Abstract
Purpose
Despite substantial animal evidence, cell therapy in humans remains in its infancy. The purpose of this study was to examine the potential therapeutic effects and safety of cell therapy in the treatment of tendon disorders.
Methods
According to the PRISMA guideline, a systematic review was performed on clinical studies concerning cell therapy in tendon disorders. A comprehensive search including the 5 databases of MEDLINE, Embase, Scopus, Web of Science, and Cochrane Library until December 2021 was carried out and associated with hand searching. The quality of the eligible studies was assessed using the tools suggested by Cochrane recommendations. Qualitative synthesis was performed in 2 tables and discussed separately for rotator cuff, elbow, patella, Achilles, and gluteal tendons.
Results
Through 6017 records, 22 studies were included in the qualitative synthesis, including 658 patients. All the studies administered autologous cells, except one that used allogenic adipose-derived mesenchymal stem cells (Allogenic AD-MSC). Almost all studies demonstrated the safety of cell injection in their follow-up period with no serious side effects or immunologic reactions, with only a few related minor adverse events in some cases.
The included studies showed the effectiveness of cell injection in tendinopathies of different sites, rotator cuff, elbow, patella, Achilles, and gluteal tendons. Among the rotator cuff studies, 4 comparative studies claimed that cell therapy is a more efficient treatment with a lower retear rate and pain level compared to the control group. However, one study found no differences between the groups. No controlled study has been performed on elbow tendinopathies, but 5 case series demonstrated the effectiveness of cell injection in elbow tendon disorders. For Achilles tendinopathies, only one randomized controlled trial (RCT) found that both cell therapy and control groups showed significant pain reduction and functional improvement with no statistical differences at the 6 months follow-up, but the cell therapy group had improved faster at earlier follow-ups. Patellar tendinopathy was studied in 2 RCTs, one did not show a significant difference and the other showed superior improvement compared to controls.
Conclusion
Cell therapy showed promising results and the available evidence suggests that it is safe at several sites of tendon disease. Based on available evidence, cell therapy should be suggested in specific conditions at each site. To approve cell therapy for tendon diseases, randomized clinical trials are required with a large sample size and long-term follow-ups.
Level of evidence
IV
Similar content being viewed by others
Introduction
As widespread and chronic disorders, tendinopathies can cause severe economic, social, physical, and psychological burden for patients [1, 2]. It is estimated that lower limb tendinopathy occurs at an incidence of 11.83 and a prevalence of 10.52 per 1000 person-years, respectively. This number increased in the sporting population up to 14.4% and in elite volleyball players up to 45% [1]. Due to insufficient blood supply, tendon tissue cannot efficiently repair its defect and reform [3]. Moreover, the tendon tends to form fibrous tissue and scars in the healing process, thus leading to adhesion formation [4]. Therefore, tendinopathy and tendon rupture impairs the patient’s ability and function [3]. Thus, the treatment of tendon disorders is a significant challenge for orthopaedic surgeons [4]. Various treatments, both operative and non-operative, for the restoration of tendon function have been discussed [5, 6]. Currently, operative repair is the treatment of choice after the failure of conservative treatments [5].
Cell therapy, performed through prepared cells injection, shows encouraging results [7,8,9,10]. The most common cells used in this method are mesenchymal stem cells (MSCs), multipotent stem cells primarily found in bone marrow and capable of differentiating into bone, tendon, cartilage, muscle, ligament, fat, and marrow stroma. MSCs can be applied to the injury site or delivered on a scaffold [1, 11]. Other cells can be used in tendinopathy such as human embryonic stem cells (hESCs), bone marrow cells, bone marrow mononuclear cells (BMMCs), and adipose-derived stem cells (ASCs) [2]. The rationale behind cell therapy for tendon disorders is that fibroblastic cells derive from undifferentiated MSCs. Cells of this type are responsible for tendon healing through synthesizing collagen after tendon damage [3, 4, 12].
The results of previous studies using cell therapy for tendon healing were promising, and cell injections such as MSC demonstrated a significant pooled effect size for pain and functional scores, as well as structural healing in radiologic and arthroscopic investigations [13,14,15,16,17]. Some studies have presented superior radiological and clinical outcomes for cell therapy in tendon disease [14], while others have claimed faster healing regardless of similar outcomes in the end [15]. Although previous studies have supported cell therapy, some have encountered serious limitations such as non-randomized allocation and unavoidable selection biases, low quality of the method, heterogeneity, disagreement over the details of the method, and short-term follow-ups [9]. A recent meta-analysis reviewed 4 prospective studies, suggesting the high efficacy of MSC therapy in tendon disorder and its promising outcome in respect to radiologic, arthroscopic, and functional parameters [18]. In addition, a systematic review of stem cell therapy identified 8 trials with significant bias, and thus, they could not conclude that it is safe [9].
Regardless of massive animal evidence, cell therapy in humans is in its infancy. This systematic review provides a summary of the current findings on the potential therapeutic effect of cell therapy and its safety in healing tendon disorders. This study sought to compare the beneficial effect of cell therapy based on the injury site, and the type of cells injected and their source.
Materials and methods
Search strategy and screening
The study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (see Additional file 1) [19]. A protocol for this review has been registered in the International Prospective Register of Systematic Reviews (PROSPERO); registration ID: CRD42021251539; https://www.crd.york.ac.uk/PROSPERO).
The 5 databases of MEDLINE/PubMed, EMBASE, SCOPUS, Web of Science, and Cochrane Library were searched for clinical studies that applied cell therapy for tendon disease treatment from inception until March 22, 2021. We updated our search on December 26, 2021. Our search strategy consisted of numerous keywords and database-specific subject heading vocabulary to identify studies regarding cell therapy for tendon disease, which includes these concepts without any prior restriction: “Cell therapy” AND “Tissue Engineering” OR “Regenerative Medicine” AND “Tendon.” The thorough search strategy is available in the supplementary material (Tables S1, S2, and S3, (see Additional file 2)). The search query was changed to some extent based on the search rules of each database. Citation searching and forward citation screening were performed on the potentially eligible articles, and a reference was included when it met our eligibility criteria.
