Abstract
We applied stable carbon and nitrogen isotopic analyses to understand the faunivory of the four sympatric wild Paradoxurinae civet species in Borneo, which share similar ecological characteristics. We also employed compound-specific nitrogen isotope analysis of amino acids to estimate these species’ trophic positions (TPs). The bulk stable isotope analysis revealed distinctly lower nitrogen isotope ratios in binturongs than in the other three species. This suggests that binturongs exhibit the lowest degree of faunivory among the four species. Binturongs had the lowest TP estimated from the nitrogen isotope ratios of amino acids (2.0–2.1), followed by small-toothed palm civets (2.4–2.5), masked palm civets (2.7), and common palm civets (2.9). These results suggest that there is little faunivory in binturong and variations in faunivory in the other species. Although the number of samples measured for the nitrogen isotope ratios of amino acids is small (n = 2 for each species), our results suggest that the varying degree of consumption of animal food sources, such as insects, is the key mechanism of niche partitioning in these four Paradoxurinae civet species in Borneo. Such subtle but essential differences in closely related sympatric species would maintain high biodiversity in tropical regions.

Similar content being viewed by others
1 Introduction
Carbon and nitrogen stable isotope analyses of animal tissues are effective for estimating the actual food sources consumed by individual animals. In terrestrial environments, the δ13C values of C3 plants are lower than those of C4 plants (Smith and Epstein 1971; O’Leary 1988), and such a difference is reflected in the consumers (Cerling et al. 1997). The δ15N values of bulk tissues (δ15Nbulk values) of organisms exhibit a stepwise increase with an increase in the trophic position (TP) in a food web (Minagawa and Wada 1984; Schoeninger and DeNiro 1984). Although the precise estimation of the TP with the δ15Nbulk values is limited in some cases by fluctuations in baseline δ15N values of the ecosystem and physiological changes in diet–tissue offset values, compound-specific nitrogen isotope analysis of individual amino acids (hereafter CSIA-AA) provides quantitative estimates of TP in individual animals (Chikaraishi et al. 2007, 2011; Steffan et al. 2013; Naito et al. 2016; Ohkouchi et al. 2017). Amino acids can be categorized into “source” and “trophic” amino acids, and the former slightly fractionates 15N (< 0.5‰) during trophic transfer, whereas the latter is highly enriched in 15N (~ 6–8‰) in each trophic step (Popp et al. 2007; O’Connell 2017; Ohkouchi et al. 2017). Therefore, the δ15N values of each amino acid from an individual provide information about both the baseline of the ecosystem and the individual's TP (McClelland and Montoya 2002; Chikaraishi et al. 2007). In this study, we applied these isotopic methods to investigate whether there is dietary partitioning among four sympatric wild Paradoxurinae civet species in Borneo.
The coexistence mechanism of closely related sympatric species remains one of the major themes in ecological science. In general, interspecific competition for resources among closely related sympatric species is intense because they have similar morphology, physiology, behavior, and ecology (Simberloff and Dayan 1991; Pianka 2000). As a consequence of the interspecific competition, resource partitioning typically occurs between closely related sympatric species (Pianka 2000).
Many scholars have focused on the coexistence mechanisms of mammalian carnivores because they form community structures such as apex or mesopredators (Ritchie and Johnson 2009). In mammalian carnivores, sympatric species typically exhibit differences in body size and behavior, which are typically reflected in dietary, spatial, and temporal partitioning (Vanak et al. 2013; Lovari et al. 2015; Karanth et al. 2017; de Satgé et al. 2017; Hearn et al. 2018; Nakabayashi et al. 2021). Carnivore species richness is high in southeast Asia and central and southeast Africa (Loyola et al. 2009). Among them, rainforests in Asia have a significantly larger number of sympatric carnivore species than those in the Neotropics and Africa (Corlett 2007). Civets (family Viverridae) are notable in their relatively greater number of sympatric species (Burgin et al. 2020). Civets are mammalian carnivorans that are widely distributed across Asia and Africa (Jennings and Veron 2009). There are four subfamilies in Viverridae: Paradoxurinae, Hamigalinae, Viverridae, and Genettidae. Paradoxurinae and Hamigalinae are distributed only in Asia (Jennings and Veron 2009). Eight species of civets coexist in Asian rainforests and share similar behaviors, such as nocturnal and solitary behaviors.
Four civet species belonging to the subfamily Paradoxurinae inhabit Borneo, with notable dietary, spatial, and temporal overlaps. These Paradoxurinae species include binturongs (Arctictis binturong), masked palm civets (Paguma larvata), common palm civets (Paradoxurus philippinensis), and small-toothed palm civets (Arctogalidia trivirgata), which weigh 6–10, 2.5–3, 1.7–2.7, and 1.5–2.6 kg, respectively (Yasuma and Andau 2000; Nakabayashi et al. 2017). Genetic evidence and morphology of the perineal glands support the monophyly of Paradoxurinae civet species within the Viverridae family (Gaubert et al. 2005; Patou et al. 2008). Given that the estimated divergence time of Paradoxurinae from the ancestral clade was 35.5–21.9 million years ago (Mya) (Patou et al. 2008), speciation occurred before Borneo was isolated as an island during the last glacial maxima (17,000 years ago, Voris 2000). The estimated divergence times of small-toothed palm civets, binturongs, masked palm civets, and common palm civets were 21.9, 15.9, 10.7, and 7.1 Mya, respectively (Patou et al. 2008). Radio-tracking and camera-trapping studies of these species have revealed that they occur sympatrically even in small areas (Brodie and Giordano 2011; Nakabayashi et al. 2017). Feeding observations have shown that the temporal activity patterns of these civets overlap significantly in Borneo (Nakabayashi et al. 2016). Information about the diets of civets is scarce, but they are generally omnivorous (Jennings and Veron 2009). A dental morphological study suggests that the diets of civets are dependent on subfamilies. Viverridae is more carnivorous than Paradoxurinae (Anders 2005). The dental morphology of two Paradoxurinae civet species, common palm civets and binturongs demonstrates that they are specialists in fruit crushing rather than generalists within Viverridae (Anders 2005).
