, macrophages and mast cells, were found to express MMP-9, contributing to the occurrence of skin squamous cell carcinogenesis [117]. Furthermore, MSCs were reported to be regulated by a variety of cytokines and chemokines in tumour tissues, mediating cell proliferation, angiogenesis, and metastasis [118]. Evidence suggests that MSCs can inhibit the growth of tumour cells in a healthy microenvironment and that only after acquiring tumour-like gene mutations can secrete factors to promote tumour progression [119]. In the lung adenocarcinoma (A549 cells) experiments conducted in vitro, soluble factors secreted by MSCs in the tumour microenvironment could promote the growth and metastasis of cancer cells [120]. The reduced tumorigenesis is hypothesised to be related to the methylation of genes in MSCs, as indicated in the research where DNA methylation in mesenchymal stem cells was found to be capable of regulating the expression and activation of PTEN, promoting osteogenic effects and reducing tumorigenesis [121]. Similarly, the abnormal DNA methylation in MSCs could affect the progression of myeloma, and the treatment of abnormal DNA methylation could exert an antimyeloma and osteogenic effect [122].
The clinical application of MSCs has another problem, at present, no consensus has been reached on the best single dose of MSC injection in clinical practice. In the treatment of osteoarthritis, focal cartilage defects, etc., studies have suggested that a single high dose (1.0 × 108) administered on the patients with osteoarthritis can significantly improve the clinical symptoms and therapeutic outcomes of radiology and arthroscopy [123]. Surely, the efficiency of MSC transplantation needs to be examined in the clinical context. In the study of bone tissue repair, intramedullary injected MSCs could reach the bone marrow quickly, but the apoptosis rate was significantly high during the migration to the damaged site; therefore, the ultimate number of MSCs at the damaged site was significantly small [124]. Although ideal results have been achieved in the animal models in which OP was treated, the systemic involvement of OP and the existence of adverse events associated with MSCs still restrict its clinical application to OP treatment.
Conclusion
OP is a systemic metabolic bone disease characterized by low BMD caused by abnormal bone metabolism. At present, the clinical approaches mainly involve exercise intervention and drug therapy, the former delaying bone loss in the early stage of the disease, and the latter having a therapeutic effect; however, long-term drug use can cause serious side effects. So far, great progresses have been achieved in the treatment of clinical diseases with stem cells, which are characterized by unique biological advantages in terms of proliferation, differentiation, and immune regulation, among other properties. However, there are still many challenges to be addressed in the practical application of MSC therapy. Overall, MSC-based stem cell therapy is relatively safe for the treatment of local bone injury. Actually, the potential of this method in OP treatment is unlimited, but the fate, tumorigenicity and ethical problems of clinical treatment of stem cells limit its clinical application. Further preclinical research is needed to clarify its long-term safety and effectiveness. With a growing understanding of exosomes, we speculate that stem cell-derived exosomes could be used as a feasible strategy to replace stem cell therapy to fight osteoporosis. Therefore, we suggest that in the near future researches much importance should be attached to the clinical transformation of mesenchymal stem cell therapy and the stem cell-derived exosomes in the treatment of OP.
Availability of data and materials
Not applicable.
Abbreviations
- OP:
-
Osteoporosis
- BM:
-
Bone marrow
- BMD:
-
Bone mineral density
- MSCs:
-
Mesenchymal stem cells
- BM-MSCs:
-
Bone marrow-derived mesenchymal stem cells
- AD-MSCs:
-
Adipose tissue-derived MSCs
- UC-MSCs:
-
Umbilical cord-derived MSCs
- BMI:
-
Body mass index
- BMAC:
-
Bone marrow aspirate concentrate
- RANKL:
-
Receptor activator of nuclear factor-κ B ligand
- OPG:
-
Osteoprotegerin
- OVX:
-
Ovariectomized
- PTH:
-
Parathyroid hormone
- LRP5/6:
-
LDL receptor related protein 5/6
- M-CSF:
-
Macrophage-colony stimulating factor
- RNA:
-
Ribonucleic acid
- DNA:
-
Deoxyriboncleic acid
- lncRNAs:
-
Long non-coding RNAs
- TGF-β:
-
Transforming growth factor-β
- FGF:
-
Fibroblast growth factor
- EGF:
-
Epidermal growth factor
- VEGF:
-
Vascular epidermal growth factor
- ONFH:
-
Osteonecrosis of the femoral head
References
Thambiah SC, Yeap SS. Osteoporosis in South-East Asian countries. Clin Biochem Rev. 2020;41(1):29–40.
