Abstract
Background
Galliformes are widely distributed throughout the world and economically important to humans as domesticated animals or gamebirds. They are at a unique position for advancing knowledge and techniques of wildlife conservation as the barometer of the status of applied ecology. Populations of many galliform species have declined mainly due to habitat loss and over-hunting. An assessment of knowledge of Galliformes could help to provide guidelines for future research and conservation strategies.
Methods
Using the Web of Science search engine, we conducted a literature review of galliform-related articles published from 1990 to 2016. We used the “research area” option to filter articles focused on the zoology, environmental sciences ecology, biodiversity conservation, forestry, behavioral sciences, reproductive biology, biochemistry and molecular biology, cell biology, genetics and heredity, evolutionary biology, physiology and developmental biology. We then checked duplication based on the title, abstract and full text. In addition, we examined the reference lists of selected studies to include the publications that were missed by above searching.
Results
We retained 1874 articles related to the Galliformes from the initial 243,128 publications that were found. About 91.4% focused on one or two species, and 85.0% were conducted within a short duration, typically 1–2 years. The majority of the articles concentrated on macroscopic ecology (55.5%), mainly focusing on habitat selection or habitat use. With recent advances of molecular biology, the studies of taxonomy and phylogenetics rose quickly in last two decades. The study of physiology and biochemistry was no longer limited to simple description but expanded to the mechanisms of phenotype and micro-evolutionary potential. An additional area receiving increasing attention is the conservation of Galliformes, with the assessment of the conservation status and conservation management effectiveness of Galliformes (e.g. species diversity and genetic diversity) becoming the focus.
Conclusions
The studies on Galliformes have made great achievements since 1990, but there are still gaps, particularly in macroscopic ecology, molecular genetics, and conservation. There is an urgent need to enhance long-term monitoring and analysis of population dynamics, and applying different disciplines to galliform conservation. Moreover, life history information of many galliform species is still lacking, which has hindered conservation efforts and effectiveness. In addition, multidiscipline studies and new technologies are not common for galliform studies, and should be encouraged.
Similar content being viewed by others
Background
The Galliformes is one of the most important avian groups throughout the world (del Hoyo et al. 1994; Zhang et al. 2003), and have played a beneficial role to humans as they are widely domesticated and hunted for food, plumage and trading (Fuller and Garson 2000). Galliformes have cultural importance as seen in ancient literatures and artworks (e.g. the characters of “pheasant” and “chicken” appeared in oracle inscriptions in the Shang Dynasty of China) (Peters et al. 2016). In addition, many galliform specimens were captured by some naturalists and explorers from the start of nineteenth century to the 1960s. Hence, it contributed partly to the accelerated decline of some Galliformes because of the great interest in the gorgeous looking and economic value of wild animals (Hennache 2009). Besides hunting, many species of Galliformes have also been threatened by habitat loss (Lawes et al. 2006; Zhou et al. 2015a), human disturbance (Storch 2013), and urbanization (McNew and Sandercock 2013). In particular, the population of many species of Galliformes declined dramatically (Kurhinen et al. 2009; Johnson et al. 2014), such as the Hazel Grouse (Bonasa bonasia), Reeves’s Pheasant (Syrmaticus reevesii) and Tibetan Eared-pheasant (Crossoptilon harmani) (Lu and Zheng 2007; Rhim 2010; Zhou et al. 2015a).
Galliformes have been in a unique position to advance wildlife conservation and research (McGowan and Garson 1995; McGowan et al. 2012) because of their close relationship with human and some species being model animals in animal/avian studies (del Hoyo et al. 1994; Fuller and Garson 2000). Since 1975, the conservation and research of Galliformes have been greatly promoted after the establishment of the World Pheasant Association (WPA) (Tang 1990; Moss et al. 2010). During this time, many techniques (e.g. DNA testing and artificial insemination) were also developed and applied in the research of Galliformes (Gee 1983; Hennache 2009). A brief summary on galliform research before 1989 was presented in the 4th International Symposium on Galliformes in 1989 (Tang 1990). Although there are some recent reviews of Galliformes with focuses on either one topic (e.g. taxonomy or phylogeny) (Crowe et al. 2006; Zheng 2015) or targeted a single species (Moss et al. 2010), the global research status and study areas of Galliformes were not well documented.
Here, we reviewed the literatures on Galliformes published since 1990, and aimed to (1) review the current study areas on Galliformes, (2) analyze the potential implications of deficiency in the knowledge for a complete understanding of Galliformes, and (3) provide suggestions for future research on Galliformes.
Methods
We conducted a search of the literatures on Galliformes published during 1990‒2016. The search engine, Web of Science, was used for collecting articles with the key words “Galliformes”, “Megapodiidae”, “Cracidae”, “Meleagrididae”, “Tetraonidae”, “Odontophoridae”, “Numididae”, “Phasianidae”, and the names of each genus of Galliformes. The genera of Galliformes (Table 1) were decided according to the IOC World Bird List (Gill and Donsker 2016) and eBird/Clements Checklist (Clements et al. 2016).
We used the “Refine Results” option in Web of Science to filter articles and retained the articles written in English. Then we used the “research area” option to filter the articles focused on the zoology, environmental sciences ecology, biodiversity conservation, forestry, behavioral sciences, reproductive biology, biochemistry and molecular biology, cell biology, genetics and heredity, evolutionary biology, physiology, and developmental biology. Topics focusing on agriculture, psychology, virology, medical science, surgery, energy fuels, history, social issues, business economics and food science that was not related to our topic were removed. Finally, all the articles retained were checked manually based on their titles, abstracts and full texts to reduce duplications and were confirmed the research species were not domesticated. The PRISMA flow diagram (Moher et al. 2009) showed the procedure used for selection of studies for this systematic review (Fig. 1).
For the retained articles, we collected information including author(s), country of author(s), title, abstract, year, study object and research content for each article, and used the country of the first author to report the origin of study. We divided authors’ countries into seven regions: Asia (China, Japan, Korea, etc.), Europe (Finland, Spain, United Kingdom, etc.), Africa (South Africa, Nigeria, etc.), Latin America (Brazil, Mexico, etc.), Middle East (Iran, Turkey, etc.), United States of America/Canada, Australia/New Zealand (Marzluff 2016). Meanwhile, the papers were grouped into six subject areas based on the contents (Table 2). Seven articles on fossil studies were classified into the group of taxonomy and phylogenetics, as those articles had a closer relationship with phylogenetics.
We used SPSS 21.0 (SPSS Inc., Chicago, IL, USA) for data analysis. We employed Spearman correlation analysis to assess the relationship between the number of articles in each region and the number of genus in the corresponding region. In order to test whether there was a significant influence of the 23rd International Ornithological Congress held in Beijing in 2002 on the research of Galliformes, we used independent samples t test to compare the number of articles published each year before and after 2003 in this study.
Results
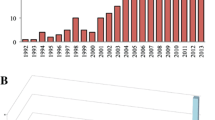
Of the 1874 retained articles, nearly half (49.4%) were from United States of America/Canada, and followed by Europe (26.7%), Asia (14.6%), Latin America (3.6%), Africa (2.1%), Australia/New Zealand (2.0%), and Middle East (1.7%). The average growth rate was 37.9% over the years, and the number of articles after 2003 had a great increase compared with that before 2003 (Independent samples t test, t25 = − 20.7, p < 0.001) (Fig. 2). Regions with more genera of Galliformes had more publications (Fig. 3, Spearman correlation analysis, r = 0.937, p = 0.002).
Most studies (85.0%) were conducted within a short duration, typically 1–2 years, and 91.4% of all studies focused on one or two species. There were 224 studies concentrating on Sage Grouse (Centrocercus urophasianus), 150 on Wild Turkey (Meleagris gallopavo), and 145 on Northern Bobwhite (Colinus virginianus). Recently, an increasing number of long-term or multiple species studies occurred. Sun et al. (2007) monitored the Chinese Grouse (Tetrastes sewerzowi) in Gansu Province for more than 30 years, and the study interests have covered habitat preference, home range and movement, and nest site selection. Clawson et al. (2015) conducted a 50-year study of the abundance and hunting effect of Wild Turkeys in Missouri, USA, and found that the number of turkeys had reached the maximum capacity of the local environment in the 1980s. In addition, those studies on multiple species usually focused on the phylogenetic relationship among the species (e.g. Crowe 2010; Galla and Johnson 2015).