Observational studies have revealed that fig fruits, hereafter figs, dominate the diets of these Paradoxurinae civet species (Nakabayashi et al. 2016; Nakabayashi and Ahmad 2018; Nakabayashi 2020). Fig fruits represent approximately 75.6% of the observed feeding patches for these civet species (Nakabayashi 2020). Although there are species-specific differences in the use of plant parts (e.g., tree bark sap, nectar, oil palm pith, and unripe fruit), these civet species typically eat the fruits of the same plant species (Nakabayashi 2020). Observational studies have suggested that faunivory (the generic term for feeding on animal flesh [carnivory] and insects [insectivory]) is rare in these four civet species because no faunivory had been observed in three binturong individuals during a total of 951 days of individual tracking (Nakabayashi and Ahmad 2018) and only ~ 5% (n = 3/55) of fecal samples of common palm civets contained visible remains of arthropods (Nakabayashi 2020).
Observational evidence of extensive dietary overlaps and little faunivory in the four civet species contradicts ecological and physiological expectations. Differentiating food resources is one of the most fundamental strategies for resource partitioning in ecological communities (Azevedo et al. 2006). Multiple sympatric species typically exhibit difficulty in coexisting if their spatial and temporal activity patterns and food sources overlap extensively (Pianka 2000). This is especially true in the tropical forests of Borneo, where the availability of fruits in this region is scarce compared with other Sundaic regions (Wich et al. 2011). It is also expected that a diet almost exclusively consisting of fruits will not meet the nutritional and energetic demands of civets. This is attributed to their typical morphology as mammalian carnivores, such as carnassial teeth (Anders 2005) and simple digestive tracts without polysaccharide fermentation (Lambert et al. 2014), which primarily limit the types and amounts of ingestible fruits (Nakabayashi 2015). Therefore, we expected unrevealed dietary partitioning in these civet species even though there are other examples of carnivores with extremely specialized herbivorous diets (e.g., pandas) or those that eat large amounts of fruits (e.g., some ursids, mustelids, canids, and procyonids (Draper et al. 2022).
A complete picture of the diet of Bornean sympatric civet species remains unclear even though systematic observational studies have been conducted on the diet of civets (masked palm civets in China: Zhou et al. 2008; common palm civets in Borneo: Nakashima et al. 2013; binturongs in Borneo: Nakabayashi and Ahmad 2018; Nakabayashi 2020). In Borneo, a fecal content analysis revealed the consumption of animal materials by common palm civets (Nakashima 2010); however, 92% of the analyzed feces contained seeds. Furthermore, the feces of civets are difficult to obtain for fecal content analysis because several civet species, such as small-toothed palm civets and binturongs, defecate higher in the canopy (Nakabayashi et al. 2019; M Nakabayashi personal observation), making it difficult to detect these feces on the ground. Thus, it is not clear whether or the extent to which Bornean civets consume animal materials. Because regional differences may affect diets, investigating the dietary breath of Paradoxurinae civets in Borneo may contribute to our understanding of the coexistence mechanism of closely related sympatric species. Opportunistic feeding of small insects is difficult to observe, and their faunivory is underestimated, particularly in regard to feces in the canopy. Even if their fecal samples are obtained, soft-bodied insects such as larvae and annelids are easily digested and thus are morphologically undetectable by identifying macro remains in feces. Dietary estimation methods in field-based research, such as fecal analysis and direct observation of animal feeding typically provide a “snapshot” of reality. Thus, it is difficult to comprehensively determine animal diets and ecological resource use (Moreno-Black 1978; Dickman and Huang 1988; Gales and Cheal 1992).
In this study, we hypothesized that the consumption of small animals, such as arthropods, is underestimated in the diets of civets and that their contribution to dietary protein intake is important despite their low detectability. We tested this hypothesis using stable isotope analyses. We conducted CSIA-AA on representative samples (i.e., two individuals per species) to obtain a perspective on species-specific differences in the degree of faunivory, which is reflected in TPs. Because the nitrogen isotope ratios of consumer tissues mostly represent those of proteins in food sources, we investigated the dietary protein contribution rather than the energy contribution in this study. Furthermore, this study focused on dietary niche partitioning that appears in TPs.
2 Materials and methods
2.1 Study sites
We conducted this study in the Danum Valley Conservation Area (Danum) and Maliau Basin Conservation Area (Maliau) in Sabah, northeastern Borneo (Supplementary Figure S2) from May 2012 to May 2014, and from November 2015 to June 2018, respectively. Danum (4°57ʹN, 117°48ʹE) is a 438 km2 protected area, and 90% of this area comprises mature lowland evergreen dipterocarp forest between 180 and 900 m a.s.l. (Marsh and Greer 1992; Newbery et al. 1999). The study area in Danum was located around the eastern boundary of the protected area. Maliau (4°49′N, 116°54′E) is a 588 km2 protected area, including lowland dipterocarp forests and at least 12 forest types between 300 and 1675 m a.s.l. (Hazebroek et al. 2004). The study area in Maliau was outside the basin and in a selectively logged dipterocarp forest.
2.2 Sample collection
We trapped civet species using box traps (Supplementary Text 1.1) set on the ground or branches at heights ranging from 3 to 35 m. In total, we captured six individuals of two civet species from Danum and 21 individuals of five civet species from Maliau (Supplementary Table S1). The hairs were pulled out from their skin and used for stable isotope analyses. The trapping and handling of the animals followed the guidelines of the American Society of Mammalogists (Sikes et al. 2016). Because seasonality was not clear during the study period (The Royal Society Southeast Asia Rain Forest Research Program, https://www.searrp.org/scientists/available-data/), seasonal dietary changes were not considered in this study. Regarding the unclear seasonality, the Paradoxurinae civet species studied generally do not exhibit clear seasonal molting in Borneo.
We collected insects and fruits as potential food samples for the civet species. Two insect species (a dung beetle and a weevil) were found near the base camp, and two fig species (Ficus caulocarpa and Ficus annulata) were obtained at a height of 3 m from trees in a phenological survey plot. Based on behavior observations of the civets, they strongly depend on figs as their diet (Nakabayashi 2020). Therefore, we selected figs as representative plant food items.
Sample collection, transfer, and analyses were approved by Sabah Biodiversity Centre (Access Licence JKM/MBS.1000–2/2JLD.4(170), JKM/MBS.1000–2/2JLD.6(50), JKM/MBS.1000–2/2JLD.4(170), JKM/MBS.1000–2/2JLD.7(64), and Transfer Licence JKM/MBS.1000–2/3(66), JKM/MBS.1000–2/3 JLD.3(100)).