All records were imported to the Covidence online systematic review software (https://www.covidence.org). After removing duplicates, 2 reviewers (S.P.M and Z.V) separately screened all the imported articles to find eligible studies based on the distinct inclusion/exclusion criteria. A study was included if it had gained 2 “yes” votes in both steps of title/abstract screening and consequent full-text screening. For full-text papers not included in the analysis, the reasons for exclusion were documented. Here and in the following sections, any conflicts were resolved through discussion and consulting with the corresponding author (M.H.N).
Inclusion and exclusion criteria
All original studies that had administered any type of cells to treat patients with tendon disease were included in the review. There was no restriction on the route through which cells were administered. The included studies had to represent the patient-reported outcome measures (PROMs) or paraclinical imaging investigations as to their primary outcome. The exclusion criteria included lack of administration of cells as intervention, non-English articles, non-human studies (animal or in vitro studies), patients with another major interfering morbidity, case reports, reviews, congress abstracts, commentaries, and book chapters, and lack of availability of the full-text (after attempts to receive the text from the authors or the journal via Email). No other restrictions were applied to the inclusion of studies.
Assessment of study quality
Again the same 2 reviewers independently evaluated the selected studies in terms of their quality and risk of bias. The Cochrane Risk of Bias tool V 2.0. (RoB 2) [20] was used for randomized controlled trials (RCTs). This tool assesses the study based on the 6 subsets of selection bias, performance bias, detection bias, attrition bias, reporting bias, and other potential biases [20]. According to the Cochrane recommendations, studies were rated in each topic as high, low, or unclear. Finally, the overall estimation of the study quality was expressed as high risk, low risk, or some concern.
To evaluate the quality of non-RCTs, here we assumed clinical trials that groups were not divided randomly, the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Quasi-experimental studies [21] was used. The National Institutes of Health (NIH) quality assessment tool for case-series studies was used for the case studies without a control group [22]. This tool evaluates the quality of case studies based on selection, comparability, and description of the population, intervention, result, and statistical method. Both NIH and JBI tools described appraising the articles with 9 questions. Each question received a score of 1 (yes) or 0 (no). Greater scores are considered as high quality. A score of ≥8, 5-7, and ≤ 4 was associated with high, moderate, and low quality.
Levels of evidence
The level of evidence was written as declared in the original study. The Centre for Evidence-Based Medicine (CEBM) guideline in Oxford, UK, was used to detect the level of evidence of studies that had not provided it [23].
Data collection and abstraction
Data was extracted from the selected studies and entered into a pre-designed table for evidence synthesis by 2 reviewers (S.P.M and Z.V) independently. The characteristics and conclusions are presented in one table, and the study’s raw results in another. The extracted information included the first author’s family name, year of publication, country, type of study design, study groups and their population, sex, age, type and site of injury, size of the lesion, injected cell type and source, follow-up duration, number of cells injected and route, surgery procedure, outcome measure, results and outcomes, and the study’s conclusion and level of evidence. For the studies that had not reported their results as numbers in a table, reviewers extracted the approximate values from the figures and diagrams or emailed the corresponding author to receive the data. Furthermore, any adverse events related to cell injection, including abnormal signs, symptoms, or diseases, were extracted.
Data synthesis and statistical analysis
Kappa (κ) values were used to assess the inter-reviewer agreement for article screening. Based on a priori category classification, substantial agreement was κ > 0.60, moderate agreement 0.21 < κ < 0.60, and slight agreement κ < 0.21 [24]. Descriptive statistics (e.g., means, ranges, and variance measures) are presented where applicable. A meta-analysis was not performed due to the small number of studies with similar outcome measures and high heterogeneity.
Results
Search results
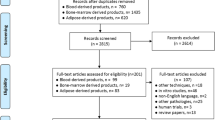
A total of 6017 records were retrieved from the search and hand searching (Fig. 1). After removing duplicates, 2543 studies were screened by title and abstracts. Subsequently, the remaining 262 articles were evaluated by full-text for eligibility, and 22 were selected [14, 15, 25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44]. In terms of the title and abstract screening (κ = 0.89; 95% CI:, 0.86 - 0.92), as well as the full text (κ = 0.85; 95% CI,: 0.81 - 0.89) there was excellent agreement across reviewers. Three relevant case reports were excluded; their results are presented in the supplementary material (table S6, (see Additional file 2)) [45,46,47]. Moreover, 2 articles were excluded [48, 49] because a new update with a longer follow-up was published [30, 44]. A technical note was excluded because of the lack of follow-up outcomes [18]. The details of the study selection process are illustrated in Fig. 1.
Included studies
The selected studies consist of 658 patients cumulatively, were published between 2009 and 2021, and were performed in the USA (n = 6), South Korea (n = 5), UK (n = 2), Brazil (n = 2), Spain (n = 2), Italy (n = 1), France (n = 1), Australia (n = 1), Argentina (n = 1), and India (n = 1). The studies consist of 13 case series (interventional study without control group), 6 RCTs, and 3 non-RCTs (clinical trials with non-randomized control groups). The characteristics of the studies are presented in Table 1 and divided based on the site of the injury (rotator cuff: 10 studies; elbow: 5 studies; Achilles: 3 studies; patellar: 3 studies; gluteal: 1 study) (Table 1).
Methodological quality
The overall quality assessment of the included studies is presented in Table 2; 15 studies have high methodological quality (68.2%), 4 of them have moderate quality or some concern exists regarding biases (18.2%), and 3 studies seemed to have low methodological quality (13.6%).
Outcomes
The conclusions of the included studies are presented in Table 1, and the raw results in table S7. All the studies, as previously mentioned, included direct whole-cell injection to the injury site (tendon damage site), and studies that injected extracts from the cells like secretome or biologics (not a “whole-cell”) were excluded. Only Lee et al. [36] used allogenic cells.