A majority of Galliforme research concentrated on macroscopic ecology, followed by molecular ecology, physiology and biochemistry, taxonomy and phylogenetics, conservation and some other field research (Fig. 4). The early studies on Galliformes mainly focused on physiology (e.g. Onyeyili et al. 1992; Onyeanusi et al. 1993), descriptions of reproductive biology (e.g. Follett and Pearce 1990; Follett et al. 1992; Ancel and Visschedijk 1993), and identifying molecular markers (e.g. Hanotte et al. 1991; Matzke et al. 1992). However, more research began to focus on macroscopic ecology (n = 1026) since 2003, with the proportions rising rapidly over the study period (Fig. 5).
Macroscopic ecology
The research on the macroscopic ecology of Galliformes mainly concentrated on habitat selection or habitat use (34.1%), reproductive ecology (22.3%), and population studies (25.3%), and there is a rising trend (Fig. 6).
As habitat use has a direct impact on species survival and individual fitness (e.g. Block and Brennan 1993), many articles assessed habitat characteristics of Galliformes, such as topography (e.g. Tirpak et al. 2008; Zhou et al. 2015a), vegetation type (e.g. Chávez-león et al. 2004; Dzialak et al. 2011; Anich et al. 2013) and climate change (e.g. Kvasnes et al. 2014). The home range or territory of the Galliformes and the influence factors, including habitat characteristics, were also interested by many researchers at the early stage (e.g. Iqubal et al. 2003). It has been found that the home range sizes of Galliformes varied with the gender, seasons, breeding period, and food abundance (e.g. Fearer and Stauffer 2003; Xu et al. 2009; Wang et al. 2012b; Janke and Gates 2013).
The application of Species Distribution Models in analysis of spatio-temporal variations of habitat selection or habitat suitability became popular especially at the beginning of the 21st century (Jones 2001; Fearer and Stauffer 2003; Xu and Zhang 2011; Coates et al. 2016; Li et al. 2016). Lots of researchers have processed the studies on Galliformes at multiple spatial scale (e.g. Dzialak et al. 2012; Ross et al. 2016), and their results showed that the habitat use patterns of some species varied at different spatial scales (Dzialak et al. 2012), whereas those of some species were similar at different spatial scales (Thogmartin 1999). As regards the temporal scale, researchers conducted these studies at different time intervals, including different seasons, life history stages or years under the background of climate changes, which further influenced the perception of habitat availability and habitat selection (Jones 2001; Dzialak et al. 2011; Kvasnes et al. 2014).
Habitat loss or fragmentation have negative impacts on many Galliformes, especially pheasants (e.g. Jones 2001; Deng and Zheng 2004; Lawes et al. 2006), and can negatively influence population distribution (e.g. Deng and Zheng 2004; Zhou et al. 2015a), nest survival (e.g. Goddard and Dawson 2009) and increase individual mortality (e.g. Robinson et al. 2016). Specially, more and more research has paid attention to the impact of the human footprint or human disturbance on Galliformes (e.g. Froese et al. 2015; Tanner et al. 2015; Zhang et al. 2015; Smith et al. 2016).
Reproductive ecology is also an important aspect of macroscopic ecological studies on Galliformes (Jones 2001). In additional to recording breeding parameters like egg size, clutch size and incubation period (Hernández et al. 2003), there are more efforts focusing on breeding habitat use or nest site selection (Jones 2001). A great number of results stated that the vegetation canopy density was one of the main factors related to nest site selection of pheasants (e.g. McNew and Sandercock 2013; Wu et al. 2013). However, it was controversial about the influence of the vegetation cover on the nest fate (Lu and Zheng 2003; Rhim 2012; Khalil et al. 2016). Synthetic reviews suggested that high nest survival rate may be attributed to the extended breeding season (Jansen and Crowe 2005) and available supplemental food sources (Sandoval and Barrantes 2012). Meanwhile, the predation (Ellis-Felege et al. 2013; Carpio et al. 2014; Capdevila et al. 2016; Lyly et al. 2016), competition (Robel et al. 2003; Hämäläinen et al. 2012), extreme weather condition (Kobayashi and Nakamura 2013) and temperature effects (Xu et al. 2008) were likely to be the principal causes of nest failure.
As the main natural factors, those causes mentioned above contributed to the decrease in population size and density (e.g. Sučić 2008; Rolstad et al. 2009). For the non-natural factors, a general consensus emerged that hunting and human disturbance were the most important reasons of the rapid decline of the population size and density of Galliformes (e.g. Franco et al. 2006; Stiver et al. 2008; Hörnell et al. 2014). However, some researchers hold different opinions that reasonable hunting and moderate interference have no significant influence on population density and survival rate (Williams et al. 2004) as the species were found to modify their behaviors and spatial movements to increase their habitat use (Brøseth and Pedersen 2010). The self-regulating mechanism of maintaining the relatively stable population continues to fascinate ecologists of population ecology (Moss et al. 2010).
Molecular ecology
Basic molecular genetics are used to study genetic diversity differences among populations to verify the ecological theories (Bouzat 2000), whereas recent studies turned to changes of the genetic structure under different circumstances (e.g. Bellinger et al. 2003; Gu et al. 2012; Dong et al. 2013). For instance, Huang et al. (2007) found that the genetic diversity of Rusty-necklaced Partridge (Alectoris magna) increased with latitude, altitude, and climate stability, whereas habitat fragmentation (Benedict et al. 2003) reduced genetic diversity of ptarmigan populations. Huang et al. (2005) showed that the peripheral populations that were not isolated exhibited higher genetic diversity than isolated populations. Low genetic variation and diversity were often considered to contribute to the extinction of species when population size was small (Johnson and Dunn 2006). As an important source of genetic variation in populations, introgressive hybridization is widespread (Barilani et al. 2007b). The genetic integrity of the Rusty-necklaced Partridge was shown to be at risk from introgressive hybridization, and the introgressive hybridization may disrupt local adaptations in natural populations (Barilani et al. 2007a) and pollute the gene pool of wild populations (Barilani et al. 2007b). Although unidirectional introgression did not reduce genetic diversity of some species like partridges, it affected the balance of gene flow among populations (Chen et al. 2016).
The methods of the genetic diversity research have been used to address questions based on morphological traits, biochemical markers, molecular markers, and information from whole genome sequencing (Powell et al. 1996). In recent years, applications using molecular markers, such as Restriction Fragment Length Polymorphism (RFLP) and Simple Sequence Repeat microsatellite (SSR) for testing the species differences in genetic structures have become popular. The technique based on microsatellite markers has become one of the most advanced techniques of analyzing molecular markers due to the high polymorphism (Vignal et al. 2002); and the publications accounted for 26.6% of all the articles in this category. Zhou and Zhang (2009) assessed the isolation and characterization of microsatellite markers of Temminck’s Tragopan (T. temminckii), a threatened species in China, which provided means for studying gene flow and genetic diversity of the species. Some studies employed nuclear or mitochondrial marker to study phylogenetic relationships, such as Birks and Edwards (2002) studied the phylogeny of the megapodes (Megapodiidae) based on nuclear and mitochondrial DNA sequences and showed an early split within the megapodes, leading to two major clades. Others used the molecular technique for sex identification, which facilitated the assessment of the sexual ration and related questions in wild population. Wang and Zhang (2009) designed a pair of primers (sex1/sex2) for sex identification in Brown Eared-pheasant (C. mantchuricum) based on the mechanism of PCR amplification of CHD fragments; these primers were found to be more sensitive than P2/P8 and can also be used for sex identification in other species of Phasianidae and Passeriformes.
Taxonomy and phylogenetics
Researchers have paid more attention to the taxonomy and phylogenetics of Galliformes (Moulin et al. 2003; Lu 2015). Most studies of taxonomic status were conducted by using genetic methods. For example, Chang et al. (2008) discovered that phylogeographic monophyly and large genetic distance existed between the Hainan Peacock-pheasant and the Grey Peacock-pheasant (Polyplectron bicalcaratum katsumatae) by using molecular markers, including the mitochondrial cytochrome b gene and one loci. However, only several articles tested the taxonomy and phylogenetics by using morphological methods. As the morphological features of species might vary considerably with diet and habitat, the traditional morphological identification technology also had obvious defects, which require professional ornithologists to review a large amount of literatures for identification (Kayvanfar et al. 2015).