Albright F, Smith PH, Richardson AM. Postmenopausal osteoporosis: its clinical features. JAMA. 1941;116(22):2465–74.
Nordin BE. Osteoporosis and calcium deficiency. Proc Nutr Soc. 1960;19:129–37.
Smith R. Osteoporosis at the Tivoli. The third international symposium on osteoporosis. October 1990—Copenhagen, Denmark. J Bone Joint Surg Br. 1991;73(3):525–6.
Peck WA. Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1993;94(6):646–50.
Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet. 2019;393(10169):364–76.
Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29(11):2520–6.
Zeng Q, Li N, Wang Q, Feng J, Sun D, Zhang Q, et al. The prevalence of osteoporosis in China, a nationwide, multicenter DXA survey. J Bone Miner Res. 2019;34(10):1789–97.
Qaseem A, Forciea MA, McLean RM, Denberg TD. Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(11):818–39.
Bolland MJ, Grey A, Avenell A. Effects of vitamin D supplementation on musculoskeletal health: a systematic review, meta-analysis, and trial sequential analysis. Lancet Diabetes Endocrinol. 2018;6(11):847–58.
Vargas-Franco JW, Castaneda B, Rédiní F, Gómez DF, Heymann D, Lézot F. Paradoxical side effects of bisphosphonates on the skeleton: what do we know and what can we do? J Cell Physiol. 2018;233(8):5696–715.
Arjmand B, Sarvari M, Alavi-Moghadam S, Payab M, Goodarzi P, Gilany K, et al. Prospect of stem cell therapy and regenerative medicine in osteoporosis. Front Endocrinol. 2020;11:430.
Donsante S, Palmisano B, Serafini M, Robey PG, Corsi A, Riminucci M. From stem cells to bone-forming cells. Int J Mol Sci. 2021;22(8):3989.
Tsiapalis D, O’Driscoll L. Mesenchymal stem cell derived extracellular vesicles for tissue engineering and regenerative medicine applications. Cells. 2020;9(4):991.
Waugh EJ, Lam MA, Hawker GA, McGowan J, Papaioannou A, Cheung AM, et al. Risk factors for low bone mass in healthy 40–60 year old women: a systematic review of the literature. Osteoporos Int. 2009;20(1):1–21.
Del Puente A, Esposito A, Del Puente A, Costa L, Caso F, Scarpa R. Physiopathology of osteoporosis: from risk factors analysis to treatment. J Biol Regul Homeost Agents. 2015;29(3):527–31.
Caffarelli C, Alessi C, Nuti R, Gonnelli S. Divergent effects of obesity on fragility fractures. Clin Interv Aging. 2014;9:1629–36.
Bachmann KN, Fazeli PK, Lawson EA, Russell BM, Riccio AD, Meenaghan E, et al. Comparison of hip geometry, strength, and estimated fracture risk in women with anorexia nervosa and overweight/obese women. J Clin Endocrinol Metab. 2014;99(12):4664–73.
Kim KC, Shin DH, Lee SY, Im JA, Lee DC. Relation between obesity and bone mineral density and vertebral fractures in Korean postmenopausal women. Yonsei Med J Yonsei Med J. 2010;51(6):857–63.
Tanaka S, Kuroda T, Saito M, Shiraki M. Overweight/obesity and underweight are both risk factors for osteoporotic fractures at different sites in Japanese postmenopausal women. Osteoporos Int. 2013;24(1):69–76.
Nakamura Y, Nakano M, Suzuki T, Sato J, Kato H, Takahashi J, et al. Two adipocytokines, leptin and adiponectin, independently predict osteoporotic fracture risk at different bone sites in postmenopausal women. Bone. 2020;137: 115404.
Mpalaris V, Anagnostis P, Anastasilakis AD, Goulis DG, Doumas A, Iakovou I. Serum leptin, adiponectin and ghrelin concentrations in post-menopausal women: is there an association with bone mineral density? Maturitas. 2016;88:32–6.
