Abstract
Purpose
The aim of this systematic review is to explore the current available knowledge about tendon disorders and orthobiologics derived by preclinical experiments to evaluate their role and efficacy in the different stages and conditions related to the tendon healing processes.
Methods
The systematic review was performed according to the PRISMA guidelines. Different electronic databases (MEDLINE, Web of Science, EMBASE) were searched for studies investigating orthobiologics (PRP and cell-based products from adipose tissue or bone marrow) in animal models or veterinary clinical trials for tendon pathologies (complete/partial tendon ruptures, rotator cuff tears, tendinopathy, enthesis-related injuries). Data regarding the specific product used, the treatment site/pathology, the host and the model were collected. The results were classified into the following categories: histological, biomechanical, molecular and imaging.
Results
A large pool of preclinical studies on tendon disorders have been found on platelet-rich plasma (PRP), while data about stromal vascular fraction (SVF) and bone marrow concentrate (BMAC) are still limited and frequently focused on expanded cells, rather than orthobiologics prepared at the point of care.
The effect of PRP is related to an acceleration of the healing process, without improvements in the final structure and properties of repaired tendon. Cell-based products have been reported to produce more durable results, but the level of evidence is currently insufficient to draw clear indications.
Conclusions
The preclinical results about orthobiologics applications to tendon pathologies would support the rationale of their clinical use and encourage the performance of clinical trials aimed to confirm these data in human subjects.
Similar content being viewed by others
Background
Tendon injuries, especially those affecting Achilles, forearm extensor, patellar and rotator cuff tendons, represent a very common condition [9, 55, 112]. At present, management strategies are limited in terms of both success and scientific robustness [3]. The term “tendon disorders” includes a wide range of pathologies, including partial or complete ruptures (deriving from traumatic injuries or late-stage degenerative conditions), inflammation with early stage tissue degeneration (often associated with overuse) [2, 67], and, thus, the treatment choice may significantly vary depending on the specific condition. For example, ruptures require in most cases a surgical intervention, while other conditions may benefit from conservative therapies in order to control symptoms and possibly halt the degenerative process [28, 58]. In the scenario of non-surgical approaches, regenerative medicine aims to promote and sustain tissue repair by exploitation of the self-healing ability of the body [6, 25]. Regenerative medicine comprises a number of approaches, including the application of orthobiologics, i.e. blood-derived and cell cell-based products (from bone marrow and adipose tissue-derived), that represent a ready-to-use and cutting-edge strategy to enhance tissue healing [1, 23]. Blood-derived products comprise plasma- or serum-based whole blood derivatives. In general, they are well tolerated and provide reduction of pain and inflammation. The mechanism of action of these products relies on their content of cytokines and growth factors that stimulates cell proliferation and modulates the inflammatory response [34]. During the last 30 years, several formulations have been developed, all with different properties. Autologous PRP is the most used, but even considering only this product, preparation protocols may significantly vary, depending on the depletion or concentration of leukocytes and the activation of platelets [5]. Among cell-based products, bone marrow represents the most traditional source of mesenchymal stem/stromal cells (or Medicinal Signaling Cells, MSCs) in the adult human body [39]. The rationale of MSCs use in regenerative medicine relies on their ability to contribute to tissue healing through restoration of tissue homeostasis [16, 84]. In fact, the MSCs are able to release paracrine effectors promoting healing by the modulation of the response to injury of tissue resident and immune cells response [113]. These properties are shared by all the MSCs, regardless of their origin [4, 98, 106]. Adipose tissue popularity as a source for regenerative medicine products is gaining momentum thanks to its relative abundance and ease of harvest. Bone marrow aspirate concentrate (BMAC), freshly isolated stromal vascular fraction (SVF), as well as micro/nano-fragmented adipose tissue, represent intraoperative and minimally manipulated solutions for the application of MSCs-based therapies. Compared to culture-expanded cells, characterized by a higher homogeneity, these products includes different cell types together with MSCs, such as hematopoietic cells from bone marrow, or epithelial cells and pre-adipocytes from adipose tissue [12]. Other approaches, such tissue engineering techniques [91] and products derived from embryonic annexes [44, 121] have been proposed, but their use in these conditions is less represented in current literature and the analysis of these techniques is beyond the aims and scope of the present document.