Rotator cuff
All studies, except one [35], showed the safety of cell injection in rotator cuff tendinopathies without serious adverse effects [14, 28,29,30, 32, 34, 37]. The RCT by Lamas et al. on autologous MSCs in a xenogenic scaffold (OrthADAPT™) for repairing full-thickness rotator cuff tears was terminated because both groups showed adverse effects [35]; 1 patient (8%) in the control group (Scaffold) and 3 patients (23%) in the intervention group (Scaffold + MSC) experienced postoperative complications. Supraclavicular cysts and subacromial inflammatory tissue were observed in these patients. About 60% of both groups experienced re-rupture. The complications experienced in the 2 study groups were not associated with the autologous MSCs, but rather with the scaffold (OrthADAPT™) [35].
Cells used in the rotator cuff repair studies consist of the following type of cells: BMMC [28], bone marrow-derived mesenchymal stem cells (BM-MSCs) [14, 35], adipose-derived mesenchymal stem cells (AD-MSC) [30, 33], bone marrow concentrate (BMC) [25, 32, 37], and uncultured, autologous, fresh, unmodified, adipose-derived regenerative cells (UA-ADRCs) [29].
The included studies have demonstrated that cell injection in tendon disorders yielded beneficial effects. Four studies claimed that cell therapy was a more efficient treatment compared to the control group (MSC and UA-ADRCs) [14, 29, 32, 35], with a lower rate of retear in surgical patients [14, 34], less pain, and higher function [29, 32, 35]. However, Kim YS et al. observed no differences between the cell therapy and control groups in terms of pain, ROM, and functional scores at the final follow-up (28 months) [34]. Nevertheless, the retear rate in MRI was significantly lower (28.5% vs. 14.3% retear; P < 0.001) in the cell therapy group and better outcomes were observed in this group at earlier follow-ups. In another study, Kim SJ et al. reported no significant reduction in tear size in the study groups, although substantial improvement was observed in pain and function in the cell injected group [32].
Elbow
All studies showed the safety of cell injection in elbow tendinopathies without serious or clinically significant adverse effects [27, 31, 36, 41, 44]. However, Lee et al. reported a minor effusion in the elbow joint in 2 of the 12 patients with recalcitrant lateral elbow tendinopathy 52 weeks after allogenic AD-MSC injection [36]. Khoury et al. followed 18 patients with recalcitrant lateral elbow tendinopathy for 6 months after autologous AD-MSC injection; they observed a subcutaneous hematoma at the injection site in 2 participants [31]. In elbow tendon repair studies, the following types of cells are used: AD-MSCs [31, 36], autologous tenocytes [44], and tenocyte-like cells [27].
In addition, all the studies demonstrated the effectiveness of cell injection in elbow tendon disorders [27, 31, 36, 41, 49]. Lee et al. studied the two groups of low-dose (106 cells) and high-dose (107 cells) allogenic AD-MSC injection in patients with lateral elbow tendinopathy, and found no significant differences in function and pain between the groups. However, the improvement in pain and function was faster in the high-dose group, which illustrates the efficacy of cell therapy [36].
Achilles
All studies demonstrated that cell injection in Achilles tendinopathies was safe without serious adverse effects during the follow-up periods [15, 42, 43]. Moreover, all the studies demonstrated the beneficial effects of cell injection in tendon disorders. Cells used in the Achilles repair studies consist of the following type of cells: BMC [42], adipose-derived tissue stromal vascular fraction (AD-tSVF) [43], and stromal vascular fraction (SVF) [15].
Tate-Oliver and Alexander [43] administered AD-tSVF to 3 patients with Achilles tendinosis and interstitial tears, and Stein et al. [42] augmented 27 Achilles tendon tears with BMC injection. Neither study had a control group [42, 43], and the results of both demonstrated a reduction in pain [42, 43], no retear or re-occurrence [42, 43], structural improvement in the damaged tendon, and the ability to do light activities or return to sports [42, 43] in patients compared to pre-treatment. Findings of the only RCT on recalcitrant non-insertional Achilles tendinopathy treated with SVF (intervention) or platelet-rich plasma (PRP; controls) [15] showed a significant reduction in the VAS pain score and improvement of functional scores (VISA-A and AOFAS) in both groups compared to baseline. No significant differences were detected in the final follow-up (6 months), but the SVF group improved faster. This means that the SVF group participants showed significantly better outcomes in the shorter follow-ups (15 and 30 days follow-up). Radiological data (MRI and US) showed no improvement in either group [15].
Patellar
All studies showed the safety of cell injection in patellar tendinopathies with no serious adverse effect [26, 38, 39]. Nevertheless, Rodas et al. study comparing BM-MSC and Lp-PRP injections on patients with chronic patellar tendinopathy reported a few mild side effects (one in each group), mostly musculoskeletal such as myalgia and arthralgia [39]. Cells used in patellar tendon repair studies consist of the following type of cells: tenocyte-like cells [26], bone marrow mononuclear cell (BM-MNC) [38], and MSC [39].
All the studies demonstrated the beneficial result of cell injection in patellar tendon disorders [26, 38, 39]. Rodas et al., in their RCT, compared BM-MSC treatment with Lp-PRP treatment as a control group in patients with refractory patellar tendinopathies [39]. They concluded that both treatments successfully reduce pain and improve the VISA-P score with no significant difference. Nevertheless, the BM-MSC group was superior in terms of structural healing in the ultrasound and MRI imaging. In another RCT by Clarke et al. on patients with refractory patellar tendinosis, the cell therapy group (tenocyte-like collagen-producing cells) was superior to the control group (autologous plasma alone) in terms of VISA score with a faster response [26].
Gluteal
There was only one study on gluteal tendinopathy, in which they used BMC in the intervention group and corticosteroid in the control group [40]. This technique was safe and effective in significantly reducing the VAS score and Lequesne score compared to the control group.