2.3 Stable isotope analyses
Samples were treated for stable isotope analyses following previously described protocols (Campbell et al. 2017). In brief, hair and insect samples were defatted with chloroform and methanol. Strands of hair and insects/plant powder were used for both bulk stable isotope analysis and CSIA-AA. Carbon and nitrogen stable isotope ratios (δ13C and δ15N, respectively) of bulk hair, insect, and plant samples (~ 0.6 mg) were measured by Shoko Science, Co., Ltd. using an elemental analyzer-isotope ratio mass spectrometer. Compound-specific nitrogen isotope analysis of amino acid (δ15NAA) was performed for selected hair, insect, and plant samples. Samples for CSIA-AA were prepared using the amino acid derivatization procedures described by Chikaraishi et al. (2015). The δ15N values of individual amino acids were determined using a gas chromatograph coupled with an isotope ratio mass spectrometer (GC/C/IRMS) at the Japan Agency for Marine-Earth Science and Technology (JAMSTEC) (Ishikawa et al. 2018, 2022). The detailed methods are described in Supplementary Text 1.2.
The TP value was calculated on the basis of the stable nitrogen isotope ratios of glutamic acid and phenylalanine (δ15NGlu and δ15NPhe, respectively) as follows (Chikaraishi et al. 2011, 2014):
Glutamic acid and phenylalanine were used for TP calculation in this study even though recent meta-analyses and feeding experiments have proposed other combinations of amino acids for TP calculations (e.g., Ramirez et al. 2021; Whiteman et al. 2021). The metabolism of these amino acids has been extensively investigated, and their positions in nitrogen metabolism represent one of the most obvious trophic and source amino acids (Ohkouchi et al. 2017; Ohkouchi 2023). Theoretically, the TPs of primary producers (i.e., plants), primary consumers (i.e., obligate plant eaters, such as herbivorous animals), and secondary consumers (i.e., obligate eaters of primary consumers, such as obligate insectivorous animals) are expected to be 1, 2, and 3, respectively. The propagated errors of the TPs were calculated using the methods of Chikaraishi et al. (2009) and Ishikawa et al. (2022).
3 Results
3.1 Stable carbon and nitrogen isotope ratios of bulk tissues
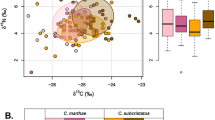
The results of stable isotope analyses are presented in Supplementary Table S1 and summarized in Table 1. First, the δ15Nbulk values of civets and plants were higher in Maliau than in Danum (Fig. 1). Mann‒Whitney U tests revealed that the δ13Cbulk (U = 36, p = 0.003) and δ15Nbulk (U = 1, p = 0.009) values of common palm civets from Maliau (n = 10) were significantly higher (+ 0.4‰ and + 1.0‰, respectively) than those from Danum (n = 4). Similarly, the δ15Nbulk values of small-toothed palm civets (n = 2) and plant fruits (n = 5) from Maliau were + 1.0‰ and + 3.5‰ higher than those from Danum (n = 2 and 2), respectively, even though no statistical tests were conducted because of the small sample size. Because of the more comprehensive coverage of the civet taxa and the larger number of obtained samples, CSIA-AA was performed only on the samples from Maliau. The results obtained from Maliau are described subsequently.
The mean δ13Cbulk and δ15Nbulk values of the civet species ranged from − 26.1‰ to − 22.6‰ and from 4.8‰ to 8.6‰, respectively (Table 1; Fig. 2). Among the five civet species in Maliau, binturong exhibited the lowest δ13Cbulk and δ15Nbulk values, indicating that the TP of binturongs is lower, even though the feeding experiments were inconsistent with this assumption (Supplementary Text 2). The δ13Cbulk and δ15Nbulk values of binturongs and the other civets were similar to those of squirrel/porcupine and mouse, respectively (Fig. 2).
The mean δ13Cbulk and δ15Nbulk values of fig fruits were − 29.8 ± 0.9‰ and 2.0 ± 1.0‰, respectively (Table 1). Compared with the δ15Nbulk values of fig fruits (Ficus spp.), those of binturong and the other civets in Maliau were 2.8‰ and 5.5‰–6.6‰ higher, respectively (Table 1). The δ13Cbulk and δ15Nbulk values of insects varied widely (Fig. 2), hindering the estimation of faunivory from bulk stable isotope ratios.
3.2 Nitrogen isotope ratios of amino acids
The degree of faunivory was quantitatively estimated using CSIA-AA (Table 2; Supplementary Table S2; Fig. 3). The δ15NPhe values, which primarily reflect the δ15N of primary producers, were distributed within 4.9‰ (8.6‰–13.5‰) among the analyzed samples in Maliau, with the exception of the dung beetle (16.2‰). The δ15NGlu values, which typically increase with increasing TP, were higher in civets (9.0‰–14.6‰) and the weevil (11.9‰) than in fig fruits (3.8‰ and 4.6‰).
δ15NGlu and δ15NPhe of Paradoxurinae civet species from Maliau and their potential food sources. Sample IDs and estimated TPs calculated based on Eq. (1) are also shown
The TPs calculated from the CSIA-AA of fig fruits were 0.8 and 1.2, and those of the civets ranged from 2.0 to 2.9 (Table 2; Fig. 3). Binturong exhibited the lowest TP (2.0 and 2.1) compared with the other species: common palm civets (2.9 and 2.9), masked palm civets (2.7 and 2.7), and small-toothed palm civets (2.4 and 2.5). Their point measurements of TPs (n = 2 for each species) revealed species-specific clusters with no overlap (Fig. 4). The TP was not associated with δ13Cbulk in these civets (Supplementary Figure S.3). The estimated TPs of insects were 2.6 and 2.7 for the weevil and the dung beetle, respectively (Table 2). The results show taxonomic differences in the degree of faunivory, even though the small sample size prevents further statistical tests.
4 Discussion
Common palm civets, masked palm civets, and small-toothed civets exhibited similar δ13Cbul even though they exhibited slightly different TPs (Supplementary Figure S3). Furthermore, their slight differences in TPs were not associated with δ15Nbulk (Fig. 2). This suggests that the bulk isotope ratios do not necessarily reflect the TPs of these non-binturong Paradoxurinae civet species even though the small sample size impedes rigorous interpretation, as will be discussed later. Conversely, binturongs exhibited different δ13Cbulk values compared with the other civet species, suggesting that binturongs rely on different nutritional sources compared to the other civet species. This argument is supported by the CSIA-AA of the selected samples, which indicates that the TPs of binturongs are lower than those of the other three species.
