Abstract
Background and Purpose
Intravascular injection of mesenchymal stem cells (MSCs) has been found to cause considerable vascular obstructions which may lead to serious outcomes, particularly after intra-arterial injection. However, the underlying mechanisms have been poorly understood.
Methods
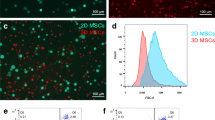
In this study, we fractionated MSCs that had been cultured in monolayer for six passages into small (average diameter = 17.9 μm) and large (average diameter 30.4 μm) populations according to their sizes, and examined their vascular obstructions after intra-internal carotid artery injection in rats and mice in comparison with MSCs derived from 3D spheroids which were uniformly smaller in size (average diameter 12.6 μm).
Results
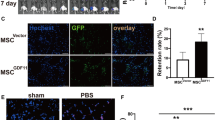
We found that 3D MSCs did not cause detectable infarct in the brain as evidenced by MRI scan and TTC stain, 2D MSCs in small size caused a microinfarct in one of five animals, which was co-localized to the area of entrapped MSCs (labeled with DiI), while 2D MSCs in large size caused much larger infarcts in all five animals, and substantial amounts of DiI-positive MSCs were found in the infarct. Meanwhile, corresponding neurological defects were observed in the animals with stroke. In consistence, injection of 2D MSCs (average diameter 26.5) caused a marked loss of cortical neurons and their axons in Thy1-GFP transgenic mice and the activation of microglia in CX3CR1-GFP transgenic mice in the area with MSC entrapment.
Conclusions
Our results suggest that the size of MSCs is a significant cause of MSC caused vascular obstructions and stroke.





Similar content being viewed by others
References
Hacke, W., Kaste, M., Bluhmki, E., Brozman, M., Davalos, A., Guidetti, D., et al. (2008). Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. The New England Journal of Medicine, 359, 1317–1329.
Zhang, Z. G., & Chopp, M. (2009). Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. Lancet Neurology, 8, 491–500.
Li, Y., Chen, J., Wang, L., Lu, M., & Chopp, M. (2001). Treatment of stroke in rat with intracarotid administration of marrow stromal cells. Neurology, 56, 1666–1672.
van Velthoven, C. T. J., Sheldon, R. A., Kavelaars, A., Derugin, N., Vexler, Z. S., Willemen, H. L. D. M., et al. (2013). Mesenchymal stem cell transplantation attenuates brain injury after neonatal stroke. Stroke, 44, 1426–1432.
Horwitz, E. M. (2006). MSC: a coming of age in regenerative medicine. Cytotherapy, 8, 194–195.
Prockop, D. J. (1997). Marrow stromal cells as stem cells for nonhematopoietic tissues. Science, 276, 71–74.
Chamberlain, G., Fox, J., Ashton, B., & Middleton, J. (2007). Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells, 25, 2739–2749.
Keating, A. (2012). Mesenchymal stromal cells: new directions. Cell Stem Cell, 10, 709–716.
Chen, J., Li, Y., Wang, L., Zhang, Z., Lu, D., Lu, M., et al. (2001). Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke, 32, 1005–1011.
Mangi, A. A., Noiseux, N., Kong, D., He, H., Rezvani, M., Ingwall, J. S., et al. (2003). Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nature Medicine, 9, 1195–1201.
Salem, H. K., & Thiemermann, C. (2010). Mesenchymal stromal cells: current understanding and clinical status. Stem Cells, 28, 585–596.
Uccelli, A., Moretta, L., & Pistoia, V. (2008). Mesenchymal stem cells in health and disease. Nature Reviews Immunology, 8, 726–736.
Wu, Y., & Zhao, R. C. (2012). The role of chemokines in mesenchymal stem cell homing to myocardium. Stem Cell Reviews, 8, 243–250.
Lee, R. H., Pulin, A. A., Seo, M. J., Kota, D. J., Ylostalo, J., Larson, B. L., Semprun-Prieto, L., Delafontaine, P., & Prockop, D. J. (2009). Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell, 5, 54–63.
Toma, C., Wagner, W. R., Bowry, S., Schwartz, A., & Villanueva, F. (2009). Fate of culture-expanded mesenchymal stem cells in the microvasculature: in vivo observations of cell kinetics. Circulation Research, 104, 398–402.
Bliss, T., Guzman, R., Daadi, M., & Steinberg, G. K. (2007). Cell transplantation therapy for stroke. Stroke, 38, 817–826.
Fischer, U. M., Harting, M. T., Jimenez, F., Monzon-Posadas, W. O., Xue, H., Savitz, S. I., et al. (2009). Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells and Development, 18, 683–692.
Guzman, R., De Los Angeles, A., Cheshier, S., Choi, R., Hoang, S., Liauw, J., et al. (2008). Intracarotid injection of fluorescence activated cell-sorted CD49d-positive neural stem cells improves targeted cell delivery and behavior after stroke in a mouse stroke model. Stroke, 39, 1300–1306.