While orthobiologics have been used extensively for the treatment of bone and cartilage diseases, the application to tendon disorders of this large variety of products is under-investigated. The aim of this systematic review is to explore the current available knowledge about orthobiologics for the treatment of tendon disorders in in vivo preclinical studies to evaluate their efficacy in the different tendon conditions. Given the little knowledge available from the clinical trials in this field, the evidence provided by preclinical studies may allow for the identification of the most promising applications in each different condition, in order to guide further clinical research on a subset of specific products and strategies.
Methods
Sources and study selection
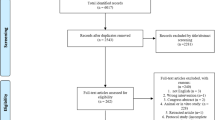
This systematic review was prepared according to the PRISMA guidelines and Cochrane Collaboration methodology [88]. Three electronic databases (MEDLINE, Web of Science, EMBASE) were searched on September 30th, 2021. In addition, further reports were manually identified in the reference list of previously published systematic reviews addressing this topic. Figure 1 reports the flowchart representing the studies selection process. Supplementary Table 1 provides the list of keywords and MeSH terms used for data retrieval.
The screening process was focused on identifying preclinical studies or veterinary clinical trials investigating the effects of blood-derived products, cell-based products derived from adipose tissue and bone marrow in tendon disorders. The following reports were excluded during the screening process: in vitro studies or clinical trials on human subjects; studies evaluating the effects of orthobiologics on other tissues; studies investigating other techniques; documents written in languages other than English. Study selection was performed by two independent researchers. Figure 1 reports the PRISMA flow chart of the study selection process.
Blood-derived products
The search for articles reporting preclinical studies concerning blood-derived products and tendons retrieved 89 papers after title/abstract screening. Further 10 papers were identified by manual search within the references of full-texts included in the analysis. They were all evaluated by abstract screening, and 37 were excluded because considered non-inherent for various reasons or because they did not satisfy inclusion/exclusion criteria. Then, 62 studies were included in the qualitative analysis.
Bone marrow-derived products
The search for studies reporting the efficacy of cultured mesenchymal stem cells or bone marrow aspirate concentrates (BMAC) in tendon conditions retrieved 19 documents. Of these, 8 were excluded after title/abstract screening, resulting in 11 studies available for analysis.
Adipose tissue-derived products
Seventy-eight studies were identified during database search after title/abstract screening and 5 more were found among references of full-texts included in the analysis. Sixty-two studies were excluded because considered non-inherent for various reasons or because they did not satisfy inclusion/exclusion criteria, resulting in 21 studies included in the analysis.
Data collection and risk of bias evaluation
Product specifications, treatment site (or specific pathology), type of host and model, and experimental results (histological, biomechanical, molecular and imaging findings) were collected for all studies included in the qualitative analysis.
Risk of bias was determined for all studies using SYRCLE’s tool for animal studies. Two independent investigators rated the risk of bias for each study as low, high or unclear, depending on specific items dedicated to the identification of selection, performance, detection, attrition and reporting biases [52].
Results
Blood-derived products
Selected studies and overall evaluation
Among the 62 selected studies investigating the effects of blood-derived products, the vast majority were based on rat (n = 28) or rabbit (n = 26) models. Three and 2 studies respectively used horse and sheep models, while only one study was conducted in mice. In addition, two veterinary trials involving dogs were selected. Thirty-three studies used models of tendon ruptures, 8 addressed spontaneous or experimentally-induced tendinopathy, 10 investigated the rotator cuff tears and 6 studies involved tendon-bone junctions. The effect of PRP alone was evaluated in 57 studies, while 9 reports on PRP in addition to other therapies (drugs or cells). In 6 cases, PRP was used in combination with scaffolds. Platelet-rich fibrin (PRF) and plasma rich in growth factors (PRGF) were evaluated in 4 and 1 studies, respectively. According to SYRCLE’s tool, 22 studies were at high risk of bias, 9 due to the lack of control group and 13 due to lack of randomization and/or blinding of caregivers and outcome assessment (Fig. 2). The first study about this topic was published in 2009, with not significant increase or decrease was observed in the following years, with a peak of 10 studies published in 2017.