Fassio A, Idolazzi L, Rossini M, Gatti D, Adami G, Giollo A, et al. The obesity paradox and osteoporosis. Eat Weight Disord. 2018;23(3):293–302.
Herman BC, Cardoso L, Majeska RJ, Jepsen KJ, Schaffler MB. Activation of bone remodeling after fatigue: differential response to linear microcracks and diffuse damage. Bone. 2010;47(4):766–72.
Boyce BF, Xing L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res Ther. 2007;9(Suppl 1):1.
Ono T, Hayashi M, Sasaki F, Nakashima T. RANKL biology: bone metabolism, the immune system, and beyond. Inflamm Regen. 2020;40:2.
Nusse R, Clevers H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169(6):985–99.
Reyes-Garcia R, Mendoza N, Palacios S, Salas N, Quesada-Charneco M, Garcia-Martin A, et al. Effects of daily intake of calcium and vitamin D-enriched milk in healthy postmenopausal women: a randomized, controlled, double-blind nutritional study. J Womens Health. 2018;27(5):561–8.
Reid IR, Horne AM, Mihov B, Stewart A, Garratt E, Wong S, et al. Fracture prevention with zoledronate in older women with osteopenia. N Engl J Med. 2018;379(25):2407–16.
Fung P, Bedogni G, Bedogni A, Petrie A, Porter S, Campisi G, et al. Time to onset of bisphosphonate-related osteonecrosis of the jaws: a multicentre retrospective cohort study. Oral Dis. 2017;23(4):477–83.
Black DM, Abrahamsen B, Bouxsein ML, Einhorn T, Napoli N. Atypical femur fractures: review of epidemiology, relationship to bisphosphonates, prevention, and clinical management. Endocr Rev. 2019;40(2):333–68.
Kendler DL, Marin F, Zerbini CAF, Russo LA, Greenspan SL, Zikan V, et al. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet. 2018;391(10117):230–40.
Cipriani C, Irani D, Bilezikian JP. Safety of osteoanabolic therapy: a decade of experience. J Bone Miner Res. 2012;27(12):2419–28.
Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359–81.
Tobeiha M, Moghadasian MH, Amin N, Jafarnejad S. RANKL/RANK/OPG pathway: a mechanism involved in exercise-induced bone remodeling. Biomed Res Int. 2020;2020:6910312.
Khosla S, Hofbauer LC. Osteoporosis treatment: recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 2017;5(11):898–907.
López-Jornet P, Camacho-Alonso F, Molina-Miñano F, Gomez-Garcia F. Bisphosphonate-associated osteonecrosis of the jaw. Knowledge and attitudes of dentists and dental students: a preliminary study. J Eval Clin Pract. 2010;16(5):878–82.
Papapoulos S, Lippuner K, Roux C, Lin CJ, Kendler DL, Lewiecki EM, et al. The effect of 8 or 5 years of denosumab treatment in postmenopausal women with osteoporosis: results from the FREEDOM Extension study. Osteoporos Int. 2015;26(12):2773–83.
Cosman F, Crittenden DB, Adachi JD, Binkley N, Czerwinski E, Ferrari S, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375(16):1532–43.
Chouinard L, Felx M, Mellal N, Varela A, Mann P, Jolette J, et al. Carcinogenicity risk assessment of romosozumab: a review of scientific weight-of-evidence and findings in a rat lifetime pharmacology study. Regul Toxicol Pharmacol. 2016;81:212–22.
McClung MR, Grauer A, Boonen S, Bolognese MA, Brown JP, Diez-Perez A, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370(5):412–20.
Boggild MK, Gajic-Veljanoski O, McDonald-Blumer H, Ridout R, Tile L, Josse R, et al. Odanacatib for the treatment of osteoporosis. Expert Opin Pharmacother. 2015;16(11):1717–26.
Nakamura T, Shiraki M, Fukunaga M, Tomomitsu T, Santora AC, Tsai R, et al. Effect of the cathepsin K inhibitor odanacatib administered once weekly on bone mineral density in Japanese patients with osteoporosis–a double-blind, randomized, dose-finding study. Osteoporos Int. 2014;25(1):367–76.
Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6(2):230–47.