Harting, M. T., Jimenez, F., Xue, H., Fischer, U. M., Baumgartner, J., Dash, P. K., et al. (2009). Intravenous mesenchymal stem cell therapy for traumatic brain injury. Journal of Neurosurgery, 110, 1189–1197.
Freyman, T., Polin, G., Osman, H., Crary, J., Lu, M., Cheng, L., et al. (2006). A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. European Heart Journal, 27, 1114–1122.
Vulliet, P. R., Greeley, M., Halloran, S. M., MacDonald, K. A., & Kittleson, M. D. (2004). Intra-coronary arterial injection of mesenchymal stromal cells and microinfarction in dogs. Lancet, 363, 783–784.
Walczak, P., Zhang, J., Gilad, A. A., Kedziorek, D. A., Ruiz-Cabello, J., Young, R. G., et al. (2008). Dual-modality monitoring of targeted intraarterial delivery of mesenchymal stem cells after transient ischemia. Stroke, 39, 1569–1574.
Li, Z., Liu, C., Xie, Z., Song, P., Zhao, R. C., Guo, L., et al. (2011). Epigenetic dysregulation in mesenchymal stem cell aging and spontaneous differentiation. PLoS One, 6, e20526.
Bartosh, T. J., Ylostalo, J. H., Mohammadipoor, A., Bazhanov, N., Coble, K., Claypool, K., et al. (2010). Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proceedings of the National Academy of Sciences of the United States of America, 107, 13724–13729.
Wu, Y., Ip, J. E., Huang, J., Zhang, L., Matsushita, K., Liew, C.-C., et al. (2006). Essential role of ICAM-1/CD18 in mediating EPC recruitment, angiogenesis, and repair to the infarcted myocardium. Circulation Research, 99, 315–322.
Yang, G., Pan, F., Parkhurst, C. N., Grutzendler, J., & Gan, W. B. (2010). Thinned-skull cranial window technique for long-term imaging of the cortex in live mice. Nature Protocols, 5, 201–208.
Powner, M. B., Vevis, K., McKenzie, J. A., Gandhi, P., Jadeja, S., & Fruttiger, M. (2012). Visualization of gene expression in whole mouse retina by in situ hybridization. Nature Protocols, 7, 1086–1096.
Tsai, L. K., Wang, Z., Munasinghe, J., Leng, Y., Leeds, P., & Chuang, D. M. (2011). Mesenchymal stem cells primed with valproate and lithium robustly migrate to infarcted regions and facilitate recovery in a stroke model. Stroke, 42, 2932–2939.
Li, L., Jiang, Q., Ding, G., Zhang, L., Zhang, Z. G., Li, Q., et al. (2009). Effects of administration route on migration and distribution of neural progenitor cells transplanted into rats with focal cerebral ischemia, an MRI study. Journal of Cerebral Blood Flow & Metabolism, 30, 653–662.
Stammers, A. D. (1926). The blood count and body temperature in normal rats. The Journal of Physiology, 61, 329–336.
Janowski, M., Lyczek, A., Engels, C., Xu, J., Lukomska, B., Bulte, J. W., et al. (2013). Cell size and velocity of injection are major determinants of the safety of intracarotid stem cell transplantation. Journal of Cerebral Blood Flow and Metabolism, 33, 921–927.
Eckert, M. A., Vu, Q., Xie, K., Yu, J., Liao, W., Cramer, S. C., et al. (2013). Evidence for high translational potential of mesenchymal stromal cell therapy to improve recovery from ischemic stroke. Journal of Cerebral Blood Flow and Metabolism, 33, 1322–1334.
Döppner, T., & Hermann. (2010). Mesenchymal stem cells in the treatment of ischemic stroke: Progress and possibilities (p. 157). Stem Cells and Cloning: Advances and Applications.
Acknowledgments
This work was supported by grants from Natural Science Foundation of China (No. 30871273, 31371404, U1032003) and Shenzhen Science and Technology Innovation Committee (JC201005280597A, GJHZ20120614194251967 and JCYJ20130402145002397) to Y Wu.
Conflict of interest
The authors have declared that no conflict of interest exists.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jianfeng Ge and Ling Guo contributed equally to this work
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary video 1
Trafficking of MSCs. Prior to the injection of MSCs, FITC-Dextran was injected into the tail vein of wild type Balb/C mice to illuminate the vasculature. DiI-labeled MSCs were injected into the carotid artery. The trafficking of DiI-MSCs was recorded by a video camera under fluorescence microscope. MSCs derived from 3D spheroids moved fast and smoothly in the blood vessels, while MSCs derived from monolayers moved much slower and some stopped moving in the blood vessels. (MP4 4095 kb)
Rights and permissions
About this article
Cite this article
Ge, J., Guo, L., Wang, S. et al. The Size of Mesenchymal Stem Cells is a Significant Cause of Vascular Obstructions and Stroke. Stem Cell Rev and Rep 10, 295–303 (2014). https://doi.org/10.1007/s12015-013-9492-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-013-9492-x