Histological results
Thirty-three studies showed positive histological results following the application of blood-derived products, while 4 reports demonstrated no improvement compared to controls (n = 2) or detrimental effect of the experimental products (n = 2) (Table 1). Studies performed on models of complete or partial Achilles, digital-flexor or patellar tendon ruptures consistently report enhanced appearance [114, 118, 123] with improved Movin and Bonar scores [36, 40, 122], accelerated healing [37, 65, 73, 76, 102] and minimal cartilage formation in tendon mid-portion [114]. Most studies showed faster collagen fibers maturation, with increased organization and density [47, 72, 76, 93, 102, 114, 117, 124], and a reduction of elastic fibers [37, 60, 72]. Improvement in cell morphology and density [37, 102, 118] as well as reduction of neovascularization [37, 72, 76, 114] were described too. Interestingly, while vascularization is reduced at late phase of tendon healing, it is increased in the early phases [13, 72]. Administration of PRP immediately after injury reduced inflammation and improved cell density, collagen fibers organization and epitenon thickness [24]. Overall, when applied to tendon ruptures, blood-derived products induce an acceleration of the initial phases of tendon healing with reduced inflammation and improved cell proliferation/neovascularization, while these parameters tend to be similar between experimental and control groups at the final follow up [47, 65, 76, 117]. Seven studies concerning collagenase-induced tendinopathy showed improvements in terms of fibers organization [17, 26, 45, 119], fibers dimension and neovascularization [26], overall histological appearance [20, 45, 119], while one study did not identify any effect of PRP treatment compared to platelet-derived growth factor-BB or steroids [100]. Increased cellularity was observed early after treatment in one study [17]. Leukocyte-rich PRP provided better histological results compared to leukocyte-poor formulations in one study [56]. Studies using rotator cuff repair models reported controversial results, ranging from no improvements [33] or detrimental effects on cell density and vascularization [22], to the amelioration of histological appearance [31, 64] and fibers organization [31]. Studies involving injury at the enthesis consistently reported improvements in terms of reduced inflammation and vascularity, [49] deposition of type I and type II collagen [126], fibers organization [115] and histologic appearance [127] following blood-derived products application. Only one study reported a detrimental effect of platelet-rich fibrin matrix on tissue healing with formation of fibrotic tissue [50]. The repair with fibrotic tissue may be the consequence of an excess of stimulation by growth factors resulting in an unnaturally rapid healing response. Thus, while treatment was indeed able to accelerate healing, the dosage need to be fine-tuned to ensure a high-quality tissue repair [57, 116].
Biomechanical results
Enhancement of biomechanical properties of the tissue was obtained in 20 studies, while in 5 cases no improvements were observed compared to controls (Table 1). Controversial results were observed especially in regards of the treatment of tendon ruptures, where 4 studies showed no improvements after treatment [43, 62, 122, 124]. Conversely, maximum failure load [60, 78, 101, 117], tensile strength [47, 93, 118, 123] and mechanical stress [60] improved in treated tendons according to 8 studies. Three studies investigating enthesis repair consistently reported increased strength in the treated tendons [48, 80, 127]. Two studies conducted in collagenase induced tendinopathy models were investigated, one reporting improvements after treatment [45] and the other showing no effect of PRP [100]. Reports about rotator cuff repair models consistently showed improved biomechanical properties [22] in terms of maximum failure load [31, 33, 50, 81, 115], resistance to mechanical stress [50], tensile strength [49] and stiffness [31, 33, 50]. Notably the use of frozen platelet concentrates would not allow to obtain the same results as fresh products [61, 127]. In addition, leukoocyte-rich PRP provides higher improvements in the maximum failure load compared to leukoocyte-poor PRP [56]. Together, these observations suggest that a low rate of live cells, reduced during processing or by freeze/thaw cycles, may correlate with a reduced treatment effectiveness. Interestingly, the contemporary use of NSAIDs and PRP did not provide improvements in terms of maximum failure load compared to PRP-only treated animals [81].