Discussion
This systematic review investigated the safety and efficacy of cell therapy in treating tendon disorders. Almost all included studies reported the safety of cell injection in their follow-up period with no significant side effects or immunologic reactions. They noted only a few related minor adverse events in some cases (including pain or swelling at the site of the injection [15, 25, 31, 34, 36], abdominal pain [29], musculoskeletal pain [29, 39], upper respiratory tract infection [29], mild effusion of joint [36], and subcutaneous hematoma [15, 31]).
All the studies in this review demonstrated the potential effect of cell therapy in tendon disorder treatment. Although some of the articles reported the beneficial impact of cell injection on tendinopathies and the superiority of the cell injected group compared to controls [14, 26, 28, 29, 32, 40], other RCTs and studies with a control group showed no improvement in outcomes in the treatment group compared to controls [15, 34, 39]. However, the procedure satisfied a high rate of patients [32, 38]. Our results are in line with that of the meta-analysis by Cho et al.; they reviewed only prospective studies on MSC administration in tendinopathies [13]. They analyzed 4 prospective studies and revealed a significant pooled effect size with a significant cell dose-dependent response in pain reduction.
The exact mechanism of action of the MSC effect in tendon healing is still not clear. Studies have suggested that injected stem cells survive for some weeks in the defect [50], differentiate into tenocytes [11, 51], and excrete their secretome (paracrine effect) with regenerative effects [52, 53]. Another possible mechanism is that the MSCs, on their own, release extracellular factors and cytokines, thus accelerating regeneration and modulating immune cell response [53, 54]. Considering that inflammation has a critical role in the tendon tissue damage process, the regulatory effect of MSC can potentially affect tendon tissue repair [53,54,55].
There are still concerns regarding the safety of cell injection. In previous studies with different settings, such as ischemic cardiomyopathy [56] and myocardial infarction [57], the systemic administration of both allogeneic and autologous MSC appeared safe with minimal adverse effects, including immunologic reactions [13, 56,57,58,59,60]. In line with the present study findings, other systematic reviews on tendon tissue cell therapy did not report serious adverse events in clinical and preclinical studies [13, 18, 61, 62]. However, van den Boom et al., in an article systematically reviewing the efficacy of stem cell therapy in tendon disease treatment, highlighted the potentially harmful consequences of stem cell application such as the development of malignancies in the target organ [9]. Injection of autologous hematopoietic stem cells caused tumor growth in the injected kidney of a patient with renal failure [63], and intrathecal injection of MSCs caused glioma growth in another patient with an ischemic stroke [64]. Major complications of stem cell therapy were observed in other tissues, such as infection following the receipt of umbilical cord blood-derived stem cells [65], tumor formation at the target tissue [63, 64], and worsening of the disease course in patients with macular degeneration [9]. Regarding the complications of cell therapy in tendon tissue, ectopic bone formation was observed in the rabbit model as a result of using MSC [66]. Donor site morbidity when retrieving a sufficient amount of cells is another drawback [67]. Generally speaking, studies on human tendon tissue have not illustrated any major adverse event as a result of the delivering of cells to the tendinopathy site.
Numerous studies have investigated the efficacy of cell therapy in rotator cuff conditions. As far as the available evidence indicates, cell therapy for rotator cuff tendon seems beneficial for the augmentation of rotator cuff repair surgery and for patients with partial-thickness tears who did not respond to conservative medication or physical therapy for more than 3-6 months. Liu et al., in a systematic review study in 2019, evaluated stem cell application in rotator cuff healing [61]. Although only 3 of the articles included in their study were on human subjects, their meta-analysis revealed that VAS and ASES scores at 3 months are more favorable in the stem cell group. Regarding the animal studies included in their review [61], no significant differences were observed between groups when biomechanical evaluation of the tendon was performed. However, motion analysis scores (walking distance, fast walking time, and mean walking speed) were higher in the stem cell group [61]. Our finding revealed that 4 trials favoured cell therapy [14, 29, 34, 35]; however, 1 trial revealed no superiority for the cell group over controls [34].
Lamas et al., in a double-blind RCT (only abstract) [68], assessed the safety and efficacy of autologous MSC administration accompanied by surgical repair in full-thickness rotator cuff tears. The stem cell group (N = 8) showed an improvement of 31 points in the Constant score after a year, which was significantly higher than the control group (N = 5) with an improvement of 16 points. Other assessments were comparable (VAS pain, retear rate, and repair integrity) [68]. However, 37.5% of the treatment group and 25% of the controls presented with swelling, pain, and retear requiring reoperation. Therefore, the complications of the procedure mandate further RCTs. Lamas et al. published a double-blind RCT in 2019, 4 years after their first study, on the efficacy and safety of autologous MSCs implanted in a xenogenic scaffold in repairing full-thickness rotator cuff tear [35]. They stopped the study due to adverse events in both groups (~ 60% retear) [35], which indicates that the technique of xenogenic scaffold use (OrthADAPT™) should be revised. The complications experienced in the 2 study groups (Scaffold vs. Scaffold + MSC) were not associated with autologous MSCs, but rather with the scaffold (OrthADAPT™) [35]. However, the treatment group showed a significant improvement in the Constant score compared to baseline even though it is inconclusive [35].
No well-designed RCT exists on cell therapy in elbow conditions and epicondylitis. In the elbow tendon disorders, the cell therapy again could be used as a non-surgical rescue treatment after failed first-line options for patients suffering from a partial-thickness tear of extensor tendons. Yet, the existing case studies show an improvement in pain, function, and radiology assessment in patients with refractory lateral epicondylitis [27, 31, 36, 44] and patients with no history of treatment [41]. In the study by Khoury et al. on 18 patients with recalcitrant lateral elbow tendinopathy, pain reduction and improved function were witnessed after injection of autologous ASCs under US guide [31]. Moreover, structural healing was verified using MRI radiology. The use of allogenic AD-MSC in another trial also had comparable results and was safe for patients in terms of immunologic rejection [36]. The advantage of allogenic cell application is that there is no need to harvest cells from the patients individually. The application of cultured autologous tenocytes has the same outcome [44, 49].