Researchers also analyzed the genomes to identify phylogenetic relationships of different species (e.g. He et al. 2009; Jiang et al. 2014; Zhou et al. 2015b), aiming to clarify the relationship among genera, species or subspecies (Huang et al. 2007; Chen et al. 2015; Persons et al. 2016). For example, Ren et al. (2016) suggested that the genus Crossoptilon was the sister of the genus Lophura. The phylogenetic relationship among Phasianidae species has presented great challenges (Bush and Strobeck 2003). In 2010, based on mitochondrial genome of 34 species, Shen et al. (2010) provided evidences for clarifying the phylogenetic relationship of the Phasianidae; the conclusion was largely consistent with previous molecular studies based on mitochondrial genes and nuclear segments (Shen et al. 2014). However, the most recent studies have exhibited incongruence regarding the relationships within this order. For instance, Shen et al. (2010) suggested a derived position for turkeys and grouse within the Phasianidae, and placed them sister to each other, while Wang et al. (2013) stated that the turkey and grouse formed a sister group nesting inside the Phasianidae based on data from 88 galliform species and four anseriform outgroups. Some of these inconsistencies may reflect the types of data (mitochondrial or nuclear DNA data) used in analysis (Wang et al. 2013). Therefore additional research, such as fossil records, is needed for better understanding the phylogeny of Galliformes (Thomas 2015).
Physiology and biochemistry
Recently the researches of the physiological and biochemical aspect of Galliformes are not limited to the simple description of organs (e.g. nose, intestine), and a series of studies focus on the morphological structure and the mechanism of organs (Kadhim et al. 2010; Bourke and Witmer 2016). For instance, Charvet and Striedter (2008) collected the embryos of the Northern Bobwhite (C. virginianus) and the Budgerigar (Melopsittacus undulatus) at various stages to examine whether the differences in brain region size were due to the different species in cell cycle rates. The results showed that the tectum was initially much smaller but then grew more extensively in parakeets than in quail, and species in adult brain proportions can be traced back to cell cycle kinetics. The researchers also analyzed the kinematics as movements were the mechanically complex activities, which improved our understanding of how these muscles modulate mechanical function (Daley et al. 2009).
A number of studies investigated physiological coping mechanism to the stress response of Galliformes in wild environment. Some evidence proved that the acute stress can be caused by the sudden prey and human interference. Jankowski et al. (2014) found that the amount of grazing was positively associated with the content of cortisol metabolites on Sage Grouse. In term of the chronic stress, the change of seasons and circadian rhythms were the important impact factors, and they would cause basal corticosterone secreted variation (Follett et al. 1992). By affecting the hypothalamic–pituitary–gonadal (HPG) axis, corticosterone can inhibit the reproduction of Galliformes. Moreover, the effect of corticosterone on reproductive was not only on the decrease content of sex hormone, but also on the offspring sex ratio (Pike and Petrie 2006).
In general, the hormone levels were influenced by the body size, gender, and were associated with the species of Galliformes (e.g. Jankowski et al. 2014; Corfield et al. 2016). Some evidence also showed that maternal hormones were a good pathway to influence offspring development. For instance, the female Common Quail (Coturnix coturnix) with high concentration of corticosterone could transfer corticosterone to yolk, and may alter offspring growth and adult phenotype (Hayward and Wingfield 2004). Herrington et al. (2016) suggested yolk hormones of maternal origin in Northern Bobwhite have a positive effect on the physiological characteristics of offspring.
Conservation
This category specialized in assessment of the conservation status and policy effectiveness of the species of Galliformes on both the species diversity and genetic diversity, and it accounted for 6.7% of remaining articles. Most (45.2%) were conducted by the researchers in the United States of America/Canada, followed by Europe (31.0%). The conservation biologists have made great efforts to improve the conservation effectiveness on Galliformes at different levels. Some researchers analyzed the genetic structure or variation to assess the genetic diversity and then provided suggestions to maintain genetic variability (e.g. Schulwitz et al. 2014), while other scientists studied approaches to increase the individual or population survival rate (e.g. Bernardo et al. 2014; Blomberg 2015). Those measures were focused on habitat protection by establishing the protected areas through programs such as the Conservation Reserve Programs (CRP) in the USA (e.g. Lupis et al. 2005), breeding programs (e.g. Apa and Wiechman 2016), and reintroduction projects (e.g. Baruch-Mordo et al. 2013; Gama et al. 2016). Almost all these articles suggested that more actions should be carried out to maintain the integrity and continuity of habitats (e.g. Bro et al. 2004), and they believed that those actions could contribute to creating favorable living conditions for Galliformes (Gama et al. 2016). Unfortunately, a number of articles also showed that many species were not well protected because of lacking effective local managements and reasonable financial provision (Fuller and Garson 2000; Baruch-Mordo et al. 2013) or the effective conservation techniques (e.g. Apa and Wiechman 2016). In particular, hunting was an important negative impact factor in relation to galliform conservation as it was evident that hunting pressure has contributed to the large part of threatened species (e.g. Fuller and Garson 2000; Blomberg 2015).
Others
This category was split into two main themes, i.e. ethology (n = 42) and research review (n = 10). Given that the territorial behavior, flocking behavior, and foraging behavior were categorized into macroscopic ecology as they were often related to ecological environment, the ethology category mainly included social behavior (e.g. Wells et al. 2014; Krakauer et al. 2016), vocal behavior (e.g. Garcia et al. 2012), and imitative learning (e.g. Akins and Zentall 1996). By analyzing the results, it showed that the method using playback of vocalisations has been widely used to survey the behaviors of Galliformes. Using playback, the researchers identified subadults, males and females of the species, analyzed the population structure (Van Niekerk 2010), directionality (Garcia et al. 2012), and tested whether and how the playback calls attracted the mating partners (Van Niekerk 2010).
The reviews of grouse research suggested that the species and topics varied with time, but more recently conservation and the effect of human disturbance on grouse became hot topics (Höglund 2009; Moss et al. 2010; Storch 2013). The remaining articles summarized the conservation status and species extinctions of Galliformes, which provided a basis for better protection of Galliformes. Many species of the grouse, like Sage Grouse, remained listed for protection (McGowan et al. 2009, 2012). Therefore, the researchers called for the more knowledge and improvement of research techniques to study the endangered and poor-known species, and make great efforts to eliminate the negative impacts on biodiversity (Storch 2013).
Discussion
Our study analyzed the galliform-related articles from 1990 to 2016, and the results showed that most articles were from the United States of America, Canada, and Europe. Although the vast majority studies focused on one or two species and were of a short duration, it is gratifying to note that the total number of species being studied, articles and the duration of study period were increasing, and the topic range is more extensive, which was similar to the patterns found for the research on grouses (Moss et al. 2010). Zheng (2015) suggested that galliform research has rapidly progressed since 2000. Our results showed that the year of 2003 was a turning point for the great increase of publications related to the Galliformes, which might be attributed to the language barriers and lack of good communication among researchers from non-English speaking countries, especially in China (Myles and Cheng 2003) before 2003. In 2002, the 23rd International Ornithological Congress was held in Beijing, which might make researchers to recognize the importance of international cooperation and communication, especially for Chinese researchers (Myles and Cheng 2003; Walter 2004). After that, more and more researches on the Galliformes in China were published in English (Zheng 2015).
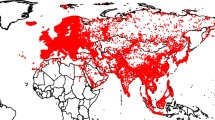
Different countries and regions hold some different species of Galliformes (Johnsgard 1999), and our results also show that different countries are inclined to conduct research on the species unique to the region (Fig. 3). For example, 77.6% of turkey (Meleagris) research occurred in the United States, as turkey occurs only in North America and Central America (e.g. Mock et al. 2002; McJunkin et al. 2005; Brautigam et al. 2016). Most of the studies on Francolinus spp. occurred in Pakistan and South Africa (Cohen et al. 2012; Khan and Mian 2013), while nearly two-thirds of the literatures of the genus Syrmaticus were from China (e.g. Zhan and Zhang 2005; Jiang et al. 2007; Ashizawa et al. 2014; Zhou et al. 2015a), as they were mainly distributed in China and Japan.