Song N, Scholtemeijer M, Shah K. Mesenchymal stem cell immunomodulation: mechanisms and therapeutic potential. Trends Pharmacol Sci. 2020;41(9):653–64.
Kyurkchiev D, Bochev I, Ivanova-Todorova E, Mourdjeva M, Oreshkova T, Belemezova K, et al. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J Stem Cells. 2014;6(5):552–70.
Linero I, Chaparro O. Paracrine effect of mesenchymal stem cells derived from human adipose tissue in bone regeneration. PLoS ONE. 2014;9(9): e107001.
Shao J, Zhang W, Yang T. Using mesenchymal stem cells as a therapy for bone regeneration and repairing. Biol Res. 2015;48:62.
Kim GB, Shon OJ. Current perspectives in stem cell therapies for osteoarthritis of the knee. Yeungnam Univ J Med. 2020;37(3):149–58.
Jin YZ, Lee JH. Mesenchymal stem cell therapy for bone regeneration. Clin Orthop Surg. 2018;10(3):271–8.
Sabol RA, Bowles AC, Côté A, Wise R, Pashos N, Bunnell BA. Therapeutic potential of adipose stem cells. Adv Exp Med Biol. 2021;1341:15–25.
Li X, Duan L, Liang Y, Zhu W, Xiong J, Wang D. Human umbilical cord blood-derived mesenchymal stem cells contribute to chondrogenesis in coculture with chondrocytes. Biomed Res Int. 2016;2016:3827057.
Wang T, Liu Q, Tjhioe W, Zhao J, Lu A, Zhang G, et al. Therapeutic potential and outlook of alternative medicine for osteoporosis. Curr Drug Targets. 2017;18(9):1051–68.
Jiang Y, Zhang P, Zhang X, Lv L, Zhou Y. Advances in mesenchymal stem cell transplantation for the treatment of osteoporosis. Cell Prolif. 2021;54(1): e12956.
Aghebati-Maleki L, Dolati S, Zandi R, Fotouhi A, Ahmadi M, Aghebati A, et al. Prospect of mesenchymal stem cells in therapy of osteoporosis: a review. J Cell Physiol. 2019;234(6):8570–8.
Hu L, Yin C, Zhao F, Ali A, Ma J, Qian A. Mesenchymal stem cells: cell fate decision to osteoblast or adipocyte and application in osteoporosis treatment. Int J Mol Sci. 2018;19(2):360.
Kong Y, Zhao Y, Li D, Shen H, Yan M. Dual delivery of encapsulated BM-MSCs and BMP-2 improves osteogenic differentiation and new bone formation. J Biomed Mater Res A. 2019;107(10):2282–95.
Freitas GP, Lopes HB, Souza ATP, Oliveira PGFP, Almeida ALG, Souza LEB, et al. Cell therapy: effect of locally injected mesenchymal stromal cells derived from bone marrow or adipose tissue on bone regeneration of rat calvarial defects. Sci Rep. 2019;9(1):13476.
Ichioka N, Inaba M, Kushida T, Esumi T, Takahara K, Inaba K, et al. Prevention of senile osteoporosis in SAMP6 mice by intrabone marrow injection of allogeneic bone marrow cells. Stem Cells. 2002;20(6):542–51.
Takada K, Inaba M, Ichioka N, Ueda Y, Taira M, Baba S, et al. Treatment of senile osteoporosis in SAMP6 mice by intra-bone marrow injection of allogeneic bone marrow cells. Stem Cells. 2006;24(2):399–405.
Ocarino Nde M, Boeloni JN, Jorgetti V, Gomes DA, Goes AM, Serakides R. Intra-bone marrow injection of mesenchymal stem cells improves the femur bone mass of osteoporotic female rats. Connect Tissue Res. 2010;51(6):426–33.
Huang S, Xu L, Zhang Y, Sun Y, Li G. Systemic and local administration of allogeneic bone marrow-derived mesenchymal stem cells promotes fracture healing in rats. Cell Transplant. 2015;24(12):2643–55.
Wang J, Zhou L, Zhang Y, Huang L, Shi Q. Mesenchymal stem cells—a promising strategy for treating knee osteoarthritis. Bone Joint Res. 2020;9(10):719–28.