Modulation of gene expression, protein production and molecular pathways
The treatment with blood-derived products was able to enhance the gene expression and protein deposition of collagen type I, as reported by 8 different studies involving both tendon rupture models and collagenase-induced tendinopathy [20, 45,46,47, 56, 60, 74, 119]. This effect, together with increased expression of Scleraxis and Tenascin, appears to be mediated by the FAK/ERK1,2 cascade [20]. Conversely, no consensus was observed concerning collagenase type III expression after treatment with blood derived products [60, 74], even if reduction was frequently reported [45, 46, 119]. Metalloproteases (MMPs) expression appeared reduced after treatment in collagenase-induced tendinopathy [45, 46, 119] and ex vivo experiments [14, 79] but not in tendon ruptures [60]. Reduction of inflammatory mediators such as IL-6 [45, 46, 119], TNFα, IL-8, IL-6 [92] and PGE2 [125] were frequently reported after treatment with blood-derived products. In particular, the inhibition of PGE2 appeared to be mediated by HGF (known component of PRP), leading to a reduced expression of downstream inflammatory effectors such as COX1 and COX2 [125]. The expression of the growth factors TGFβ and IGF, as well as the tendon specific marker TNMD, is increased in the first phases of tendon healing in treated animals, whilst they all decreased at later time-points [47, 60, 73,74,75]. In a model of rotator cuff tear, an increase in BMP-2 after treatment, possibly benefitting the healing of the bone-tendon interface was reported [115].
Imaging and behavior evaluations
Imaging techniques and behavior assessments demonstrated overall positive results using blood-derived products for the treatment of tendon-bone injury, tendon ruptures and collagenase induced tendinopathy, with 7 studies reporting satisfactory results and 1 showing no improvement compared to controls (Table 1). Blood-derived treatments were able to sustain healing, reduce neovascularization, improve tendon structure, and bone formation at the enthesis as observed by ultrasound [26, 114], MRI T2 mapping [40] and micro-CT [127] evaluations. Conflicting results were observed in term of the effect of leukocyte-rich and leukocyte-poor PRP, assessed by MRI T2 mapping, with one study showing better results in tendons treated with leucocyte poor-PRP compared to leucocyte rich-PRP treated samples [119], and another study showing the opposite [56]. Veterinary case series in dogs with spontaneous rotator cuff tendinopathy confirmed the positive results in terms of ultrasound-evaluated echogenicity and heterogeneity correlating with improvements in owner-assessed function score [51]. In a spontaneous model of calcaneal tendon rupture, the use of PRP effectively improved function and quality of life owner-assessed scores, as well as restoring the limb native anatomical condition [96].
Bone marrow-derived products
Selected studies
Rabbit models were the most used across the 11 selected studies (n = 4), followed by horse (n = 3), rat (n = 2) mouse (n = 1) and dog models (n = 1). Concerning the pathological models, 3 focused on tendon ruptures, 2 on tendinopathy,2 on rotator cuff tears and 3 studies addressed lesions of the enthesis. Six studies employed BMAC, comprising 1 study combining this product with a scaffold and another study combining it with PRP. Five studies used in vitro cultured bone marrow (BM)-MSCs, 4 of which in combination with scaffolds or fibrin glue. The analysis performed using SYRCLE’s Risk of Bias tool, identified 5 studies at high risk of bias, due to the lack of a control group (n = 2) or differences between groups at baseline (n = 3). Only 1 randomized study and 2 studies with blinded outcome assessors were identified among the selected studies (Fig. 3). The reports about the application of bone marrow-based product in tendon pathologies are generally older than articles about blood-derived products, with the first study published in 2002, and only 5 studies published after 2012.
Histology and immunohistochemistry
Overall, 7 studies reported positive results in histological appearance of tendons treated with bone marrow-derived products, while 1 study reported a detrimental effect. One study showed mixed results (Table 1). In the context of tendon ruptures, treatments based on scaffolds seeded with cultured bone marrow-MSCs demonstrated superior cell proliferation and improved tissue formation compared to controls, especially in the initial phases of tendon healing [32, 87], with improved deposition of collagen type I and type III. Nevertheless, at the final stage the repaired tissue appeared to be similar to controls [87]. Other studies reported the ectopic formation of bone in some samples, rising questions about the appropriateness of the procedure [7]. Studies investigating tendon-to-bone repair using BM-MSCs with scaffolds or BMAC added with bone morphogenetic protein-2 (BMP-2) showed improved integration, mineralization [54], bone appearance and density [63, 104], increased fibrocartilage formation [104], deposition of collagen type II and presence of SOX9+ cells [129] compared to controls. One study reported an increased presence of macrophages in treated tendons [129], suggesting possible enhancement of immune reaction. BMAC applied to rotator cuff disorders improved fibers continuity and orientation [69]. In a horse spontaneous model of digital flexor tendon disorder, the use of BM-MSCs in marrow supernatant was able to improve the histological appearance in terms of vascularity, cell density, fiber organization, as well as GAG and water content [99] compared to saline.