There are increasing studies on the conservation and ethology of Galliformes in recent years (Fuller and Garson 2000), whereas such studies in our results just occupied a small part (9.0%). As a matter of fact, a great number of articles regarding macroscopic ecology have discussed the conservation implications of their results, and they are classified into macroscopic ecology due to their primary objectives. Similarly, the articles on territorial behavior, behavioral ecology, flocking behavior and foraging behavior were all related to ecology and thus we regarded them as behavioral ecology under the category macroscopic ecology.
Future directions
Although studies on Galliformes have made great achievements, there are still some gaps in macroscopic ecology, molecular genetics and conservation. Galliformes still faces many threats, including climate change, human population growth, deforestation and hunting behaviors (Fuller and Garson 2000; Deng and Zheng 2004; Zheng 2015). Based on the trends of current avian research, we make following suggestions for future research of Galliformes.
Galliformes conservation
As a highly threatened taxon in the world, the conservation of Galliformes is a significant topic of the global change, and it is more important in developing countries for increasing conflicts between wildlife and human beings. A clear and science-based plan is needed to improve Galliformes conservation (Watson and Venter 2017). Also, long-term monitoring and comprehensive surveys of the populations and habitats of Galliformes should be conducted (Fuller and Garson 2000), which will help to assess the dynamics of the populations and habitat use patterns for habitat suitability at multi-scales (Zheng 2002; Gregory and Beck 2014), and to build a comprehensive database of Galliformes to improve the conservation effort and management effectiveness (Jones 2001; Zheng 2015). Although a number of management policies and conservation programs have been implemented in some regions, most assessments just focused on small scales or restricted topics with limited implications (Brymer et al. 2016). A more comprehensive monitoring and assessment programs are therefore needed for better use of resources to achieve species or community level conservation goals.
In addition, Galliformes conservation studies were mainly at the macro and descriptive levels historically, with the molecular genetic mechanisms involved less (Vignal et al. 2002). This study found that the number of the articles on genetic studies was more than that on the species conservation, but most of them having provided limited suggestions or guidelines for conservation. Therefore, interdisciplinary and synthetic approaches of molecular ecology and any other fields should be integrated to promote the development of new knowledge and techniques, so as to fit the present and future needs of conservation (Gama et al. 2016).
Climate change and adaptive plan
Global climate change is considered as one of the major threats to biodiversity (Feng et al. 2015), and there is strong evidence that climate change limited the reproduction of some species of Galliformes (Selås et al. 2011), and may have already deduced several species’ extinctions (Heller and Zavaleta 2009). Mantyka-pringle et al. (2013) suggested that climate change has negatively interacted with habitat loss, and synergistically continues to pose direct and indirect impacts to species, even contributes to the degradation of biodiversity (Jetz et al. 2007). However, climate change adaptation work was still mainly at the conception stage (Heller and Zavaleta 2009), and most research so far just provided general adaptation recommendations without considering the size and location of each threat (Watson et al. 2013), and few recommendations suggested a process that managers could use to develop an adaptive plan and evaluate its effectiveness (Heller and Zavaleta 2009). As such, there will be a need for specific biodiversity-oriented adaptation planning, from short to long term and from precautionary and robust to more risky or deterministic, to respond to both rapid directional change and tremendous uncertainty (Heller and Zavaleta 2009; Rao et al. 2013; Watson et al. 2013).
The life history of Galliformes
Understanding the pattern of change in life history characteristics is the central goal of evolutionary ecology (Martin 1996), and it is also the basis for understanding bird evolution and adaptation to the environment (Wang et al. 2012a). However, while many researchers devoting great efforts to genomics rather than life history in recent years (Zheng 2015), the information on the natural histories of many Galliformes, as of other birds, is still lacking (Lu 2015). Xiao et al. (2016) analyzed all the available information for three key breeding parameters for nearly 10,000 species of birds in the world, and they found that the information of the reproductive parameters was available for only one-third of these birds. Therefore, research on the natural history of birds should be encouraged to fill these knowledge gaps (Jimenez et al. 2014).
Cross-disciplinary studies and application of new technologies
Understanding the scientific questions in ornithological studies not only requires the knowledge of ecology and genetics, but also cell biology, physiology and biochemistry, etc. Multidisciplinary and multiple technology approaches will be more effective to solve the complicated questions of Galliformes, compared to isolated, single-dimensional studies (Fuller and Garson 2000). The interactions among ornithologists and between ornithologists and scientists of other fields or natural resources managers will benefit or are even necessary for the development of new theories and techniques.
Over the past two decades, researchers have undertaken a lot of work on the application of new technologies (Powell et al. 1996). It is an ongoing challenge to use new technologies to answer the key questions about bird conservation (Wang et al. 2012a). With the development of molecular techniques and computer science applications, ornithological studies are acquiring new tools (Caravaggi et al. 2017). Although molecular technologies have made great breakthroughs in genetic diversity (Huang et al. 2005), taxonomy (Moulin et al. 2003) and phylogenetic (Wang et al. 2013), there is still a need to develop the simple and accurate molecular techniques, such as molecular markers, to inject new impetus into genetic research (Vignal et al. 2002). With the development of whole genome sequencing, it is becoming implementable using population genome to identify the genes linked to local adaptation, which may provide evidences for conservation management (Campbell-Staton et al. 2017). In recent years, computer-centric “3S integration” technology has been developed rapidly and adopted by many researchers. The integrated application of this technology allows for regional investigation and dynamic monitoring, which saves time and human and material resources (Caravaggi et al. 2017), and the work has expanded to experimental data processing and modeling to explain mechanisms such as dispersal and population differentiation (O’Brien and Kinnaird 2008). Research has also evaluated the ecological environment to provide a more scientific basis for bird habitat protection planning and associated decision-making. The world is becoming “smaller” with the development of new technologies and artificial intelligence, and exploring frontier research techniques for study, monitoring, and analyzing patterns and mechanisms of Galliformes ecology is becoming a necessity.
Conclusions
By reviewing galliform-related articles published from 1990 to 2016, our results showed that the average growth rate was 37.9% over the years. Macroscopic ecology, taxonomy and phylogenetics were the major topics of the studies on Galliformes, accounting for a large part of the current research and research on molecular ecology was on the rise. However, despite the progresses, there is a lack of studies directly applying new knowledge to the conservation of Galliformes, given that the group of birds are facing increased threatens. Moreover, the research on life history represented only a small proportion in the literatures reviewed, with the fact that the knowledge of life history of many galliform species is still missing. Future studies that investigate the basic life history and conduct long-term monitoring of galliform populations and those incorporating different disciplines and new technologies should be encouraged, not only for a better understanding of them, but for better making effective conservation measures.
References
Akins CK, Zentall TR. Imitative learning in male Japanese quail (Coturnix japonica) using the two-action method. J Comp Psychol. 1996;110:316.
Ancel A, Visschedijk AJ. Respiratory exchanges in the incubated egg of the domestic guinea fowl. Resp Physiol. 1993;91:31–42.
Anich NM, Worland M, Martin KJ. Habitat use by spruce grouse in northern Wisconsin. Wildl Soc B. 2013;37:766–77.
Apa AD, Wiechman LA. Captive-breeding of captive and wild-reared Gunnison sage-grouse. Zoo Biol. 2016;35:70–5.
Ashizawa K, Kawaji N, Tanaka A, Nagase D, Matsumoto Y, Tatemoto H, Tatemoto H, Tsuzuki Y. Population fluctuation and habitat preference of Ijima’s Copper Pheasant Syrmaticus soemmerringii ijimae: an endemic, ‘near threatened’ Japanese subspecies. Ornithol Sci. 2014;13:77–81.
Barilani M, Bernard-Laurent A, Mucci N, Tabarroni C, Kark S, Perez G, Jose A, Randi E. Hybridisation with introduced chukars (Alectoris chukar) threatens the gene pool integrity of native rock (A. graeca) and red-legged (A. rufa) partridge populations. Biol Conserv. 2007a;137:57–69.
Barilani M, Sfougaris A, Giannakopoulos A, Mucci N, Tabarroni C, Randi E. Detecting introgressive hybridisation in rock partridge populations (Alectoris graeca) in Greece through Bayesian admixture analyses of multilocus genotypes. Conserv Genet. 2007b;8:343–54.