Hu BT, Chen WZ. MOTS-c improves osteoporosis by promoting osteogenic differentiation of bone marrow mesenchymal stem cells via TGF-β/Smad pathway. Eur Rev Med Pharmacol Sci. 2018;22(21):7156–63.
Wang QL, Li HF, Wang DP, Liu ZY, Xiao WW, Xu LL, et al. Effect of GGCX on the differentiation function of osteoporosis bone marrow mesenchymal stem cells through regulating TGFβ/smad signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23(17):7224–31.
Ren C, Gong W, Li F, Xie M. Pilose antler aqueous extract promotes the proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells by stimulating the BMP-2/Smad1, 5/Runx2 signaling pathway. Chin J Nat Med. 2019;17(10):756–67.
Feng C, Xiao L, Yu JC, Li DY, Tang TY, Liao W, et al. Simvastatin promotes osteogenic differentiation of mesenchymal stem cells in rat model of osteoporosis through BMP-2/Smads signaling pathway. Eur Rev Med Pharmacol Sci. 2020;24(1):434–43.
Zhao P, Xiao L, Peng J, Qian YQ, Huang CC. Exosomes derived from bone marrow mesenchymal stem cells improve osteoporosis through promoting osteoblast proliferation via MAPK pathway. Eur Rev Med Pharmacol Sci. 2018;22(12):3962–70.
Jiang Y, Wu W, Jiao G, Chen Y, Liu H. LncRNA SNHG1 modulates p38 MAPK pathway through Nedd4 and thus inhibits osteogenic differentiation of bone marrow mesenchymal stem cells. Life Sci. 2019;228:208–14.
Shen JJ, Zhang CH, Chen ZW, Wang ZX, Yang DC, Zhang FL, et al. LncRNA HOTAIR inhibited osteogenic differentiation of BMSCs by regulating Wnt/β-catenin pathway. Eur Rev Med Pharmacol Sci. 2019;23(17):7232–46.
Yuan Z, Li Q, Luo S, Liu Z, Luo D, Zhang B, et al. PPARγ and Wnt signaling in adipogenic and osteogenic differentiation of mesenchymal stem cells. Curr Stem Cell Res Ther. 2016;11(3):216–25.
Shen G, Ren H, Shang Q, Zhao W, Zhang Z, Yu X, et al. Foxf1 knockdown promotes BMSC osteogenesis in part by activating the Wnt/β-catenin signalling pathway and prevents ovariectomy-induced bone loss. EBioMedicine. 2020;52: 102626.
Cong Q, Jia H, Biswas S, Li P, Qiu S, Deng Q, et al. p38α MAPK regulates lineage commitment and OPG synthesis of bone marrow stromal cells to prevent bone loss under physiological and pathological conditions. Stem Cell Reports. 2016;6(4):566–78.
Wang Q, Wang CH, Meng Y. microRNA-1297 promotes the progression of osteoporosis through regulation of osteogenesis of bone marrow mesenchymal stem cells by targeting WNT5A. Eur Rev Med Pharmacol Sci. 2019;23(11):4541–50.
Zhang HL, Du XY, Dong QR. LncRNA XIXT promotes osteogenic differentiation of bone mesenchymal stem cells and alleviates osteoporosis progression by targeting miRNA-30a-5p. Eur Rev Med Pharmacol Sci. 2019;23(20):8721–9.
Mushahary D, Spittler A, Kasper C, Weber V, Charwat V. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytometry A. 2018;93(1):19–31.
Park JS, Piao J, Park G, Yoo KS, Hong HS. Osteoporotic conditions influence the activity of adipose-derived stem cells. Tissue Eng Regen Med. 2020;17(6):875–85.
Li CY, Wu XY, Tong JB, Yang XX, Zhao JL, Zheng QF, et al. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res Ther. 2015;6(1):55.
Chen HT, Lee MJ, Chen CH, Chuang SC, Chang LF, Ho ML, et al. Proliferation and differentiation potential of human adipose-derived mesenchymal stem cells isolated from elderly patients with osteoporotic fractures. J Cell Mol Med. 2012;16(3):582–93.