4.1 Trophic positions of Paradoxurinae civet species
Our findings show that non-binturong civet species have high δ15Nbulk values and higher estimated TPs, suggesting that their consumption of animal foods may have been underestimated. The δ13Cbulk and δ15Nbulk values of civets suggest that their diet varies by species, which is supported by the TP values estimated using CSIA-AA. Estimating the degree of faunivory based on these δ15Nbulk values of possible food sources is difficult due to variations in baseline isotope ratios and trophic discrimination. In other words, it is not clear whether the higher δ15Nbulk values of a civet species are due to higher trophic levels or increased dietary δ15Nbulk values. It is not feasible to collect and analyze dozens of dietary items from all civets (Nakabayashi 2020) to determine a stable isotopic baseline. As previously mentioned, CSIA-AA provides information about baseline isotope ratios and TPs, enabling quantitative estimation of TPs even from a limited set of representative samples.
The measured TP of plant-eating insects was higher (≥ 2.6) than that expected for obligate plant eaters (i.e., 2), and a small amount of insectivory can inflate the TPs of civet species. The higher than expected insect TPs may be due to the consumption of microorganisms with higher TPs than autotrophic organisms (Steffan et al. 2015, 2019). If dung beetles and weevils assimilated proteins from intestinal bacteria from feces or microbes grown on decaying wood, their TPs would be higher than expected for a strictly herbivorous insect. In addition, the TPs of masked palm civets (2.7) and common palm civets (2.9) were higher than those of insects, confirming their faunivory.
The point measurements of TP of the civet species ranged from 2.0 to 2.9, and each species exhibited clustered TPs that did not overlap with those of the other species (Fig. 3). Although our critical assumption is that the TP estimates of two individuals per species represent their respective species, this result suggests possible species-specific differences in the degree of faunivory among Paradoxurinae civet species. The CSIA-AA results illustrated overall faunivory in common palm civets, masked palm civets, and small-toothed palm civets, and the tendency of faunivory was stronger in this order if the individuals being analyzed truly represented the diet of each species. In contrast to the other civet species, the TP of binturongs was nearly one level lower even within the same subfamily, suggesting that their diet consisted almost entirely of plant-based materials such as fruits.
The diets of these civet species can be discussed in greater detail based on the existing knowledge from observational studies. First, we used the hairs of two intensively radio-tracked female binturong individuals for the analysis (Nakabayashi et al. 2016; Nakabayashi and Ahmad 2018; Nakabayashi 2020). According to the results of individual tracking of these binturongs, spanning over 900 days in total, 79%–86% of their diets consisted of fig fruits, and faunivory was not observed (Nakabayashi and Ahmad 2018). Therefore, the low δ15Nbulk values and TP of binturongs, which are similar to those of plant-eating animals, compared with the other three Paradoxurinae species simply reflect their fruit-dominated diet (Figs. 2, 3, and 4). In addition, given that fig fruits generally contain pollinating fig wasps inside (Harrison et al. 2003), fig wasps seem to contribute little to protein intake in binturongs despite their large consumption of fig fruits (Nakabayashi et al. 2019). Popowics (2003) noted that binturong dentition is small compared with the body size and exhibits a decrease in shearing and crushing functions. Binturongs typically feed on mature fig fruits; thus, the use of large teeth to process large hard fruits may not be necessary. Our stable isotopic results, along with the aforementioned observational studies, imply that faunivory seldom occurs in wild binturongs, at least in females. The results are similar to those obtained by individual tracking (Nakabayashi and Ahmad 2018) and conclusions based on more than 700 h of observations of animals that visited fruiting fig trees in Borneo (Leighton and Leighton 1983; Shanahan 2000).
Compared with binturongs, the difference in TP was less clear for the other civet species. Additional samples should be analyzed using CSIA-AA to investigate dietary niche partitioning in TPs further. Nevertheless, these civet species may exhibit occasional species-specific feeding behaviors, especially in small-toothed palm civets and binturongs. Common palm civets exhibited similar TP (2.9) to masked palm civets (2.7), equivalent to an omnivory. Scat analyses of common palm civets revealed that they occasionally consume rodents in Borneo (Nakashima et al. 2010, 2013; Colon and Sugau 2012), which is supported by our CSIA-AA results. These two species are genetically close compared with the other two Paradoxurinae species (Patou et al. 2008; Zhou et al. 2017) and exhibit overlap in several food items (Nakabayashi 2020). However, competition can be mitigated by their habitats being different in altitude. Although the occurrence records of common palm civets are concentrated in the lowlands, those of masked palm civets are concentrated in the highlands at an altitude of over 700 m in Borneo (Mathai et al. 2010; Brodie and Giordano 2011; Nagano et al. 2019; Nakabayashi et al. 2021). Geographical differences may be a more critical niche partitioning factor than diet for these two species. The measured TP for small-toothed palm civets (2.4 and 2.5) is between the TP ranges of common palm civets and binturongs, indicating their unique dietary niche among Paradoxurinae civets as omnivores consuming both plant and animal materials. This is partly supported by their unique dietary habits of feeding on tree bark sap, nectar, oil palm pith, and unripe fruits which the other Paradoxurinae civets do not consume in Borneo (Nakabayashi 2020). Contributions from these unique food sources increase the overall dietary protein contribution of plants and reduce the TP of small-toothed palm civets compared with common palm civets and masked palm civets.
4.2 Ecological significance
Our isotopic results suggest faunivory in several Paradoxurinae civet species in Borneo, which has never been confirmed in previous observational studies (e.g., Harrison 1961). While Paradoxurinae civet species are thought to largely depend on plant foods (Nakabayashi 2020), their dental morphology (Anders 2005) and digestive tract anatomy (Gahkod 1878; Liu et al. 1997; McGrosky et al. 2016) show clear characteristics of Carnivora. Such adaptations are not unique to civets, because some other mammals in Carnivora largely depend on plant foods, such as extant giant pandas and extinct cave bears (Naito et al. 2020). However, this study suggests that the postulated assumption of a plant-dominated diet only applies to binturongs among the Paradoxurinae civet species in Borneo. Common palm civets, masked palm civets, and small-toothed palm civets exhibited TP values greater than 2.4, suggesting an omnivorous diet (Table 2, Fig. 4). Considering that small-toothed palm civets were the first to evolve from the group containing these four species, followed by binturongs and masked palm civets (Patou et al. 2008), the possible differences in TP cannot be explained by the evolutionary relationships among the subject species. In Viverridae civets, larger species feed on fibrous low-quality food (Gittleman 1985), such as figs. Binturongs have the largest body size and cannot efficiently digest fruits (Crapo et al. 2002; Lambert et al. 2014), indicating that they require large amounts of food to extract sufficient energy for survival and reproduction compared with the other three species (Kleiber 1961). Because figs reproduce fruits year-round and have large crop sizes (Harrison et al. 2003), they are among the most stable food resources in Bornean rainforests where fruit production is unstable and low (Wich et al. 2011). Therefore, binturongs may strongly depend on figs as their diet, at least in Borneo. Because Asian rainforests possess a significantly larger number of sympatric carnivore species than other tropical regions (Corlett 2007), the strong dependence of binturongs on plant foods may reduce competition for faunivorous diets among sympatric carnivores.