Biomechanical results
Positive biomechanical results were reported by all the six studies that included this kind of evaluation following administration of bone marrow-derived products for tendon pathologies (Table 1). The effects on the biomechanical properties of tendons appeared to be related to an improvement of maximum stress/load to failure and tensile stiffness in different contexts including experimental defects of the tendon mid-portion and of tendon-bone junction [7, 54, 63, 87]. These results were obtained with either cultured cells seeded on scaffolds or BMAC. BMAC was able to improve ultimate load to failure of teared rotator cuff in a rabbit model [69], as well as structural stiffness in a naturally occurring injury of digital flexor tendon in horses [99]. Elasticity was not affected by treatment with BM-derived products [32, 99].
Molecular pathways
BMAC was able to induce the expression of collagen type I, decorin and COMP in horse digital flexor tendon at comparable levels with respect to blood derived products (PRP, PPP, serum), while contemporary reducing the expression of collagen type III, MMP-3, MMP-13 [95, 99]. The observed differences between BMAC and PRP could be explained by the higher content of growth factors in BMAC, compared to blood-derived products [69].
Imaging and functional outcomes
All studies evaluating imaging (n = 1) or behavior/functional (n = 2) outcomes of BM-derived products for tendon pathologies reported positive results (Table 1). Scaffolds seeded with BM-MSCs were are able to improve the radiographic appearance and bone mineral density in a model of enthesis injury [104]. Dogs receiving autologous cancellous bone scaffold supplemented with bone marrow for the reconstruction of tendon-to-bone junction showed a 90% recovery of preoperative weight bearing at 16 weeks after surgery [54]. Treatment with freshly isolated BM-MSCs added to PRP was able to improve lameness and allowed for returning to activity in 85% of race horses affected by spontaneous suspensory ligament desmopathy or superficial flexor [105].
Adipose tissue-derived products
Selected studies
Most of the studies concerning adipose-derived products were conducted in rats, followed by rabbits (n = 4), horses (n = 2), mice (n = 1) and sheep (n = 1). Eleven studies addressed models of tendon ruptures, 5 focused on spontaneous or experimentally-induced tendinopathy and 5 focused on lesions of the tendon-bone junctions. In 5 cases, the site of injury was the supraspinatus tendon (2 tendon ruptures, one collagenase induced tendinopathy, one detachment of tendon from bone). Cultured adipose derived mesenchymal stem cells (ASCs) were frequently used either alone (n = 8) or in combination with scaffolds/hydrogels (n = 6) or with other treatments (biophysical stimulation, Vitamin D, PDGF; n = 3). Four studies used freshly isolated stromal vascular fraction (SVF) alone (n = 2) or in combination with fibrin glue (n = 2). Risk of bias analysis performed using SYRCLE’s tool demonstrated high risk only in 3 studies, due to lack of randomization and blinding of outcome assessment. Notably the frequency of randomized studies is higher among reports investigating adipose-derived products compared to studies about blood- and bone marrow-derived products (Fig. 4). The time-distribution of these studies resembles the one observed for blood-derived products, with the first article published in 2011 and no clear trend afterwards.
Histology and immunohistochemistry
Positive histological findings after treatment of diseased tendons with adipose tissue-derived products were found by 15 studies, while in 2 cases no improvements compared to controls were reported (Table 1). Studies reporting the use of ASCs seeded on scaffolds or associated to surgical repair for the treatment of tendon ruptures showed improved morphology [66, 120], fibers organization and collagen deposition [11, 29], or reduced fatty infiltration [85]. ASCs were able to effectively colonize decellularized scaffolds, and this is considered crucial to allow scaffold integration and healing [8]. Only one study used ASCs without scaffold or surgery for tendon repair, reporting no differences between treated and control samples [42]. Four studies applying ASCs treatment in experimental models of collagenase-induced tendinopathy demonstrated reduced inflammation [19] and cellularity [35], improvement of tissue appearance, Bonar score, fibers thickness and organization [19, 35, 59, 86], with reduced neovascularization and tissue degeneration [35, 86]. Five studies investigated the role of SVF or ASCs in the treatment of tendon-bone junction injuries, using surgical repair and scaffolds. Four of them showed improved formation of mature fibrocartilage with enhanced collagen deposition [18, 80, 97], increase in the Collagen type I/type III ratio and improved tissue appearance [71]. One study reported a reduction of inflammation with no effect on matrix organization [108]. Noteworthy, these surgical studies showed that ASCs are able to colonize scaffolds and to differentiate into osteoblast/chondrocytes, providing a direct contribution to tissue healing [18, 80].