Skin-derived tenocyte-like cells were used by Connell et al. to treat refractory lateral epicondylitis [27]. Symptoms relief and structural healing, and no retear were observed in their 12 participants [27]. In the study by Singh et al., BMC, which mainly consists of BM-MSC, was used in patients with lateral epicondylosis, and was found to be effective in terms of the Patient-Rated Tennis Elbow Evaluation (PRTEE) score after 3 months [41]. No comparative clinical trial has been undertaken to determine which of the abovementioned cell types is more efficient in clinical use. Furthermore, no study has compared cell therapy with a control group. Thus, further investigations are necessary to approve cell therapy as a treatment option.
Cell therapy might also improve Achilles tendinopathies. However, Achilles tendinopathy patients are very heterogeneous, and no recommendation can be drawn from them. The RCT by Usuelli et al. revealed the safety of adipose-derived SVF injection for chronic Achilles tendinopathy with minor adverse effects at the site of the adipose tissue harvest [15]. Although both groups of SVF and PRP showed healing effects in the treatment of Achilles tendinopathy, the stem cell group recovered faster [15]. No other studies exist concerning cell injection in the treatment of the human Achilles tendon disorder. Superb results with early weight-bearing and no retear were observed in other case studies on BMC plus surgical tendon repair [42, 43].
Preclinical investigations on rats have presented promising results [69,70,71,72,73]. Okamoto et al. compared the ultimate failure load of the Achilles tendon among the BMC treatment, MSC treatment, and non-treated groups. The BMC group showed greater improvement at the final stage and that possibly the other hematopoietic stem cells are responsible for the better function of MSCs [71]. Machova Urdzikova et al. treated collagenase-induced Achilles with human MSCs and compared them to controls [70]. The MSC group illustrated a more organized ECM structure and vascularization and were safe in the rats [70]. Yao et al. also noted the faster healing of the rat’s Achilles tendon using sutures seeded with bone marrow-derived stem cells [74]. Chong et al. only reported histological and biomechanical improvement of the Achilles tendon in the early stage of administrating BM-MSCs to the transacted tendon in the rabbit model [69].
Cell injection for patellar tendinopathy is not well studied. Likewise shoulder and elbow, chronic patellar tendinopathies that do not respond to nonoperative treatment or rehabilitation for more than 3-6 months should be considered for cell therapy. Two RCTs [26, 39] on the use of BM-MSC and tenocyte-like cell (derived from dermal fibroblast) showed satisfactory healing of the tendon tissue. In the study by Rodas et al. on 20 patients with chronic patellar tendinopathy, although clinical outcomes of the cell group (BM-MSC) and active control group (Lp-PRP) were similar, the structural regeneration in radiology was only observed in the cell group [39]. Thus, the cell group may benefit more in the long term.
Using dermal fibroblast for tendon engineering has been discussed, and the potential positive effect has been established in preclinical studies [75]. These cells were harvested with minimal donor site morbidity and showed tendon regeneration in animal studies [75]. In a human study by Connell et al. [27], tenocyte-like cells derived from skin fibroblasts were injected safely to treat patients with lateral epicondylitis, resulting in symptom subsidence in 11 of the 12 patients and structural healing in the US.
This systematic review faced serious limitations, mainly due to the poor design of the included articles. Many studies included in the review lack control groups, and the ones containing control groups are on a small population, thus leading to the limited power of the studies. More than half of the included studies followed the patient for a year or less, and many for only 3-6 months, which is insufficient to provide compelling evidence. The authors recommend the follow-up of the patients for a more extended time using radiological and laboratory modalities to ensure the procedure’s safety. Studies used various types of cells from diverse sources, bone marrow, adipose tissue, or skin. Many studies in this review administered BMC [14, 15, 25, 32, 33, 37, 38, 40, 42, 43], which contains MSCs, but also contains other biologic factors and platelets. Furthermore, an unknown number of cells were injected. In future research with accurate cell counts, the optimum dose and hazardous dose of MSC injections and other types of cell injections can be determined. Moreover, in most of the included studies, patients who had refractory tendon disease were studied. If milder cases were recruited in future studies, the results could be more representative. In addition, the quality assessment of the studies was performed using three different tools, which may lead to a biased comparison among them. Finally, in many cases, surgical groups were compared with non-surgical groups, and these two types of patients represent two different populations.
Conclusion
According to the clinical studies in this systematic review, cell-based therapy for different tendinopathies appears to be safe. Numerous studies have demonstrated the potential benefits of cell injection in tendinopathy treatments, but there are currently no convincing RCTs with large sample sizes and sufficient follow-up intervals to demonstrate their effectiveness conclusively. As far as the available evidence indicates, cell therapy for rotator cuff tendon seems beneficial for the augmentation of rotator cuff repair surgery and for patients with partial-thickness tears who did not respond to conservative medication or physical therapy for more than 3-6 months. In the elbow tendon disorders, the cell therapy again could be used as a non-surgical rescue treatment after failed first-line options for patients suffering from a partial-thickness tear of extensor tendons. Likewise, chronic patellar tendinopathies that do not respond to nonoperative treatment or rehabilitation for more than 3-6 months should be considered for cell therapy. However, Achilles tendinopathy patients are very heterogeneous, and no recommendation can be drawn from them. Based on available evidence, cell therapy should be suggested in specific conditions at each site.
Availability of data and materials
Not applicable.