Direct observations of food items in wild civet species are typically difficult, and previous field-based observational studies, such as focal individual observations and fecal content analysis (Nakabayashi 2020), have failed to detect dietary partitioning in Paradoxurinae civet species. The behaviors of most civets are not observable because of their nocturnal, solitary, and semiarboreal habits above 10–60 m canopies (Nakabayashi et al. 2017; M Nakabayashi personal observation). This study shows that stable isotope analysis, along with direct observations, is useful for revealing the entire diet of the subject species. In addition, metagenomic and metaproteomic analyses of feces can be used further to identify detailed food items of mammalian species (e.g., Mallot et al. 2017; Tsutaya et al. 2021). The application of isotopic and biomolecular analyses, such as those performed in this study, to animal species that are difficult to observe or have been studied little can reveal hidden mechanisms of coexistence and facilitate efficient conservation approaches, particularly for noncharismatic medium-sized and small mammals (Trimble and Van Aarde 2010; Troudet et al. 2017).
Isotope analyses have revealed nuanced dietary partitioning in various sympatric terrestrial mammalian taxa, such as Malagasy lemurs (Dammhahn and Kappeler 2014), great apes (Oelze et al. 2014), bats (Campbell et al. 2017; Oelbaum et al. 2019), rodents, and Bovidae (Djagoun et al. 2020). In general, dietary overlaps in sympatric carnivores are extensive, particularly in confamilial species (Arbogast et al. 2017; Webster et al. 2021), due to the morphological and physiological limitations of digestible diets (Stevens and Hume 2004). In this context, the inclusion of and dependence on plant foods, in addition to faunal foods, enable Paradoxurinae civet species to compensate for their spatial, temporal, and taxonomic overlaps and similarities (Nakabayashi et al. 2016, 2017) and occur sympatrically. Such subtle yet important dietary differences in closely related sympatric species support the high biodiversity of tropical regions (Whitmore 1984). However, anthropogenic disturbances can easily disrupt these subtle differences by increasing competition for food (e.g., fruits) (Meijaard et al. 2005) and potentially impairing coexistence mechanisms and biodiversity.
4.3 Limitations of this study
Two major limitations of this study should be addressed in future studies to provide a comprehensive picture of the diet of Paradoxurinae civet species. First, the molting patterns and timing of body hairs in Bornean civets should be clarified. To the best of our knowledge, there is no available information about the molting patterns and timing of civet species. Consequently, it remains unclear how far back and for how long the analyzed hairs reflect the diet of these civets. Although the dietary seasonality of Bornean civets is minimal, understanding the temporal resolution of hair growth and its variation is crucial for integrating isotopic results with behavioral observations (Dalerum and Angerbjörn 2005).
Second, this study did not thoroughly investigate the variations in the stable isotope ratios and TPs of potential arthropod prey species (Chikaraishi et al. 2011). Diverse arthropod species exhibit various life-history strategies and TPs. The TPs of faunivorous arthropods exceed 2, and their consumption can inflate the TP, resulting in an overestimation of the degree of faunivory of civet species. To accurately estimate the extent of faunivory, it is essential to identify the primary insect species consumed by civets and to collect and analyze these species.
5 Conclusions
The diets and TPs of four Paradoxurinae civet species in Maliau, Borneo were investigated using bulk stable isotope analysis of hair and CSIA-AA. Although the TP of binturongs (~ 2.0) indicates that their diet almost entirely comprises plant foods, the TPs of small-toothed palm civets, masked palm civets, and common palm civets (2.4–2.9) suggest their omnivorous diets. These results support our hypothesis that faunivory is more common than previously assumed in Paradoxurinae civet species in Borneo and imply that the degree of faunivory systematically differs among sympatric species. Such subtle dietary differences would enable the coexistence of closely related civet species and ensure high biodiversity in tropical regions. This study addressed ecological questions through the cross-disciplinary application of novel techniques developed in the field of geochemistry. New methods of isotope analysis (CSIA-AA) are increasingly being used in various research fields beyond geochemistry, and it is expected that, as demonstrated in this study, CSIA-AA will lead to new findings that are unexplored by conventional bulk isotope analysis.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Anders U (2005) Ökomorphologie südostasiatischer Viverridae (Schleichkatzen) -Spezialisierungen im Gebiss aufgrund von Ernährungspräferenzen. Johann Wolfgang Goethe-Universität Frankfurt am Main, Diploma
Arbogast BS, Hodge A-MC, Brenner-Coltrain J (2017) Stable isotope analysis of dietary overlap between the endangered red wolf and sympatric coyote in northeastern North Carolina. Southeast Nat 16:283–296
Azevedo F, Lester V, Gorsuch W, Lariviere S, Wirsing A, Murray D (2006) Dietary breadth and overlap among five sympatric prairie carnivores. J Zool 269:127–135
Brodie J, Giordano A (2011) Small carnivores of the Maliau Basin, Sabah, Borneo, including a new locality for Hose’s Civet Diplogale hosei. Small Carniv Conserv 44:1–6
Burgin CJ, Wilson DE, Mittermeier RA, Rylands AB, Lacher TE, Sechrest WS (2020) Illustrated checklist of the mammals of the world. Vol. 2 Eulipotyphla to Carnivora. Lynx Edicions, Barcelona.