Baruch-Mordo S, Evans JS, Severson JP, Naugle DE, Maestas JD, Kiesecker JM, Falkowski MJ, Hagen CA, Reese KP. Saving sage-grouse from the trees: a proactive solution to reducing a key threat to a candidate species. Biol Conserv. 2013;167:233–41.
Bellinger MR, Johnson JA, Toepfer J, Dunn P. Loss of genetic variation in greater prairie chickens following a population bottleneck in Wisconsin, USA. Conserv Biol. 2003;17:717–24.
Benedict NG, Oyler-McCance SJ, Taylor SE, Braun CE, Quinn TW. Evaluation of the eastern (Centrocercus urophasianus urophasianus) and western (Centrocercus urophasianus phaios) subspecies of sage-grouse using mitochondrial control-region sequence data. Conserv Genet. 2003;4:301–10.
Bernardo CSS, Desbiez ALJ, Olmos F, Collar NJ. Reintroducing the red-billed curassow in Brazil: population viability analysis points to potential success. Nat Conserv. 2014;12:53–8.
Birks SM, Edwards SV. A phylogeny of the megapodes (Aves: Megapodiidae) based on nuclear and mitochondrial DNA sequences. Mol Phylogenet Evol. 2002;23:408–21.
Block WB, Brennan LA. The habitat concept in ornithology theory and application. Curr Ornithol. 1993;11:35–91.
Blomberg EJ. The influence of harvest timing on greater sage-grouse survival: a cautionary perspective. J Wildl Manag. 2015;79:695–703.
Bourke JM, Witmer LM. Nasal conchae function as aerodynamic baffles: experimental computational fluid dynamic analysis in a turkey nose (Aves: Galliformes). Resp Physiol Neurobiol. 2016;234:32–46.
Bouzat JL. The importance of control populations for the identification and management of genetic diversity. Genetica. 2000;110:109–15.
Brautigam KJ, Osborne DC, White JD. Photographic evidence and chronology of nest parasitism by a Wild Turkey (Meleagris gallopavo). Wilson J Ornithol. 2016;128:204–7.
Bro E, Mayot P, Corda E, Reitz F. Impact of habitat management on grey partridge populations: assessing wildlife cover using a multisite BACI experiment. J Appl Ecol. 2004;41:846–57.
Brøseth H, Pedersen HC. Disturbance effects of hunting activity in a willow ptarmigan Lagopus lagopus population. Wildl Biol. 2010;16:241–8.
Brymer ALB, Holbrook JD, Niemeyer RJ, Suazo AA, Wulfhorst JD, Vierling KT, Newingham BA, Link TE, Rachlow JL. A social–ecological impact assessment for public lands management: application of a conceptual and methodological framework. Ecol Soc. 2016;21:9.
Bush KL, Strobeck C. Phylogenetic relationships of the Phasianidae reveals possible non-pheasant taxa. J Hered. 2003;94:472–89.
Campbell-Staton SC, Cheviron ZA, Rochette N, Catchen J, Losos JB, Edwards SV. Winter storms drive rapid phenotypic, regulatory, and genomic shifts in the green anole lizard. Science. 2017;357:495–8.
Capdevila J, Puigcerver M, López S, Pérez-Masdeu E, García-Galea E. The role of nest-site selection and cereal production in differential nest predation in Common Quail Coturnix coturnix and hybrid quail C. coturnix × C. japonica. Ibis. 2016;158:784–95.
Caravaggi A, Banks PB, Burton CA, Finlay CMV, Haswell PM, Hayward MW, Rowcliffe MJ, Wood MD. A review of camera trapping for conservation behaviour research. Remote Sens Ecol Conserv. 2017;3:109–22.
Carpio AJ, Guerrero-Casado J, Tortosa FS, Vicente J. Predation of simulated red-legged partridge nests in big game estates from South Central Spain. Eur J Wildl Res. 2014;60:391–4.
Chang J, Wang B, Zhang YY, Liu Y, Liang W, Wang JC, Shi HT, Su WB, Zhang ZW. Molecular evidence for species status of the endangered Hainan peacock pheasant. Zoo Sci. 2008;25:30–5.
Charvet CJ, Striedter GF. Developmental species differences in brain cell cycle rates between northern bobwhite quail (Colinus virginianus) and parakeets (Melopsittacus undulatus): implications for mosaic brain evolution. Brain Behav Evol. 2008;72:295–306.
Chávez-león G, Velázquez A, Fregoso A, Bocco G. Habitat associations of the long-tailed wood-partridge (Dendrortyx macroura) in a managed coniferous forest in Michoacán, Mexico. Biodivers Conserv. 2004;13:1943–60.
Chen D, Liu Y, Davison GWH, Dong L, Chang J, Gao SH, Li S-H, Zhang ZW. Revival of the genus Tropicoperdix Blyth 1859 (Phasianidae, Aves) using multilocus sequence data. Zool J Linn Soc Lond. 2015;175:429–38.
Chen Y, An B, Liu N. Asymmetrical introgression patterns between rusty-necklaced partridge (Alectoris magna) and chukar partridge (Alectoris chukar) in China. Integr Zool. 2016;11:403–12.
Clawson MV, Skalski JR, Isabelle JL, Millspaugh JJ. Trends in male wild turkey abundance and harvest following restoration efforts in the southeast region of Missouri, 1960–2010. Wildl Soc B. 2015;39:116–28.
Clements JF, Schulenberg TS, Iliff MJ, Roberson DT, Fredericks A, Sullivan BL, Wood CL. The eBird/clements checklist of birds of the world: v2016 (2016). http://www.birds.cornell.edu/clementschecklist/download. Accessed 31 Dec 2016.
Coates PS, Casazza ML, Ricca MA, Brussee BE, Blomberg EJ, Gustafson KB, Overton CT, Davis DM, Niell LE, Espinosa SP, Gardner SC, Delehanty DJ. Integrating spatially explicit indices of abundance and habitat quality: an applied example for greater sage-grouse management. J Appl Ecol. 2016;53:83.
Cohen C, Wakeling JL, Mandiwana-Neudani TG, Sande E, Dranzoa C, Crowe TM, Bowie RCK. Phylogenetic affinities of evolutionarily enigmatic African galliforms: the Stone Partridge Ptilopachus petrosus and Nahan’s Francolin Francolinus nahani, and support for their sister relationship with New World quails. Ibis. 2012;154:768–80.
Corfield JR, Long B, Krilow JM, Wylie DR, Iwaniuk AN. A unique cellular scaling rule in the avian auditory system. Brain Struct Funct. 2016;221:2675–93.
Crowe T. Phylogenetic affinities of enigmatic African galliforms: the Stone Partridge Ptilopachus petrosus and Latham’s and Nahan’s’ Francolins’ Francolinus lathami and F. nahani. Cladistics. 2010;26:206.
Crowe TM, Bowie RCK, Bloomer P, Mandiwana TG, Hedderson TAG, Randi E, Pereira SL, Wakeling J. Phylogenetics, biogeography and classification of, and character evolution in, gamebirds (Aves: Galliformes): effects of character exclusion, data partitioning and missing data. Cladistics. 2006;22:495–532.
Daley MA, Voloshina A, Biewener AA. The role of intrinsic muscle mechanics in the neuromuscular control of stable running in the guinea fowl. J Physiol. 2009;587:2693–707.
del Hoyo J, Elliott A, Sargatal J. Handbook to the birds of the world. Vol. 2. New world vultures to Guineafowl. Barcelona: Lynx Edicions; 1994.
Deng WH, Zheng GM. Landscape and habitat factors affecting cabot’s tragopan Tragopan caboti, occurrence in habitat fragments. Biol Conserv. 2004;117:25–32.
Dong L, Heckel G, Liang W, Zhang Y. Phylogeography of Silver Pheasant (Lophura nycthemera L.) across China: aggregate effects of refugia, introgression and riverine barriers. Mol Ecol. 2013;22:3376–90.
Dzialak MR, Olson CV, Harju SM, Webb SL, Mudd JP, Winstead JB, Hayden-Wing LD. Identifying and prioritizing greater sage-grouse nesting and brood-rearing habitat for conservation in human-modified landscapes. PLoS ONE. 2011;6:e26273.