References
Cardoso TB, Pizzari T, Kinsella R, Hope D, Cook JL (2019) Current trends in tendinopathy management. Best Pract Res Clin Rheumatol 33:122–140
Chen HS, Chen YL, Harn HJ, Lin JS, Lin SZ (2013) Stem cell therapy for tendon injury. Cell Transplant 22:677–684
Ho JO, Sawadkar P, Mudera V (2014) A review on the use of cell therapy in the treatment of tendon disease and injuries. J Tissue Eng 5:2041731414549678
Sharma P, Maffulli N (2005) Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg Am 87:187–202
Costa-Almeida R, Calejo I, Gomes ME (2019) Mesenchymal stem cells empowering tendon regenerative therapies. Int J Mol Sci 20
Hajivandi S, Dachek A, Salimi A, Mamaghani HJ, Mirghaderi SP, Dehghani J et al (2021) Comparison of the separate and combined effects of physiotherapy treatment and corticosteroid injection on the range of motion and pain in nontraumatic rotator cuff tear: a randomized controlled trial. Adv Orthop 2021:6789453
Khorraminejad-Shirazi M, Dorvash M, Estedlal A, Hoveidaei AH, Mazloomrezaei M, Mosaddeghi P (2019) Aging: a cell source limiting factor in tissue engineering. World J Stem Cells 11:787–802
Teng C, Zhou C, Xu D, Bi F (2016) Combination of platelet-rich plasma and bone marrow mesenchymal stem cells enhances tendon–bone healing in a rabbit model of anterior cruciate ligament reconstruction. J Orthop Surg Res 11:96
van den Boom NAC, Winters M, Haisma HJ, Moen MH (2020) Efficacy of stem cell therapy for tendon disorders: a systematic review. Orthop J Sports Med 8:2325967120915857
Viganò M, Sansone V, d’Agostino MC, Romeo P, Perucca Orfei C, de Girolamo L (2016) Mesenchymal stem cells as therapeutic target of biophysical stimulation for the treatment of musculoskeletal disorders. J Orthop Surg Res 11:163
Guo X, Huang D, Li D, Zou L, Lv H, Wang Y et al (2022) Adipose-derived mesenchymal stem cells with hypoxic preconditioning improve tenogenic differentiation. J Orthop Surg Res 17:49
Babaniamansour P, Salimi M, Dorkoosh F, Mohammadi M (2022) Magnetic hydrogel for cartilage tissue regeneration as well as a review on advantages and disadvantages of different cartilage repair strategies. Biomed Res Int 2022:7230354. https://doi.org/10.1155/2022/7230354
Cho WS, Chung SG, Kim W, Jo CH, Lee SU, Lee SY (2021) Mesenchymal stem cells use in the treatment of tendon disorders: a systematic review and Meta-analysis of prospective clinical studies. Ann Rehabil Med 45:274–283
Hernigou P, Flouzat Lachaniette CH, Delambre J, Zilber S, Duffiet P, Chevallier N et al (2014) Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int Orthop 38:1811–1818
Usuelli FG, Grassi M, Maccario C, Vigano M, Lanfranchi L, Alfieri Montrasio U et al (2018) Intratendinous adipose-derived stromal vascular fraction (SVF) injection provides a safe, efficacious treatment for Achilles tendinopathy: results of a randomized controlled clinical trial at a 6-month follow-up. Knee Surg Sports Traumatol Arthrosc 26:2000–2010
Viganò M, Ragni E, Marmotti A, de Girolamo L (2022) The effects of orthobiologics in the treatment of tendon pathologies: a systematic review of preclinical evidence. J Exp Orthop 9:31
Yan Z, Yin H, Nerlich M, Pfeifer CG, Docheva D (2018) Boosting tendon repair: interplay of cells, growth factors and scaffold-free and gel-based carriers. J Exp Orthop 5:1
Neff P, Franklin DB 3rd, Jones DL, Lang SD, Nadone HR, Gilmer BB et al (2021) Transtendinous rotator cuff tear repair with bone marrow aspirate concentrate dermal allograft augmentation. Arthrosc Tech 10:e975–e980
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M et al (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. Bmj 350:g7647
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366:l4898
Tufanaru CMZ, Aromataris E, Campbell J, Hopp L (2020) Chapter 3: Systematic reviews of effectiveness. In: Aromataris E, Munn Z (eds) JBI Manual for Evidence Synthesis Available from https://synthesismanual.jbi.global. Accessed 10 Oct 2021
National Heart L, and blood institute National Heart, Lung, and Blood Institute website Study quality assessment tools. www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (Accessed 17 May 2021)
Jeremy Howick IC, Paul Glasziou, Trish Greenhalgh, Carl Heneghan, Alessandro Liberati, Ivan Moschetti, Bob Phillips, Hazel Thornton. The 2011 Oxford CEBM levels of evidence (introductory document)”. Oxford Centre for Evidence-Based Medicine. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence. Accessed 10 Oct 2021
McGinn T, Wyer PC, Newman TB, Keitz S, Leipzig R, For GG et al (2004) Tips for learners of evidence-based medicine: 3. Measures of observer variability (kappa statistic). CMAJ : Can Med Assoc J 171:1369–1373
Centeno CJ, Al-Sayegh H, Bashir J, Goodyear S, Freeman MD (2015) A prospective multi-site registry study of a specific protocol of autologous bone marrow concentrate for the treatment of shoulder rotator cuff tears and osteoarthritis. J Pain Res 8:269–276
Clarke AW, Alyas F, Morris T, Robertson CJ, Bell J, Connell DA (2011) Skin-derived tenocyte-like cells for the treatment of patellar tendinopathy. Am J Sports Med 39:614–623
Connell D, Datir A, Alyas F, Curtis M (2009) Treatment of lateral epicondylitis using skin-derived tenocyte-like cells. Br J Sports Med 43:293–298
Ellera Gomes JL, da Silva RC, Silla LM, Abreu MR, Pellanda R (2012) Conventional rotator cuff repair complemented by the aid of mononuclear autologous stem cells. Knee Surg Sports Traumatol Arthrosc 20:373–377