Campbell CJ, Nelson DM, Ogawa NO, Chikaraishi Y, Ohkouchi N (2017) Trophic position and dietary breadth of bats revealed by nitrogen isotopic composition of amino acids. Sci Rep 7:15932
Cerling TE, Harris JM, Ambrose SH, Leakey MG, Solounias N (1997) Dietary and environmental reconstruction with stable isotope analyses of herbivore tooth enamel from the Miocene locality of Fort Ternan, Kenya. J Hum Evol 33:635–650
Chikaraishi Y, Kashiyama Y, Ogawa NO, Kitazato H, Ohkouchi N (2007) Metabolic control of nitrogen isotope composition of amino acids in macroalgae and gastropods: implications for aquatic food web studies. Mar Ecol Prog 342:85–90
Chikaraishi Y, Ogawa NO, Kashiyama Y, Takano Y, Suga H, Tomitani A, Miyashita H, Kitazato H, Ohkouchi N (2009) Determination of aquatic food-web structure based on compound-specific nitrogen isotopic composition of amino acids. Limnol Oceanogr-Meth 7:740–750
Chikaraishi Y, Ogawa NO, Doi H, Ohkouchi N (2011) 15N/14N ratios of amino acids as a tool for studying terrestrial food webs: a case study of terrestrial insects (bees, wasps, and hornets). Ecol Res 26:835–844
Chikaraishi Y, Steffan SA, Ogawa NO, Ishikawa NF, Sasaki Y, Tsuchiya M, Ohkouchi N (2014) High-resolution food webs based on nitrogen isotopic composition of amino acids. Ecol Evol 4:2423–2449
Chikaraishi Y, Steffan SA, Takano Y, Ohkouchi N (2015) Diet quality influences isotopic discrimination among amino acids in an aquatic vertebrate. Ecol Evol 5:2048–2059
Colon CP, Sugau JB (2012) Notes on the diet of the Malay civet (Viverra tangalunga) and other civets in logged and unlogged lowland dipterocarp rain forests in Sabah, Borneo. The Malayan Nat J 64:69–74
Corlett RT (2007) What’s so special about Asian tropical forests? Curr Sci 93:1551–1557
Crapo C, Moresco A, Hurley S, Hanner T, Kadzere C (2002) Anatomical measurements of the digestive tract and nutrient digestibility in the Asian bear cat (Arctictis binturong). J Dairy Sci 85:251
Dalerum F, Angerbjörn A (2005) Resolving temporal variation in vertebrate diets using naturally occurring stable isotopes. Oecologia 144:647–658
Dammhahn M, Kappeler PM (2014) Stable isotope analyses reveal dense trophic species packing and clear niche differentiation in a Malagasy primate community. Am J Phys Anthropol 153:249–259
de Satgé J, Teichman K, Cristescu B (2017) Competition and coexistence in a small carnivore guild. Oecologia 184:873–884
Dickman CR, Huang C (1988) The reliability of fecal analysis as a method for determining the diet of insectivorous mammals. J Mammal 69:108–113
Djagoun CA, Sinsin B, Wrage-Mönnig N (2020) Stable isotope niche segregation between rare topi antelope (Damaliscus lunatus korrigum) and other sympatric bulk grazers in Pendjari Biosphere Reserve (Northern Benin): Implication for topi conservation. Glob Ecol Conserv 22:e00918
Draper JP, Young JK, Schupp EW, Beckman NG, Atwood TB (2022) Frugivory and seed dispersal by carnivorans. Front Ecol Evol 10:864864
Gahkod A (1878) Note on the anatomy of the binturoug (Arctictis binturong). Proc Zool Soc Lond 46:142–142
Gaubert P, Wozencraft WC, Cordeiro-Estrela P, Veron G (2005) Mosaic of convergences, noise and misleading morphological phylogenies: what’s in a Viverrid-like Carnivoran? Syst Biol 54:865–894
Gales NJ, Cheal AJ (1992) Estimating diet composition of the Australian sea lion (Neophoa cinerea) from scat analysis: an unreliable technique. Wildl Res 19:447–455
Gittleman JL (1985) Carnivore body size: ecological and taxonomic correlates. Oecologia 67:540–554
Harrison J (1961) The natural food of some Malayan mammals. Bull Nat Mus Singapore 30:5–18
Harrison RD, Hamid AA, Kenta T, Lafrankie J, Lee HS, Nagamasu H, Nakashizuka T, Palmiotto P (2003) The diversity of hemi-epiphytic figs (Ficus; Moraceae) in a Bornean lowland rain forest. Biol J Linn Soc 78:439–455
Hazebroek HP, Adlin TZ, Sinun W, Wong K (2004) Maliau basin. Natural History Publications (Borneo), Kota Kinabalu.
Hearn AJ, Cushman SA, Ross J, Goossens B, Hunter LT, Macdonald DW (2018) Spatio-temporal ecology of sympatric felids on Borneo Evidence for resource partitioning? PLoS ONE 13:e0200828
Ishikawa NF, Chikaraishi Y, Takano Y, Sasaki Y, Takizawa Y, Tsuchiya M, Tayasu I, Nagata T, Ohkouchi N (2018) A new analytical method for determination of the nitrogen isotopic composition of methionine: Its application to aquatic ecosystems with mixed resources. Limnol Oceanogr-Meth 16:607–620
Ishikawa NF, Ogawa O, Sun Y, Chikaraishi Y, Takano Y, Ohkouchi N (2022) Integrative assessment of amino acid nitrogen isotopic composition in biological tissue samples determined by GC/C/IRMS, LC × EA/IRMS, and LC × GC/C/IRMS. Limnol Oceanogr-Meth 20:531–542
Jennings AP, Veron G (2009) Family Viverridae (civets, genets, and oyans). In: Wilson DE, Mittermeier RA (eds) Handbook of the Mammals of the World. Volume 1. Carnivores. Lynx Editions, Barcelona. 174–232.