Dzialak MR, Olson CV, Harju SM, Webb SL, Winstead JB. Temporal and hierarchical spatial components of animal occurrence: conserving seasonal habitat for greater sage-grouse. Ecosphere. 2012;3:1–17.
Ellis-Felege SN, Burnam JS, Palmer WE, Sisson DC, Carroll JP. Fight or flight: parental decisions about predators at nests of northern bobwhites (Colinus virginianus). Auk. 2013;130:637–44.
Fearer TM, Stauffer DF. Relationship of ruffed grouse (Bonasa umbellus) home range size to landscape characteristics. Am Midl Nat. 2003;150:104–14.
Feng X, Lin CT, Qiao HJ, Ji LQ. Assessment of climatically suitable area for Syrmaticus reevesii under climate change. Endang Species Res. 2015;28:19–31.
Follett BK, Kumar V, Juss TS. Circadian nature of the photoperiodic clock in Japanese quail. J Comp Physiol A. 1992;171:533–40.
Follett BK, Pearce KA. Photoperiodic control of the termination of reproduction in Japanese quail (Coturnix coturnix japonica). Proc R Soc B Biol Sci. 1990;242:225–30.
Franco P, Fierro-Calderón K, Kattan G. Population densities and home range sizes of the Chestnut Wood-quail. J Field Ornithol. 2006;77:85–90.
Froese GZL, Contasti AL, Mustari AH, Brodie JF. Disturbance impacts on large rain-forest vertebrates differ with edge type and regional context in Sulawesi, Indonesia. J Trop Ecol. 2015;31:509–17.
Fuller RA, Garson PJ. Pheasants: status survey and conservation action plan 2000‒2004. In: IUCN; 2000. p. 1‒23.
Galla SJ, Johnson JA. Differential introgression and effective size of marker type influence phylogenetic inference of a recently divergent avian group (Phasianidae: Tympanuchus). Mol Phylogenet Evol. 2015;84:1–13.
Gama GM, Malhado ACM, Bragagnolo C, Correia RA, Ladle RJ. Cultural viability of reintroducing the ecologically extinct Alagoas Curassow (Pauxi mitu Linnaeus, 1766) to Northeast Brazil. J Nat Conserv. 2016;29:25–32.
Garcia M, Charrier I, Iwaniuk AN. Directionality of the drumming display of the ruffed grouse. Condor. 2012;114:500–6.
Gee GF. Avian artificial insemination and semen preservation//IFCB Symposium on breeding birds in captivity. North Hollywood: Int Found Conserv Birds; 1983. p. 375–98.
Gill F, Donsker D. IOC World Bird List (v 6.4) (2016). https://doi.org/10.14344/ioc.ml.6.4. http://www.worldbirdnames.org. Accessed 31 Dec 2016.
Goddard AD, Dawson RD. Seasonal changes in habitat features influencing nest survival of sharp-tailed grouse in northeastern British Columbia, Canada. Ecoscience. 2009;16:476–82.
Gregory AJ, Beck JL. Spatial heterogeneity in response of male greater sage-grouse lek attendance to energy evelopment. PLoS ONE. 2014;9:e97132.
Gu LY, Liu Y, Wang N, Zhang ZW. A panel of polymorphic microsatellites in the Blue Eared Pheasant (Crossoptilon auritum) developed by cross-species amplification. Chin Birds. 2012;3:103–7.
Hämäläinen A, Alatalo RV, Lebigre C, Siitari H, Soulsbury CD. Fighting behaviour as a correlate of male mating success in black grouse Tetrao tetrix. Behav Ecol Soc. 2012;66:1577–86.
Hanotte O, Burke T, Armour JA, Jeffreys AJ. Hypervariable minisatellite DNA sequences in the Indian peafowl Pavo cristatus. Genomics. 1991;9:587–97.
Hayward LS, Wingfield JC. Maternal corticosterone is transferred to avian yolk and may alter offspring growth and adult phenotype. Gen Comp Endocr. 2004;135:365–71.
He L, Dai B, Zeng B, Zhang X, Chen B, Yue B, Li J. The complete mitochondrial genome of the Sichuan Hill Partridge (Arborophila rufipectus) and a phylogenetic analysis with related species. Gene. 2009;435:23–8.
Heller NE, Zavaleta ES. Biodiversity management in the face of climate change: a review of 22 years of recommendations. Biol Conserv. 2009;142:14–32.
Hennache A. A review of captive Galliformes in European zoos. Int J Galliformes Conserv. 2009;1:23–8.
Hernández F, Henke SE, Silvy NJ, Rollins D. The use of prickly pear cactus as nesting cover by northern bobwhites. J Wildl Manag. 2003;67:417–23.
Herrington JA, Rodriguez Y, Lickliter R. Elevated yolk progesterone moderates prenatal heart rate and postnatal auditory learning in bobwhite quail (Colinus virginianus). Dev Psychobiol. 2016;58:784–8.
Höglund J. Genetic studies of black grouse with special reference to conservation biology: a review. Folia Zool. 2009;58:135.
Hörnell WM, Willebrand T, Smith AA. Seasonal movements and dispersal patterns: implications for recruitment and management of willow ptarmigan (Lagopus lagopus). J Wildl Manag. 2014;78:194–201.
Huang ZH, Liu NF, Luo SX, Long J, Xiao YA. Ecological genetics of rusty-necklaced partridge (Alectoris magna): environmental factors and population genetic variability correlations. Korean J Genetic. 2007;29:115–20.
Huang Z, Liu N, Zhou T. A comparative study of genetic diversity of peripheral and central populations of chukar partridge from northwestern China. Biochem Genet. 2005;43:613–21.
Iqubal P, Mcgowan PJK, Carroll JP, Rahmani AR. Home range size, habitat use and nesting success of Swamp Francolin Francolinus gularis on agricultural land in northern India. Bird Conserv Int. 2003;13:127–38.
Janke AK, Gates RJ. Home range and habitat selection of northern bobwhite coveys in an agricultural landscape. J Wildl Manag. 2013;77:405–13.
Jankowski MD, Russell RE, Franson JC, Dusek RJ, Hines MK, Gregg M, Hofmeister EK. Corticosterone metabolite concentrations in Greater Sage-Grouse are positively associated with the presence of cattle grazing. Rangel Ecol Manag. 2014;67:237–46.
Jansen R, Crowe TM. Relationship between breeding activity and rainfall for Swainson’s Spurfowl, Pternistis swainsonii, within southern Africa, with specific reference to the Springbok Flats, Limpopo Province, South Africa. Ostrich J Afr Ornithol. 2005;76:190–4.
Jetz W, Wilcove DS, Dobson AP. Projected impacts of climate and land-use change on the global diversity of birds. PLoS Biol. 2007;5:e157.
Jiang L, Wang G, Peng R, Peng Q, Zou F. Phylogenetic and molecular dating analysis of Taiwan Blue Pheasant (Lophura swinhoii). Gene. 2014;539:21–9.
Jiang PP, Ge YF, Lang QL, Ding P. Genetic structure among wild populations of Elliot’s Pheasant Syrmaticus ellioti in China from mitochondrial DNA analyses. Bird Conserv Int. 2007;17:177–85.
Jimenez AG, Van Brocklyn J, Wortman M, Williams JB. Cellular metabolic rate is influenced by life-history traits in tropical and temperate birds. PLoS ONE. 2014;9:e87349.
Johnsgard PA. Pheasants of the World. Washington: Smithsonian Institution Press; 1999.
Johnson FA, Hagan G, Palmer WE, Kemmerer M. Uncertainty, robustness, and the value of information in managing a population of northern bobwhites. J Wildl Manag. 2014;78:531–9.
Johnson JA, Dunn PO. Low genetic variation in the heath hen prior to extinction and implications for the conservation of prairie-chicken populations. Conserv Genet. 2006;7:37–48.
Jones J. Habitat selection studies in avian ecology: a critical review. Auk. 2001;118:557–62.
Kadhim KK, Zuki ABZ, Noordin MM, Babjee SMA, Khamas W. Light and scanning electron microscopy of the intestine of the young Red Jungle Fowl (Gallus gallus). J Anim Vet Adv. 2010;9:2729–937.
Kayvanfar N, Aliabadian M, Ghasempouri SM. Morphometric and morphological differentiation of the subspecies of Phasianus colchicus (Linnaeus, 1758) on the Iranian Plateau (Aves: Galliformes). Zool Middle East. 2015;61:9–17.