Pikuła M, Marek-Trzonkowska N, Wardowska A, Renkielska A, Trzonkowski P. Adipose tissue-derived stem cells in clinical applications. Expert Opin Biol Ther. 2013;13(10):1357–70.
Wang L, Huang C, Li Q, Xu X, Liu L, Huang K, et al. Osteogenic differentiation potential of adipose-derived stem cells from ovariectomized mice. Cell Prolif. 2017;50(2): e12328.
Ye X, Zhang P, Xue S, Xu Y, Tan J, Liu G. Adipose-derived stem cells alleviate osteoporosis by enhancing osteogenesis and inhibiting adipogenesis in a rabbit model. Cytotherapy. 2014;16(12):1643–55.
Mirsaidi A, Genelin K, Vetsch JR, Stanger S, Theiss F, Lindtner RA, et al. Therapeutic potential of adipose-derived stromal cells in age-related osteoporosis. Biomaterials. 2014;35(26):7326–35.
Jin C, Shuai T, Tang Z. HSPB7 regulates osteogenic differentiation of human adipose derived stem cells via ERK signaling pathway. Stem Cell Res Ther. 2020;11(1):450.
Fathi E, Farahzadi R. Enhancement of osteogenic differentiation of rat adipose tissue-derived mesenchymal stem cells by zinc sulphate under electromagnetic field via the PKA, ERK1/2 and Wnt/β-catenin signaling pathways. PLoS ONE. 2017;12(3): e0173877.
Zhou Z, Lu Y, Wang Y, Du L, Zhang Y, Tao J. Let-7c regulates proliferation and osteodifferentiation of human adipose-derived mesenchymal stem cells under oxidative stress by targeting SCD-1. Am J Physiol Cell Physiol. 2019;316(1):C57-69.
Koh YG, Kwon OR, Kim YS, Choi YJ, Tak DH. Adipose-derived mesenchymal stem cells with microfracture versus microfracture alone: 2-year follow-up of a prospective randomized trial. Arthroscopy. 2016;32(1):97–109.
Ding L, Tang S, Liang P, Wang C, Zhou PF, Zheng L. Bone regeneration of canine peri-implant defects using cell sheets of adipose-derived mesenchymal stem cells and platelet-rich fibrin membranes. J Oral Maxillofac Surg. 2019;77(3):499–514.
Lu L, Dai C, Zhang Z, Du H, Li S, Ye P, et al. Treatment of knee osteoarthritis with intra-articular injection of autologous adipose-derived mesenchymal progenitor cells: a prospective, randomized, double-blind, active-controlled, phase IIb clinical trial. Stem Cell Res Ther. 2019;10(1):143.
Reissis D, Tang QO, Cooper NC, Carasco CF, Gamie Z, Mantalaris A, et al. Current clinical evidence for the use of mesenchymal stem cells in articular cartilage repair. Expert Opin Biol Ther. 2016;16(4):535–57.
Um S, Ha J, Choi SJ, Oh W, Jin HJ. Prospects for the therapeutic development of umbilical cord blood-derived mesenchymal stem cells. World J Stem Cells. 2020;12(12):1511–28.
Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109(1):235–42.
Xie Q, Liu R, Jiang J, Peng J, Yang C, Zhang W, et al. What is the impact of human umbilical cord mesenchymal stem cell transplantation on clinical treatment? Stem Cell Res Ther. 2020;11(1):519.
Jin HJ, Bae YK, Kim M, Kwon SJ, Jeon HB, Choi SJ, et al. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int J Mol Sci. 2013;14(9):17986–8001.
Wang Q, Yang Q, Wang Z, Tong H, Ma L, Zhang Y, et al. Comparative analysis of human mesenchymal stem cells from fetal-bone marrow, adipose tissue, and Warton’s jelly as sources of cell immunomodulatory therapy. Hum Vaccin Immunother. 2016;12(1):85–96.
Zhang H, Tao Y, Liu H, Ren S, Zhang B, Chen H. Immunomodulatory function of whole human umbilical cord derived mesenchymal stem cells. Mol Immunol. 2017;87:293–9.
Can A, Celikkan FT, Cinar O. Umbilical cord mesenchymal stromal cell transplantations: a systemic analysis of clinical trials. Cytotherapy. 2017;19(12):1351–82.