Karanth KU, Srivathsa A, Vasudev D, Puri M, Parameshwaran R, Kumar NS (2017) Spatio-temporal interactions facilitate large carnivore sympatry across a resource gradient. Proc Royal Soc b: Biol Sci 284:20161860
Kleiber M (1961) The fire of life: an introduction to animal energetics. John Wiley and Sons, New York
Lambert JE, Fellner V, McKenney E, Hartstone-Rose A (2014) Binturong (Arctictis binturong) and Kinkajou (Potos flavus) Digestive Strategy: Implications for Interpreting Frugivory in Carnivora and Primates. PLoS ONE 9:e105415
Leighton M, Leighton DR (1983) Vertebrate responses to fruiting seasonality within a Bornean rain forest. In: Sutton SL, Whitmore TC, Chadwick AC (eds) Tropical Rain Forest: Ecology and Management. Blackwell, UK
Liu J, Huang F, Liu Z, Li W, Qu X, Mengsong K (1997) Anatomical studies of the digestive apparatus in Paguma larvata. J Hunan Agric Univ 23:578–581
Lovari S, Pokheral C, Jnawali S, Fusani L, Ferretti F (2015) Coexistence of the tiger and the common leopard in a prey-rich area: the role of prey partitioning. J Zool 295:122–131
Loyola RD, Oliveira-Santos LGR, Almeida-Neto M, Nogueira DM, Kubota U, Diniz-Filho JAF, Lewinsohn TM (2009) Integrating economic costs and biological traits into global conservation priorities for carnivores. PLoS ONE 4:e6807
Mallott EK, Garber PA, Malhi RS (2017) Integrating feeding behavior, ecological data, and DNA barcoding to identify developmental differences in invertebrate foraging strategies in wild white-faced capuchins (Cebus capucinus). Am J Phys Anthropol 162:241–254
Marsh CW, Greer AG (1992) Forest land-use in Sabah, Malaysia: an introduction to Danum Valley. Philos Trans R Soc b: Biol Sci 335:331–339
Mathai J, Hon J, Juat N, Peter A, Gumal M (2010) Small carnivores in a logging concession in the Upper Baram, Sarawak, Borneo. Small Carniv Conserv 42:1–9
McClelland JW, Montoya JP (2002) Trophic relationships and the nitrogen isotopic composition of amino acids in plankton. Ecology 83:2173–2180
McGrosky A, Navarrete A, Isler K, Langer P, Clauss M (2016) Gross intestinal morphometry and allometry in Carnivora. Eur J Wildl Res 62:395–405
Meijaard E, Sheil D, Nasi R, Augeri D, Rosenbaum B, Iskandar D, Setyawati T, Lammertink A, Rachmatika I, Wong A, Soehartono T, Stanley S, O'Brien T (2005) Life after logging: reconciling wildlife conservation and production forestry in Indonesian Borneo. CIFOR, Bogor, Indonesia
Minagawa M, Wada E (1984) Stepwise enrichment of 15N along food chains: further evidence and the relation between δ15N and animal age. Geochim Cosmochim Acta 48:1135–1140
Moreno-Black G (1978) The use of scat samples in primate diet analysis. Primates 19:215–221
Naito YI, Chikaraishi Y, Drucker DG, Ohkouchi N, Semal P, Wißing C, Bocherens H (2016) Ecological niche of Neanderthals from Spy Cave revealed by nitrogen isotopes of individual amino acids in collagen. J Hum Evol 93:82–90
Naito YI, Meleg IN, Robu M, Vlaicu M, Drucker DG, Wißing C, Hofreiter M, Barlow A, Bocherens H (2020) Heavy reliance on plants for Romanian cave bears evidenced by amino acid nitrogen isotope analysis. Sci Rep 10:6612
Nagano H, Okada K, Nakashima Y, Samejima H, Nais J, Kitayama K (2019) Habitat use of Bornean ferret badger Melogale everetti in Sabah, Malaysian Borneo. Small Carniv Conserv 57:25–33
Nakabayashi, M. (2015). Feeding ecology of three frugivorous civets in Borneo. PhD thesis, Kyoto University.
Nakabayashi M (2020) List of food plants of four sympatric Paradoxuriane civet species based on eight-year records on Borneo. Tropics 29:67–75
Nakabayashi M, Ahmad AH (2018) Short-term movements and strong dependence on figs of binturongs (Arctictis binturong) in Bornean rainforests. Eur J Wildl Res 64:66
Nakabayashi M, Ahmad AH, Kohshima S (2016) Behavioral feeding strategy of frugivorous civets in a Bornean rainforest. J Mammal 97:798–805
Nakabayashi M, Ahmad AH, Shiro K (2017) Horizontal habitat preference of three sympatric Paradoxurinae civet species in a small area in Sabah. Malaysian Borneo Eur J Wildl Res 63:2
Nakabayashi M, Inoue Y, Ahmad AH, Izawa M (2019) Limited directed seed dispersal in the canopy as one of the determinants of the low hemi-epiphytic figs’ recruitments in Bornean rainforests. PLoS ONE 14:e0217590
Nakabayashi M, Kanamori T, Matsukawa A, Tangah J, Tuuga A, Malim PT, Bernard H, Ahmad HA, Matsuda I, Hanya G (2021) Temporal activity patterns suggesting niche partitioning of sympatric carnivores in Borneo, Malaysia. Sci Rep 11:1–12
Nakashima Y, Inoue E, Inoue-Murayama M, Sukor JA (2010) High potential of a disturbance-tolerant frugivore, the common palm civet Paradoxurus hermaphroditus (Viverridae), as a seed disperser for large-seeded plants. Mamm Study 35:209–215
Nakashima Y, Nakabayashi M, Sukor JA (2013) Space use, habitat selection, and day-beds of the common palm civet (Paradoxurus hermaphroditus) in human-modified habitats in Sabah, Borneo. J Mammal 94:1169–1178
Newbery D, Kennedy D, Petol G, Madani L, Ridsdale C (1999) Primary forest dynamics in lowland dipterocarp forest at Danum Valley, Sabah, Malaysia, and the role of the understorey. Philos Trans R Soc b: Biol Sci 354:1763–1782
O’Connell T (2017) ‘Trophic’and ‘source’amino acids in trophic estimation: a likely metabolic explanation. Oecologia 184:317–326
Oelbaum PJ, Fenton MB, Simmons NB, Broders HG (2019) Community structure of a Neotropical bat fauna as revealed by stable isotope analysis: not all species fit neatly into predicted guilds. Biotropica 51:719–730
Oelze VM, Head JS, Robbins MM, Richards M, Boesch C (2014) Niche differentiation and dietary seasonality among sympatric gorillas and chimpanzees in Loango National Park (Gabon) revealed by stable isotope analysis. J Hum Evol 66:95–106
Ohkouchi N, Chikaraishi Y, Close HG, Fry B, Larsen T, Madigan DJ, McCarthy MD, McMahon KW, Nagata T, Naito YI, Ogawa NO, Popp BN, Steffan S, Takano Y, Tayasu I, Wyatt ASJ, Yamaguchi YT, Yokoyama Y (2017) Advances in the application of amino acid nitrogen isotopic analysis in ecological and biogeochemical studies. Org Geochem 113:150–174
Ohkouchi N (2023) A new era of isotope ecology: Nitrogen isotope ratio of amino acids as an approach for unraveling modern and ancient food web. Proc Japan Acad Ser B 99:131–154
O’Leary MH (1988) Carbon isotopes in photosynthesis. Bioscience 38:328–336
Patou M-L, Debruyne R, Jennings AP, Zubaid A, Rovie-Ryan JJ, Veron G (2008) Phylogenetic relationships of the Asian palm civets (Hemigalinae & Paradoxurinae, Viverridae, Carnivora). Mol Phylogenet Evol 47:883–892
Pianka ER (2000) Evolutionary ecology, 6th edn. Benjamin Cummings, San Francisco
Popowics TE (2003) Postcanine dental form in the Mustelidae and Viverridae (Carnivora: Mammalia). Journal of Morphol 256:322–341
Popp BN, Graham BS, Olson RJ, Hannides CC, Lott MJ, López-Ibarra GA, Galván-Magaña F, Fry B (2007) Insight into the trophic ecology of yellowfin tuna, Thunnus albacares, from compound-specific nitrogen isotope analysis of proteinaceous amino acids. In: Dawson TE, Siegwolf RTW (eds) Stable Isotopes as Indicators of Ecological Change. Academic Press, New York
Ramirez MD, Besser AC, Newsome SD, McMahon KW (2021) Meta-analysis of primary producer amino acid δ15N values and their influence on trophic position estimation. Method Ecol Evol 12:1750–1767
Ritchie EG, Johnson CN (2009) Predator interactions, mesopredator release and biodiversity conservation. Ecol Lett 12:982–998
Schoeninger MJ, DeNiro MJ (1984) Nitrogen and carbon isotopic composition of bone collagen from marine and terrestrial animals. Geochim Cosmochim Acta 48:625–639
Shanahan MJ (2000) Ficus seed dispersal guilds: ecology, evolution and conservation implications. PhD Thesis, University of Leeds.