Khalil S, Anwar M, Hussain I. Breeding biology of grey francolin (Francolinus pondicerianus) in salt range, Pakistan. Pak J Zool. 2016;48:115–23.
Khan WA, Mian A. Comparative population biology of black (Francolinus francolinus) and grey (F. pondicerianus) Francolins under Lal Suhanra National Park (Pakistan) conditions. Pak J Zool. 2013;45:949–58.
Kobayashi A, Nakamura H. Chick and juvenile survival of Japanese rock ptarmigan Lagopus muta japonica. Wildlife Biol. 2013;19:358–67.
Krakauer AH, Blundell MA, Scanlan TN, Wechsler MS, McCloskey EA, Yu JH, Patricelli GL. Successfully mating male sage-grouse show greater laterality in courtship and aggressive interactions. Anim Behav. 2016;111:261–7.
Kurhinen J, Danilov P, Gromtsev A, Helle P, Linden H. Patterns of black grouse, Tetrao tetrix distribution in northwestern Russia at the turn of the millennium. Folia Zool. 2009;58:168–72.
Kvasnes MAJ, Pedersen HC, Storaas T, Nilsen EB. Large-scale climate variability and rodent abundance modulates recruitment rates in Willow Ptarmigan (Lagopus lagopus). J Ornithol. 2014;155:891–903.
Lawes MJ, Fly S, Piper SE. Gamebird vulnerability to forest fragmentation: patch occupancy of the Crested Guineafowl (Guttera edouardi) in Afromontane forests. Anim Conserv. 2006;9:67–74.
Li Y, Cui B, Qiu X, Ding C, Batool I. Management reference for nature reserve networks based on MaxEnt modeling and gap analysis: a case study of the brown-eared pheasant in China. Anim Biodiv Conserv. 2016;39:241–52.
Lu X, Zheng GM. Dominance-dependent microroost use in flock-living Tibetan Eared-pheasants. Ardea. 2007;95:225–34.
Lu X, Zheng GM. Reproductive ecology of Tibetan Eared Pheasant Crossoptilon harmani in scrub environment, with special reference to the effect of food. Ibis. 2003;145:657–66.
Lu X. Hot genome leaves natural histories cold. Science. 2015;349:1064.
Lupis SG, Messmer TA, Black T. Gunnison sage-grouse use of conservation reserve program fields in Utah and response to emergency grazing: a preliminary evaluation. Wildl Soc B. 2005;34:957–62.
Lyly MS, Villers A, Koivisto E, Helle P, Ollila T, Korpimäki E. Guardian or threat: does golden eagle predation risk have cascading effects on forest grouse? Oecologia. 2016;182:487–98.
Mantyka-pringle CS, Martin TG, Rhodes JR. Interactions between climate and habitat loss effects on biodiversity: a systematic review and meta-analysis. Global Change Biol. 2013;19:1642–4.
Martin TE. Life history evolution in tropical and south temperate birds: what do we really know? J Avian Biol. 1996;27:263–72.
Marzluff JM. A decadal review of urban ornithology and a prospectus for the future. Ibis. 2016;159:1–13.
Matzke AJ, Varga F, Gruendler P, Unfried I, Berger H, Mayr B, Matzke MA. Characterization of a new repetitive sequence that is enriched on microchromosomes of turkey. Chromosoma. 1992;102:9–14.
McGowan PJK, Owens LL, Grainger MJ. Galliformes science and species extinctions: what we know and what we need to know. Anim Biodiv Conserv. 2012;35:321–31.
McGowan PJK, Garson PJ. Status survey and conservation action Plan 1995‒1999 Pheasants. In: IUCN; 1995.
McGowan PJK, Zhang YY, Zhang ZW. Galliformes-barometers of the state of applied ecology and wildlife conservation in China. J Appl Ecol. 2009;46:524–6.
McJunkin JW, Zelmer DA, Applegate RD. Population dynamics of wild turkeys in Kansas (Meleagris gallopavo): theoretical considerations and implications of rural mail carrier survey (RMCS) data. Am Midl Nat. 2005;154:178–87.
McNew LB, Sandercock BK. Spatial heterogeneity in habitat selection: nest site selection by Greater Prairie-Chickens. J Wildl Manag. 2013;77:791–801.
Mock KE, Theimer TC, Rhodes OE, Greenberg DL, Keim P. Genetic variation across the historical range of the wild turkey (Meleagris gallopavo). Mol Ecol. 2002;11:643–57.
Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
Moss R, Storch I, Müller M. Trends in grouse research. Wildl Biol. 2010;16:1–11.
Moulin S, Randi E, Tabarroni C, Hennache A. Mitochondrial DNA diversification among the subspecies of the Silver and Kalij Pheasants, Lophura nycthemera and L. leucomelanos, Phasianidae. Ibis. 2003;145:E1–11.
Myles J, Cheng L. The social and cultural life of non-native English speaking international graduate students at a Canadian university. J Engl Acad Purp. 2003;2:247–63.
O’Brien TG, Kinnaird MF. A picture is worth a thousand words: the application of camera trapping to the study of birds. Bird Conserv Int. 2008;18:S144–62.
Onyeanusi BI, Ema AN, Ezeokoli CD, Onyeanusi JC. The structure of the Harderian gland of the guinea fowl at embryonic and post embryonic stages. Anat Histol Embryol. 1993;22:183–90.
Onyeyili PA, Egwu GO, Jibike GI, Atori JO. Plasma and red cell cholinesterase concentrations in guinea-fowl (Numuida meleagris) and Nigerian domestic fowl (Gallus domesticus). Vet Res Commun. 1992;1:173–5.
Persons NW, Hosner PA, Meiklejohn KA, Braun EL, Kimball RT. Sorting out relationships among the grouse and ptarmigan using intron, mitochondrial, and ultra-conserved element sequences. Mol Phylogenet Evol. 2016;98:123–32.
Peters J, Lebrasseur O, Deng H, Larson G. Holocene cultural history of Red jungle fowl (Gallus gallus) and its domestic descendant in East Asia. Quat Sci Rev. 2016;142:102–19.
Pike TW, Petrie M. Experimental evidence that corticosterone affects offspring sex ratios in quail. Proc R Soc B Biol Sci. 2006;273:1093–8.
Powell W, Morgante M, Andre C, Hanafey M, Vogel J, Tingey S, Rafalski A. The comparison of RFLP, RAPD, AFLP and SSR (microsatellite) markers for germplasm analysis. Mol Breeding. 1996;2:225–38.
Rao M, Htun S, Platt SG, Tizard R, Poole C, Myint T, Watson JEM. Biodiversity conservation in a changing climate: a review of threats and implications for conservation planning in Myanmar. Ambio. 2013;42:789–804.
Ren QQ, Li XF, Yuan J, Chen DS, Zhang L, Guo WW, Jiang L, Wang P, Kan XZ. Complete mitochondrial genome of the Blue Eared Pheasant, Crossoptilon auritum (Galliformes: Phasianidae). Mitochondr DNA. 2016;27:615–7.
Rhim SJ. Ecological factors influencing nest survival of hazel grouse Bonasa bonasia in a temperate forest, South Korea. For Ecol Manag. 2012;282:23–7.
Rhim SJ. Spring-season social organsization of the Hazel Grouse (Bonasa bonasia) in relation to habitat type in temperate forests of South Korea. Ornis Fennica. 2010;87:160–7.
Robel RJ, Hughes JP, Keane TD, Kemp KE. Do artificial nests reveal meaningful patterns of predation in kansas grasslands? Southwest Nat. 2003;48:460–4.
Robinson SG, Haukos DA, Plumb RT, Hagen CA, Pitman JC, Lautenbach JM, Sullins DS, Kraft JD, Lautenbach JD. Lesser prairie-chicken fence collision risk across its northern distribution. J Wildl Manag. 2016;80:906–15.
Rolstad J, Wegge P, Sivkov AV, Hjeljord O, Storaunet KO. Size and spacing of grouse leks: comparing capercaillie (Tetrao urogallus) and black grouse (Tetrao tetrix) in two contrasting Eurasian boreal forest landscapes. Can J Zool. 2009;87:1032–43.
Ross AM, Johnson G, Gibbs JP. Spruce grouse decline in maturing lowland boreal forests of New York. For Ecol Manag. 2016;359:118–25.