Biomechanical tests
Fifteen studies showed improvements in biomechanical properties of injured tendons treated with adipose tissue-derived products, while 2 studies failed to detect improvements compared to controls (Table 1). The use of ASCs-based treatments allowed for the improvement of the biochemical properties of injured tendons in several pre-clinical models of tendon ruptures and tendon-bone detachment [66, 97, 120]. ASCs and SVF in combination with surgical repair and/or scaffolds improved maximum load [10, 11, 18, 29, 71, 80, 85], stiffness [10, 18, 29, 70, 71], tensile strength [70, 107], energy absorption [10, 11, 108] and mechanical deformation capacity of treated tendons [108]. Two studies reported no differences in mechanical properties after treatments, one using ASCs-seeded scaffolds [8] and one using ASCs alone [42]. Two studies concerning models of collagenase induced-tendinopathy showed improvements in maximum load and stiffness after ASCs injections [19, 21].
Molecular pathways
ASCs and SVF were able to enhance the deposition of collagen type I and collagen type I/type III ratio, alone or in association to scaffolds and surgical repair [21, 66, 71, 86, 107]. One study reported an increase in collagen cross-linking with hydroxylysylpyridinoline after treatment with ASCs [42], while others showed improvements in the transcription of the tendon specific genes scleraxis and tenascin [21, 66], as well as of FGF and VEGF [107]. Reduction of the pro-fibrotic factor TGFβ was reported in a model of Achilles tendon lesion treated with ASCs and surgical repair [107]. Increased BMP-2 expression was observed after treatment with ASCs in a model of injured enthesis, possibly improving healing of tendon-bone junction injuries [71].
Imaging and behavior
Improvements in functional outcomes after treatment with adipose tissue-derived products were described by 2 studies. Five studies assessed treated tendons with imaging techniques, each reporting various results, ranging from positive to detrimental effect (Table 1). SVF was able to improve MRI signal-to-noise at 12 weeks after surgical repair in a model of supraspinatus and patellar tendon lesions, especially at longer follow up times [70]. Two other studies using ASCs and SVF reported controversial results, with either no differences in terms of ultrasound parameters [42], or even increased inflammation and lesion size in treated tendons [120]. One study showed that ASCs remained at the treatment site at medium terms after injection, suggesting possible prolonged action [41]. A limited number studies identified functional improvements in terms of Achilles functional index (AFI), pow print intensity, stance time and duty cycle in experimental rats treated with ASCs [8, 120]. ASCs treatment demonstrated efficacy in enhancing bone volume, trabecular number and thickness by micro-CT [18, 97], and in improving bone formation observed by radiographic evaluation [18] in models of tendon-bone junction injury.
Discussion
Overall, the findings of the studies investigating the effects of blood-, bone marrow- and adipose tissue-derived products for the treatment of tendon pathologies demonstrated that they are able to support tissue healing in a variety of experimental and clinical settings, with measurable improvements in histological, biomechanical, molecular, functional and imaging outcomes.