Jothimani G, Di Liddo R, Pathak S, Piccione M, Sriramulu S, Banerjee A. Wnt signaling regulates the proliferation potential and lineage commitment of human umbilical cord derived mesenchymal stem cells. Mol Biol Rep. 2020;47(2):1293–308.
Na T, Liu J, Zhang K, Ding M, Yuan BZ. The notch signaling regulates CD105 expression, osteogenic differentiation and immunomodulation of human umbilical cord mesenchymal stem cells. PLoS ONE. 2015;10(2): e0118168.
Qu Z, Guo S, Fang G, Cui Z, Liu Y. AKT pathway affects bone regeneration in nonunion treated with umbilical cord-derived mesenchymal stem cells. Cell Biochem Biophys. 2015;71(3):1543–51.
Liu C, Zhang H, Tang X, Feng R, Yao G, Chen W, et al. Mesenchymal stem cells promote the osteogenesis in collagen-induced arthritic mice through the inhibition of TNF-α. Stem Cells Int. 2018;2018:4069032.
Bai L, Li D, Li J, Luo Z, Yu S, Cao S, et al. Bioactive molecules derived from umbilical cord mesenchymal stem cells. Acta Histochem. 2016;118(8):761–9.
Romanov YA, Volgina NE, Vtorushina VV, Romanov AY, Dugina TN, Kabaeva NV, et al. Comparative analysis of secretome of human umbilical cord- and bone marrow-derived multipotent mesenchymal stromal cells. Bull Exp Biol Med. 2019;166(4):535–40.
Liang M, Liu W, Peng Z, Lv S, Guan Y, An G, et al. The therapeutic effect of secretome from human umbilical cord-derived mesenchymal stem cells in age-related osteoporosis. Artif Cells Nanomed Biotechnol. 2019;47(1):1357–66.
Wang L, Huang S, Li S, Li M, Shi J, Bai W, et al. Efficacy and safety of umbilical cord mesenchymal stem cell therapy for rheumatoid arthritis patients: a prospective phase I/II study. Drug Des Devel Ther. 2019;13:4331–40.
Chen C, Qu Z, Yin X, Shang C, Ao Q, Gu Y, et al. Efficacy of umbilical cord-derived mesenchymal stem cell-based therapy for osteonecrosis of the femoral head: a three-year follow-up study. Mol Med Rep. 2016;14(5):4209–15.
Cui Y, Xu B, Yin Y, Chen B, Zhao Y, Xiao Z, et al. Repair of lumbar vertebral bone defects by bone particles combined with hUC-MSCs in weaned rabbit. Regen Med. 2019;14(10):915–23.
Wang KX, Xu LL, Rui YF, Huang S, Lin SE, Xiong JH, et al. The effects of secretion factors from umbilical cord derived mesenchymal stem cells on osteogenic differentiation of mesenchymal stem cells. PLoS ONE. 2015;10(3): e0120593.
Pires AO, Mendes-Pinheiro B, Teixeira FG, Anjo SI, Ribeiro-Samy S, Gomes ED, et al. Unveiling the differences of secretome of human bone marrow mesenchymal stem cells, adipose tissue-derived stem cells, and human umbilical cord perivascular cells: a proteomic analysis. Stem Cells Dev. 2016;25(14):1073–83.
Li J, Mao Q, He J, She H, Zhang Z, Yin C. Human umbilical cord mesenchymal stem cells improve the reserve function of perimenopausal ovary via a paracrine mechanism. Stem Cell Res Ther. 2017;8(1):55.
Hendrijantini N, Hartono P, Ari MDA, Rantan FA. Human umbilical cord mesenchymal stem-cell therapy to increase the density of osteoporotic mandibular bone. Eur J Dent. 2019;13(1):58–63.
Huang H, Kolibabka M, Eshwaran R, Chatterjee A, Schlotterer A, Willer H, et al. Intravitreal injection of mesenchymal stem cells evokes retinal vascular damage in rats. Faseb J. 2019;33(12):14668–79.
Ge J, Guo L, Wang S, Zhang Y, Cai T, Zhao RC, Wu Y. The size of mesenchymal stem cells is a significant cause of vascular obstructions and stroke. Stem Cell Rev Rep. 2014;10(2):295–303.