Sikes RS, Animal C, Use Committee of the American Society of Mammalogists (2016) 2016 Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. J Mammal 97:663-688
Simberloff D, Dayan T (1991) The guild concept and the structure of ecological communities. Annu Rev Ecol Evol 22:115–143
Smith BN, Epstein S (1971) Two categories of 13C/12C ratios for higher plants. Plant Physiol 47:380–384
Steffan SA, Chikaraishi Y, Horton DR, Ohkouchi N, Singleton ME, Miliczky E, Hogg DB, Jones VP (2013) Trophic hierarchies illuminated via amino acid isotopic analysis. PLoS ONE 8:e76152
Steffan SA, Chikaraishi Y, Currie CR, Horn H, Gaines-Day HR, Pauli JN, Zalapa JE, Ohkouchi N (2015) Microbes are trophic analogs of animals. Proc Natl Acad Sci 112:15119–15124
Steffan SA, Dharampal PS, Danforth BN, Gaines-Day HR, Takizawa Y, Chikaraishi Y (2019) Omnivory in bees: Elevated trophic positions among all major bee families. Am Nat 194:414–421
Stevens CE, Hume ID (2004) Comparative physiology of the vertebrate digestive system. Cambridge University Press, Cambridge
Trimble MJ, Van Aarde RJ (2010) Species inequality in scientific study. Conserv Biol 24:886–890
Troudet J, Grandcolas P, Blin A, Vignes-Lebbe R, Legendre F (2017) Taxonomic bias in biodiversity data and societal preferences. Sci Rep 7:9132
Tsutaya T, Mackie M, Sawafuji R, Miyabe-Nishiwaki T, Olsen JV, Cappellini E (2021) Faecal proteomics as a novel method to study mammalian behaviour and physiology. Mol Ecol Resour 21:1808–1819
Vanak AT, Fortin D, Thaker M, Ogden M, Owen C, Greatwood S, Slotow R (2013) Moving to stay in place: behavioral mechanisms for coexistence of African large carnivores. Ecology 94:2619–2631
Voris HK (2000) Maps of Pleistocene sea levels in Southeast Asia: shorelines, river systems and time durations. J Biogeogr 27:1153–1167
Webster SC, Chamberlain MJ, Hinton JW, Beasley JC (2021) Isotope analysis reveals dietary overlap among sympatric canids. J Mammal 102:1222–1234
Whiteman JP, Rodriguez Curras M, Feeser KL, Newsome SD (2021) Dietary protein content and digestibility influences discrimination of amino acid nitrogen isotope values in a terrestrial omnivorous mammal. Rapid Commun Mass Spectrom 35:e9073
Whitmore T (1984) Tropical rain forests of the Par East. Clarendon Press, Oxford
Wich SA, Vogel ER, Larsen MD, Fredriksson G, Leighton M, Yeager CP, Brearley FQ, van Schaik CP, Marshall AJ (2011) Forest fruit production is higher on Sumatra than on Borneo. PLoS ONE 6:e21278
Yasuma S, Andau M (2000) Mammals of Sabah, part 2, habitat and ecology. Japan International Cooperation Agency and Sabah Wildlife Department, Kota Kinabalu.
Zhou Y, Wang SR, Ma JZ (2017) Comprehensive species set revealing the phylogeny and biogeography of Feliformia (Mammalia, Carnivora) based on mitochondrial DNA. PLoS ONE 12:e0174902
Zhou Y, Wang SR, Ma JZ (2008) Dietary shifts in relation to fruit availability among masked palm civets (Paguma larvata) in central China. J Mammal 89:435–447
Acknowledgements
The authors thank the Sabah Biodiversity Centre, the Economic Planning Unit Malaysia, Sabah Wildlife Department, Danum Valley Management Committee, and Maliau Basin Management Committee for their permission to conduct this research. We are grateful to field assistants, Azz, Kenneth, Albert, Farizal, and Taufiq for their assistance in the field. We appreciate the staffs of Hiroshima City Asa Zoological Park and Animeal for their assistance in feeding experiments on captive individuals. This study was supported by the Center for Ecological Research, Kyoto University, a Joint Usage/Research Center. We thank Prof. Yoshito Chikaraishi and an anonymous reviewer for their critical readings and helpful comments, which greatly improved the previous version of the manuscript.
Funding
This work was supported by research grants from The Inui Memorial Trust for Research on Animal Science, The Shikata Memorial Trust for Nature Conservation, The Fujiwara Natural History Foundation, JSPS Core-to-Core Program, A. Advanced Research Networks (Wildlife Research Center of Kyoto University), “Evolutionary Studies of Complex Adaptive Systems” Research Grant (Research Center for Integrative Evolutionary Science, The Graduate University for Advanced Studies (SOKENDAI)), and Grants-in-Aid for Scientific Research from JSPS (25597 and 201608680 to MN, 15J00464 and 22KK0170 to TT, 22K19857 to NFI, and 20H00208 to NO).
Author information
Authors and Affiliations
Contributions
MN and TT conceptualized the initial idea. MN obtained the field data. AHA arranged the sampling in the field. TT, YS, NOO, NFI, and NO performed and interpreted the isotope analyses. MN and TT wrote the first draft of the manuscript. All authors contributed to the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nakabayashi, M., Tsutaya, T., Ahmad, A.H. et al. Dietary partitioning in sympatric Paradoxurinae civets in Borneo suggested by compound-specific nitrogen isotope analysis of amino acids. Prog Earth Planet Sci 11, 53 (2024). https://doi.org/10.1186/s40645-024-00655-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40645-024-00655-6