Regardless the specific condition, both surgical and conservative therapies for tendon injuries are characterized by frequent failure and relapses, as well as long recovery time [68, 77, 94]. Then, the rationale behind the use of orthobiologics relies on the possibility to accelerate tissue healing while at the same time improving the quality. In fact, several aspects of orthobiologics action reported by the studies analyzed appear to address specific mechanisms in tendon healing that span across 3 continuous phases: inflammation, proliferation and remodeling [30, 111]. In the first stage, immune cells are recruited to the injury site, with activation of platelets and tissue resident progenitors. Tissue progenitors may directly differentiate into mature tenocytes to substitute cellular loss or they aid healing by orchestrating the repair process through the production of soluble mediators [53]. This phase involves different cytokines and growth factors, including IL-1β, IL-6, bFGF, IGF1, TGFβ, VEGF and PDGF [30], that are highly concentrated in blood-derived products, especially VEGF. A timely regulation of neovascularization is of crucial importance in tendon healing since it allows for rapid cell and platelet recruitment, while preventing the formation of necrotic tissue immediately after injury [82, 103]. Inflammation should then progressively decrease, and this is possibly aided by the PRP action, which inhibits the production of pro-inflammatory cytokines (IL-6, TNFα, PGE2) in later stages. The control of inflammation is considered a key features of mesenchymal stem cells from both bone marrow and adipose tissue [84], but this effect is under-reported in the studies analyzed. During the proliferation phase of tendon healing, mitosis of tissue progenitors and migration of recruited cells cause an increase in cell density; ECM deposition is initiated in a non-organized manner, with prevalence of collagen type III, proteoglycans and fibronectin [30, 109]. Tendons treated with orthobiologics demonstrated enhanced cell content and proliferation with respect to controls, and the contribution of these products in terms of growth factors - especially PDGF, IGF and FGF - represent the putative mechanism of this effect [30]. At the same time, growth factors contained within by orthobiologics would explain the increase in terms of ECM elements (collagen type I, II, III and GAG) observed in treated tendons compared to controls [90, 128]. Although an excess of growth factors bears the risk of forming fibrotic tissue, to some extent this is expected to occur during the proliferative phase [38, 109]. Indeed, the tissue and ECM remodeling phase, which is crucial for the maturation of repaired tissue and the restoration of proper tendon, should follow this stage. In this phase, collagen type III is replaced by collagen type I and the collagen fibers form cross-links [109], both events that are stimulated by orthobiologics. In addition, adipose-derived products showed the ability to increase the expression of tendon specific markers such as SCX and tenascin, favoring tissue maturation by stimulating progenitor cell differentiation into tenocytes [15, 21, 66]. Indeed, the action on tissue resident progenitors is a well-known mechanism of action of the orthobiologics, supporting the use of regenerative medicine as treatment for degenerative pathologies [89, 110]. Metalloproteases play a relevant role in the ECM-remodeling phase of physiological tendon healing. They are finely regulated in physiological conditions, but in presence of chronic inflammation a dysregulation of their expression may foster ECM degeneration and pathology progression [27]. Indeed, especially in the context of tendinopathy their inhibition allows for counteracting pathology progression and promote tissue healing [83].
The effects of each product in the treatment of tendon pathologies are summarized in Table 1. Considering the different tendon condition, different applications of orthobiologics should be considered. In case of tendon complete ruptures the use of orthobiologics in combination with surgical repair and/or scaffolds appear superior to that of orthobiologics alone. Otherwise, the use of these products for the treatment of partial ruptures showed satisfactory results even without scaffold or surgical repair. Improvements of the histologic appearance and enhancement of ECM deposition (especially collagen type I) were reported for all products, while cell-based approaches, but not blood-derived products, allowed to observe improvements in the biomechanical properties of repaired tissue. Interestingly, the lack of improvements in biomechanical properties after treatment with blood-derived products was confirmed across a variety of models, techniques and injury sites. Adipose-derived products were reported to improve imaging and functional outcomes. Ectopic bone formation was identified as a possible side effect of orthobiologics, especially in bone marrow-derived products, while adipose-based treatments were reported to reduce fibrosis. In general, the application of blood-derived products seems to accelerate healing, demonstrating superior outcomes at short follow up and similar results compared to control in the long term. On the contrary, cell-based treatments were reported to produce more durable results in terms of quality of the repaired tissue.
In the context of experimentally-induced or spontaneous tendinopathy, histological improvements were reported for all orthobiologics, while functional improvements were reported only after cell-based treatments. In particular the use of adipose-derived products enhanced the biomechanical properties of the repaired tissue as well as the deposition of tendon ECM.
Concerning rotator cuff disease, blood-derived products were reported to effectively improve imaging appearance and functional scores in treated animals. This treatment also ameliorated the biochemical properties of tendons at short-term, but not at longer follow-ups. This observation supports the hypothesis that PRP and similar products effectively accelerate healing rather than improving the final outcome. Cell-based treatment were able to improve both histological appearance and biomechanical properties of repaired tendons.
Orthobiologics appear to be especially effective in the treatment of enthesis injuries. Indeed, all products were able to improve histologic appearance, ECM deposition and biomechanical properties of repaired tendons. This is possibly due to the positive effects on fibrocartilage and bone tissue formation. In fact, while the ectopic formation of fibrocartilage or bone would represent a drawback for orthobiologics application to tendons mid-portion injuries, the increased production of elements such as collagen type II, proteoglycans and BMP-2, would have beneficial effects in the context of the enthesis.