Mitkari B, Kerkelä E, Nystedt J, Korhonen M, Jolkkonen J. Unexpected complication in a rat stroke model: exacerbation of secondary pathology in the thalamus by subacute intraarterial administration of human bone marrow-derived mesenchymal stem cells. J Cereb Blood Flow Metab. 2015;35(3):363–6.
Cui LL, Kerkelä E, Bakreen A, Nitzsche F, Andrzejewska A, Nowakowski A, et al. The cerebral embolism evoked by intra-arterial delivery of allogeneic bone marrow mesenchymal stem cells in rats is related to cell dose and infusion velocity. Stem Cell Res Ther. 2015;6(1):11.
Álvaro-Gracia JM, Jover JA, García-Vicuña R, Carreño L, Alonso A, Marsal S, et al. Intravenous administration of expanded allogeneic adipose-derived mesenchymal stem cells in refractory rheumatoid arthritis (Cx611): results of a multicentre, dose escalation, randomised, single-blind, placebo-controlled phase Ib/IIa clinical trial. Ann Rheum Dis. 2017;76(1):196–202.
Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103(3):481–90.
Hayal TB, Kıratlı B, Şişli HB, Şahin F, Doğan A. Mesenchymal stem cells as regulators of carcinogenesis. Adv Exp Med Biol. 2019;1144:147–66.
Chanda D, Kumar S, Ponnazhagan S. Therapeutic potential of adult bone marrow-derived mesenchymal stem cells in diseases of the skeleton. J Cell Biochem. 2010;111(2):249–57.
Zakaria N, Yahaya BH. Adipose-derived mesenchymal stem cells promote growth and migration of lung adenocarcinoma cancer cells. Adv Exp Med Biol. 2020;1292:83–95.
Shen WC, Lai YC, Li LH, Liao K, Lai HC, Kao SY, et al. Methylation and PTEN activation in dental pulp mesenchymal stem cells promotes osteogenesis and reduces oncogenesis. Nat Commun. 2019;10(1):2226.
Garcia-Gomez A, Li T, de la Calle-Fabregat C, Rodríguez-Ubreva J, Ciudad L, Català-Moll F, et al. Targeting aberrant DNA methylation in mesenchymal stromal cells as a treatment for myeloma bone disease. Nat Commun. 2021;12(1):421.
Jo CH, Lee YG, Shin WH, Kim H, Chai JW, Jeong EC, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells. 2014;32(5):1254–66.
Huang S, Xu L, Sun Y, Zhang Y, Li G. The fate of systemically administrated allogeneic mesenchymal stem cells in mouse femoral fracture healing. Stem Cell Res Ther. 2015;6:206.
Acknowledgements
This work was supported by National Natural Science Foundation of China (81703533); Natural Science Foundation of Shanghai (20ZR1449500); Shanghai Jiao Tong University Medical Engineering Cross Fund (YG2019GD02); Science Technology Development Fund of Shanghai Pudong New Area (PKJ2020-Y28); Subject Leadership Project of Shanghai Pudong New Area (PWRd2016-06); Featured Clinical Discipline Project of Shanghai Pudong (PWYts2018-03).
Funding
This work was funded by National Natural Science Foundation of China (81703533); Natural Science Foundation of Shanghai (20ZR1449500); Shanghai Jiao Tong University Medical Engineering Cross Fund (YG2019GD02); Science Technology Development Fund of Shanghai Pudong New Area (PKJ2020-Y28); Subject Leadership Project of Shanghai Pudong New Area (PWRd2016-06); Featured Clinical Discipline Project of Shanghai Pudong (PWYts2018-03).
Author information
Authors and Affiliations
Contributions
JS and WZ, the corresponding authors conceived of the study, participated in its design and coordination, and have critically examined and corrected the manuscript. TC and TY carried out the literature search and data analysis, participated in the design of the study, drafted the manuscript, and contributed equally to this work. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All the authors, who have read the submitted version of the manuscript, agree to submit this work to Biological Research, which has not been published before and is not being considered by other journals.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, T., Yang, T., Zhang, W. et al. The therapeutic potential of mesenchymal stem cells in treating osteoporosis. Biol Res 54, 42 (2021). https://doi.org/10.1186/s40659-021-00366-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40659-021-00366-y