Hurd JL, Facile TR, Weiss J, Hayes M, Hayes M, Furia JP et al (2020) Safety and efficacy of treating symptomatic, partial-thickness rotator cuff tears with fresh, uncultured, unmodified, autologous adipose-derived regenerative cells (UA-ADRCs) isolated at the point of care: a prospective, randomized, controlled first-in-human pilot study. J Orthop Surg Res 15:122
Jo CH, Chai JW, Jeong EC, Oh S, Yoon KS (2020) Intratendinous injection of mesenchymal stem cells for the treatment of rotator cuff disease: a 2-year follow-up study. Arthroscopy 36:971–980
Khoury M, Tabben M, Rolón AU, Levi L, Chamari K, D'Hooghe P (2021) Promising improvement of chronic lateral elbow tendinopathy by using adipose derived mesenchymal stromal cells: a pilot study. J Exp Orthop 8:6
Kim SJ, Kim EK, Kim SJ, Song DH (2018) Effects of bone marrow aspirate concentrate and platelet-rich plasma on patients with partial tear of the rotator cuff tendon. J Orthop Surg Res 13:1
Kim SJ, Song DH, Park JW, Park S, Kim SJ (2017) Effect of bone marrow aspirate concentrate-platelet-Rich plasma on tendon-derived stem cells and rotator cuff tendon tear. Cell Transplant 26:867–878
Kim YS, Sung CH, Chung SH, Kwak SJ, Koh YG (2017) Does an injection of adipose-derived mesenchymal stem cells loaded in fibrin glue influence rotator cuff repair outcomes? A clinical and magnetic resonance imaging study. Am J Sports Med 45:2010–2018
Lamas JR, García-Fernández C, Tornero-Esteban P, Lópiz Y, Rodriguez-Rodriguez L, Ortega L et al (2019) Adverse effects of xenogenic scaffolding in the context of a randomized double-blind placebo-controlled study for repairing full-thickness rotator cuff tears. Trials 20:387
Lee SY, Kim W, Lim C, Chung SG (2015) Treatment of lateral Epicondylosis by using allogeneic adipose-derived mesenchymal stem cells: a pilot study. Stem Cells 33:2995–3005
Muench LN, Kia C, Berthold DP, Uyeki C, Otto A, Cote MP et al (2020) Preliminary clinical outcomes following biologic augmentation of arthroscopic rotator cuff repair using subacromial Bursa, Concentrated bone marrow aspirate, and platelet-Rich plasma. Arthrosc Sports Med Rehabil 2:e803–e813
Pascual-Garrido C, Rolón A, Makino A (2012) Treatment of chronic patellar tendinopathy with autologous bone marrow stem cells: a 5-year-followup. Stem Cells Int 2012:953510
Rodas G, Soler-Rich R, Rius-Tarruella J, Alomar X, Balius R, Orozco L et al (2021) Effect of autologous expanded bone marrow mesenchymal stem cells or leukocyte-poor platelet-Rich plasma in chronic patellar tendinopathy (with gap >3 mm): preliminary outcomes after 6 months of a double-blind, randomized, prospective study. Am J Sports Med 49:1492–1504
Rosário DAV, Faleiro TB, Franco B, Daltro GC, Marchetto R (2021) Comparison between CONCENTRATED bone marrow aspirate and corticoid in gluteal tendinopathy. Acta Ortop Bras 29:26–29
Singh A, Gangwar DS, Singh S (2014) Bone marrow injection: a novel treatment for tennis elbow. J Nat Sci Biol Med 5:389–391
Stein BE, Stroh DA, Schon LC (2015) Outcomes of acute Achilles tendon rupture repair with bone marrow aspirate concentrate augmentation. Int Orthop 39:901–905
Tate-Oliver K, Alexander R (2013) Combination of autologous adipose-derived tissue stromal vascular fraction plus high density platelet-Rich plasma or bone marrow concentrates in Achilles tendon tears. J Prolotherapy 5:e895–e912
Wang A, Mackie K, Breidahl W, Wang T, Zheng MH (2015) Evidence for the durability of autologous tenocyte injection for treatment of chronic resistant lateral epicondylitis: mean 4.5-year clinical follow-up. Am J Sports Med 43:1775–1783
Farina KA, Kandah BA, Sowers NM, Moore GA (2021) Bone marrow aspirate concentrate injection of the achilles tendon in a competitive distance runner. J Musculoskelet Res 24:2140004
Freitag J, Shah K, Wickham J, Tenen A (2020) Effect of autologous adipose-derived mesenchymal stem cell therapy in combination with autologous platelet-rich plasma in the treatment of elbow tendinopathy. BMJ Case Rep 13:e234592
Giannotti S, Parchi PD, Colasanti GB, Agostini G, Moreschini F, Cataldi C et al (2017) Use of autologous bone marrow cells concentrate enriched with platelet-fibrin on extensor mechanism allograft reconstruction for extensor mechanism failure following total knee arthroplasty. J Biol Regul Homeost Agents 31:107–111
Jo CH, Chai JW, Jeong EC, Oh S, Kim PS, Yoon JY et al (2018) Intratendinous injection of autologous adipose tissue-derived mesenchymal stem cells for the treatment of rotator cuff disease: a first-in-human trial. Stem Cells 36:1441–1450
Wang A, Breidahl W, Mackie KE, Lin Z, Qin A, Chen J et al (2013) Autologous tenocyte injection for the treatment of severe, chronic resistant lateral epicondylitis: a pilot study. Am J Sports Med 41:2925–2932
Lee SY, Kwon B, Lee K, Son YH, Chung SG (2017) Therapeutic mechanisms of human adipose-derived mesenchymal stem cells in a rat tendon injury model. Am J Sports Med 45:1429–1439
Mazzocca AD, McCarthy MB, Chowaniec DM, Cote MP, Arciero RA, Drissi H (2010) Rapid isolation of human stem cells (connective tissue progenitor cells) from the proximal humerus during arthroscopic rotator cuff surgery. Am J Sports Med 38:1438–1447
Nemoto M, Kizaki K, Yamamoto Y, Oonuma T, Hashizume K (2013) Tenascin-C expression in equine tendon-derived cells during proliferation and migration. J Equine Sci 24:17–24
Young M (2012) Stem cell applications in tendon disorders: a clinical perspective. Stem Cells Int 2012:637836
Squillaro T, Peluso G, Galderisi U (2016) Clinical trials with mesenchymal stem cells: an update. Cell Transplant 25:829–848