Sandoval L, Barrantes G. Characteristics of male Spot-bellied Bobwhite (Colinus leucopogon) song during territory establishment. J Ornithol. 2012;153:547–54.
Schulwitz S, Bedrosian B, Johnson JA. Low neutral genetic diversity in isolated Greater Sage-Grouse (Centrocercus urophasianus) populations in northwest Wyoming. Condor. 2014;116:560–73.
Selås V, Sonerud GA, Framstad E, Kålås JA, Kobro S, Pedersen HB, Spidso TK, Wiig O. Climate change in Norway: warm summers limit grouse reproduction. Popul Ecol. 2011;53:361–71.
Shen YY, Dai K, Cao X, Murphy RW, Shen XJ, Zhang YP. The updated phylogenies of the Phasianidae based on combined data of nuclear and mitochondrial DNA. PLoS ONE. 2014;9:e95786.
Shen YY, Liang L, Sun YB, Yue BS, Yang XJ, Murphy RW, Zhang YP. A mitogenomic perspective on the ancient, rapid radiation in the Galliformes with an emphasis on the Phasianidae. BMC Evol Biol. 2010;10:132.
Smith JA, Whalen CE, Bomberger BM, Powell LA. Indirect effects of an existing wind energy facility on lekking behavior of greater prairie-chickens. Ethology. 2016;122:419–29.
Stiver JR, Apa AD, Remington TE, Remington TE, Gibson RM. Polygyny and female breeding failure reduce effective population size in the lekking Gunnison sage-grouse. Biol Conserv. 2008;141:472–81.
Storch I. Human disturbance of grouse-why and when? Wildl Biol. 2013;19:390–403.
Sučić I. Rock Partridge (Alectoris graeca) Population size on mountain Tušnica in the period between 2000 and 2007. Šumar List. 2008;132:331–6.
Sun YH, Fang Y, Jia CX, Klaus S, Swenson JE, Scherzinger W. Nest site selection of Chinese grouse Bonasa sewerzowi at Lianhuashan, Gansu, China. Wildl Biol. 2007;13:68–72.
Tang CZ. The 4th International Pheasant Symposium was held in Beijing, China. Acta Zool Sin. 1990;36:104 (in Chinese).
Tanner EP, Elmore RD, Fuhlendorf SD, Davis CA, Thacker ET, Dahlgren DK. Behavioral responses at distribution extremes: how artificial surface water can affect quail movement patterns. Rangel Ecol Manag. 2015;68:476–84.
Thogmartin WE. Landscape attributes and nest-site selection in wild turkeys. Auk. 1999;116:912–23.
Thomas GH. Evolution: an avian explosion. Nature. 2015;526:516–7.
Tirpak J, Giuliano W, Miller A. Ruffed grouse brood habitat selection at multiple scales in Pennsylvania: implications for survival. Can J Zool. 2008;86:329–37.
Van-Niekerk JH. Vocal behaviour of Crested Francolin Dendroperdix sephaena in response to playback calls. Ostrich. 2010;81:149–54.
Vignal A, Milan D, SanCristobal M, Eggen A. A review on SNP and other types of molecular markers and their use in animal genetics. Genet Sel Evol. 2002;34:275–305.
Walter JB. Presidential address: three centuries of international ornithology. Acta Zool Sin. 2004;50:880–912.
Wang N, Kimball RT, Braun EL, Liang B, Zhang ZW. Assessing phylogenetic relationships among Galliformes: a multigene phylogeny with expanded taxon sampling in Phasianidae. PLoS ONE. 2013;8:e64312.
Wang N, Zhang ZW. The novel primers for sex identification in the brown eared-pheasant and their application to other species. Mol Ecol Resour. 2009;9:186–8.
Wang Y, Xu J, Carpenter JP, Zhang Z, Zheng G. Information-theoretic model selection affects home-range estimation and habitat preference inference: a case study of male Reeves’s Pheasants Syrmaticus reevesii. Ibis. 2012a;154:273–84.
Wang Y, Zhang ZW, Zheng GM, Li JQ, Xu JL, Ma ZJ, Biancucci AL. Ornithological research: past twenty years and future perspectives in China. Biodivers Sci. 2012b;20:119–37 (in Chinese).
Watson JEM, Iwamura T, Butt N. Mapping vulnerability and conservation adaptation strategies under climate change. Nature. 2013;3:989–94.
Watson JEM, Venter O. Ecology: a global plan for nature conservation. Nature. 2017. https://doi.org/10.1038/nature24144.
Wells DA, Jones DN, Bulger D, Brown C. Male brush-turkeys attempt sexual coercion in unusual circumstances. Behav Process. 2014;106:180–6.
Williams CK, Lutz RS, Applegate RD. Winter survival and additive harvest in northern bobwhite coveys in Kansas. J Wildl Manag. 2004;68:94–100.
Wu YQ, Xu X, Liu NF, Xu F. Seasonal Changes in Habitat Use of Blue-Eared Pheasant, Crossoptilon auritum. Pak J Zool. 2013;45:1699–704.
Xiao H, Hu Y, Lang Z, Fang B, Guo W, Zhang Q, Pan X, Lu X. How much do we know about the breeding biology of bird species in the world? J Avian Biol. 2016;4:1–6.
Xu JL, Zhang XH, Sun QH, Zheng GM, Wang Y, Zhang ZW. Home range, daily movements and site fidelity of male Reeves’s pheasants Syrmaticus reevesii in the Dabie Mountains, central China. Wildl Biol. 2009;15:338–44.
Xu JL, Zhang ZW. Home range and habitat composition of male Reeves’s Pheasants in an agricultural-forest plantation landscape in central China: a preliminary report. Chin Birds. 2011;2:53–8.
Xu Y, Ran JH, Zhou X, Yang N, Yue BS, Wang Y. The effect of temperature and other factors on roosting times of Szechenyi Monal Partridges Tetraophasis szechenyii during the breeding season. Ornis Fenn. 2008;85:126–34.
Zhan XJ, Zhang ZW. Molecular phylogeny of avian genus Syrmaticus based on the mitochondrial cytochrome b gene and control region. Zool Sci. 2005;22:427–35.
Zhang L, Dong T, Xu W, Ouyang Z. Assessment of habitat fragmentation caused by traffic networks and identifying key affected areas to facilitate rare wildlife conservation in China. Wildl Res. 2015;42:266–79.
Zhang ZW, Ding CQ, Ding P, Zheng GM. The current status and a conservation strategy for species of Galliformes in China. Biodivers Sci. 2003;11:414–21 (in Chinese).
Zheng GM. A checklist on the classification and distribution of the birds of world. Beijing: Science Press; 2002 (in Chinese).
Zheng GM. Pheasants in China. Beijing: Higher Education Press; 2015 (in Chinese).
Zhou CF, Xu JL, Zhang ZW. Dramatic decline of the Vulnerable Reeves’s pheasant Syrmaticus reevesii, endemic to central China. Oryx. 2015a;49:529–34.
Zhou TC, Sha T, Irwin DM, Zhang YP. Complete mitochondrial genome of the Indian peafowl (Pavo cristatus), with phylogenetic analysis in phasianidae. Mitochondr DNA. 2015b;26:912–3.
Zhou ZT, Zhang YY. Isolation and characterization of microsatellite markers for Temminck’s Tragopan (Tragopan temminckii). Conserv Genet. 2009;10:1633–5.
Authors’ contributions
ST analyzed the data and led efforts to draft the manuscript. JX, JL, ZZ and YW conceived the ideas, improved the manuscript and directed the research. All authors read and approved the final manuscript.
Acknowledgements
We would like to thank Dr. Joanne Di Maio and Dr. Qing Zeng for their assistance with English language and grammatical editing of the manuscript and Andrew Cantrell for reviewing the draft. We also thank Mr. Pengcheng Wang, Mr. Yuanxing Ye, Ms. Xian Hou for valuable suggestions and the help of data analysis.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
This research was supported by the funded by the National Key Programme of Research and 422 Development, Ministry of Science and Technology (2016YFC0503200).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Tian, S., Xu, J., Li, J. et al. Research advances of Galliformes since 1990 and future prospects. Avian Res 9, 32 (2018). https://doi.org/10.1186/s40657-018-0124-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40657-018-0124-7