Chisari E, Rehak L, Khan WS, Maffulli N (2021) Tendon healing is adversely affected by low-grade inflammation. J Orthop Surg Res 16:700
Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY et al (2012) Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. Jama 308:2369–2379
Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP et al (2009) A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol 54:2277–2286
Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY et al (2002) Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc Natl Acad Sci U S A 99:8932–8937
Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I et al (2008) Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 371:1579–1586
Liang J, Zhang H, Hua B, Wang H, Lu L, Shi S et al (2010) Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: a pilot clinical study. Ann Rheum Dis 69:1423–1429
Liu F, Meng Q, Yin H, Yan Z (2019) Stem cells in rotator cuff injuries and reconstructions: a systematic review and Meta-analysis. Curr Stem Cell Res Ther 14:683–697
Lui PP, Ng SW (2013) Cell therapy for the treatment of tendinopathy--a systematic review on the pre-clinical and clinical evidence. Semin Arthritis Rheum 42:651–666
Thirabanjasak D, Tantiwongse K, Thorner PS (2010) Angiomyeloproliferative lesions following autologous stem cell therapy. J Am Soc Nephrol 21:1218–1222
Berkowitz AL, Miller MB, Mir SA, Cagney D, Chavakula V, Guleria I et al (2016) Glioproliferative lesion of the spinal cord as a complication of “stem-cell tourism”. N Engl J Med 375:196–198
Perkins KM, Spoto S, Rankin DA, Dotson NQ, Malarkey M, Mendoza M et al (2018) Notes from the field: infections after receipt of bacterially contaminated umbilical cord blood-derived stem cell products for other than hematopoietic or immunologic reconstitution - United States, 2018. MMWR Morb Mortal Wkly Rep 67:1397–1399
Harris MT, Butler DL, Boivin GP, Florer JB, Schantz EJ, Wenstrup RJ (2004) Mesenchymal stem cells used for rabbit tendon repair can form ectopic bone and express alkaline phosphatase activity in constructs. J Orthop Res 22:998–1003
Liu L, Hindieh J, Leong DJ, Sun HB (2017) Advances of stem cell based-therapeutic approaches for tendon repair. J Orthop Translat 9:69–75
Lamas JRT-EP, García Fernández C, Rodriguez Rodriguez L, Marco F, Fernández-Gutiérrez Lamas JR, Tornero-Esteban P, García Fernández C, Rodriguez Rodriguez L, Marco F, Fernández-Gutiérrez B (2015) A double-blind, randomized, placebo-controlled trial of mesenchymal stem cells for the treatment of patients with full-thickness rotator cuff tears [abstract]. Arthritis Rheumatol 67(suppl 10)
Chong AK, Ang AD, Goh JC, Hui JH, Lim AY, Lee EH et al (2007) Bone marrow-derived mesenchymal stem cells influence early tendon-healing in a rabbit achilles tendon model. J Bone Joint Surg Am 89:74–81
Machova Urdzikova L, Sedlacek R, Suchy T, Amemori T, Ruzicka J, Lesny P et al (2014) Human multipotent mesenchymal stem cells improve healing after collagenase tendon injury in the rat. Biomed Eng Online 13:42
Okamoto N, Kushida T, Oe K, Umeda M, Ikehara S, Iida H (2010) Treating Achilles tendon rupture in rats with bone-marrow-cell transplantation therapy. J Bone Joint Surg Am 92:2776–2784
Renzi S, Riccò S, Dotti S, Sesso L, Grolli S, Cornali M et al (2013) Autologous bone marrow mesenchymal stromal cells for regeneration of injured equine ligaments and tendons: a clinical report. Res Vet Sci 95:272–277
Schon LC, Gill N, Thorpe M, Davis J, Nadaud J, Kim J et al (2014) Efficacy of a mesenchymal stem cell loaded surgical mesh for tendon repair in rats. J Transl Med 12:110
Yao J, Woon CY-L, Behn A, Korotkova T, Park D-Y, Gajendran V et al (2012) The effect of suture coated with mesenchymal stem cells and bioactive substrate on tendon repair strength in a rat model. J Hand Surg 37:1639–1645
Liu W, Chen B, Deng D, Xu F, Cui L, Cao Y (2006) Repair of tendon defect with dermal fibroblast engineered tendon in a porcine model. Tissue Eng 12:775–788
Acknowledgements
None.
Funding
There is no funding source with authors to declare.
Author information
Authors and Affiliations
Contributions
SP. Mirghaderi, Z Valizadeh, and K Shadman contributed to the study conception and design, material preparation, data collection. MS edited the manuscript and contribute in the methodology. Z Valizadeh and SP wrote the first draft of the manuscript. Mirghaderi. Supervising, editing, and introducing the concept was performed by MH Nabian and L Oryadi-Zanjani. Th Lafosse edited the final version. All authors commented on previous versions of the manuscript and revised it. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study have approved by the institutional review board of the Tehran University of Medical Sciences (ethic code: IR.TUMS.MEDICINE.REC.1400.783).
Consent for publication
Not applicable.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
PRISMA checklist.
Additional file 2: Table S1.
PubMed keywords and Mesh terms. Table S2. Databases search results. Table S3. PubMed databases search details. Table S4. PICOT table. Table S5. Inclusion and Exclusion criteria. Table S6. Excluded case reports. Table S7. Studies’ outcomes and results.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mirghaderi, S.P., Valizadeh, Z., Shadman, K. et al. Cell therapy efficacy and safety in treating tendon disorders: a systemic review of clinical studies. J EXP ORTOP 9, 85 (2022). https://doi.org/10.1186/s40634-022-00520-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40634-022-00520-9