Several formulations of each product are available, especially concerning blood-based products. Interestingly, leukocyte rich PRP was reported to induce better results compared to leucocyte-poor counterparts. In addition, frozen/thawed PRP has limited effectiveness compared to the fresh product. Interestingly, the additional use of NSAIDs did not modify PRP effects.
Among the limitations of the present work, data about cell-based therapies are reported in a low number of studies, frequently focusing on cultured mesenchymal stem cells rather than products obtained at point of care. Thus, the effectiveness demonstrated by these approaches has limited generalizability. On the contrary, the evidence for blood-derived products is based on a large pool of studies, with consistent results. Another limitation is due to the lack of consensus about pathological models and type of product administration throughout literature, preventing the elaboration of defined strategies for the use of orthobiologics in a preclinical setting. Furthermore, the low quality of research reports, especially concerning bone marrow-derived products, may have biased the results of this systematic review.
Conclusion
Overall, the preclinical results about orthobiologics applications to tendon pathologies support the rationale of their clinical use, and encourage the performance of clinical trials aimed to confirm these results in human subjects. The effect of blood-derived products appears to be related to an acceleration of the healing process, without improvements in the final structure and properties of repaired tendon. Cell-based products have been reported to produce more durable results, although with a lower level of supporting evidences.
In particular, many reports about cell-therapies focused on the use of cultured MSCs rather than products obtained at the point of care. In consideration of the regulatory, safety-related and economic limitations to the use of cultured cells in the clinical practice, future pre-clinical trials should focus on minimally manipulated products (i.e. BMAC or SVF). Possible safety issues about the use of orthobiologics for tendon pathologies are related to the formation of fibrotic tissue (fibrocartilage) and ectopic bone. On the other hand, this possible side effect turns into an advantage when treating tendon-bone junction injuries, as confirmed by studies assessing enthesis repair.
Availability of data and materials
N/A.
References
Abat F, Alfredson H, Cucchiarini M, Madry H, Marmotti A, Mouton C, Oliveira JM, Pereira H, Peretti GM, Spang C, Stephen J, van Bergen CJA, de Girolamo L (2018) Current trends in tendinopathy: consensus of the ESSKA basic science committee. Part II: treatment options. J Exp Orthop 5:38
Aicale R, Bisaccia RD, Oliviero A, Oliva F, Maffulli N (2020) Current pharmacological approaches to the treatment of tendinopathy. Expert Opin Pharmacother 21:1467–1477
Aicale R, Oliviero A, Maffulli N (2020) Management of Achilles and patellar tendinopathy: what we know, what we can do. J Foot Ankle Res 13(1):59-69
Alonso-Goulart V, Carvalho LN, Marinho ALG, de Oliveira Souza BL, de Aquino Pinto Palis G, Lage HGD, de Lima IL, Guimarães LD, Peres LC, Silveira MM, Lopes GHNL, Ferreira LB, de Souza Castro-Filice L (2021) Biomaterials and adipose-derived Mesenchymal stem cells for regenerative medicine: a systematic review. Materials (Basel) 14:4641
Andia I, Maffulli N (2018) A contemporary view of platelet-rich plasma therapies: moving toward refined clinical protocols and precise indications. Regen Med 13:717–728
Anz AW, Bapat A, Murrell WD (2016) Concepts in regenerative medicine: past, present, and future in articular cartilage treatment. J Clin Orthop Trauma 7:137–144
Awad HA, Boivin GP, Dressler MR, Smith FNL, Young RG, Butler DL (2003) Repair of patellar tendon injuries using a cell-collagen composite. J Orthop Res 21:420–431
Aynardi M, Zahoor T, Mitchell R, Loube J, Feltham T, Manandhar L, Paudel S, Schon L, Zhang Z (2018) Orthotopic transplantation of Achilles tendon allograft in rats: with or without incorporation of autologous Mesenchymal stem cells. Cell Transplant 27:245–255
Bass E (2012) Tendinopathy: why the difference between tendinitis and Tendinosis matters. Int J Ther Massage Bodyw 5:14–17
Behfar M, Javanmardi S, Sarrafzadeh-Rezaei F (2014) Comparative study on functional effects of allotransplantation of bone marrow stromal cells and adipose derived stromal vascular fraction on tendon repair: a biomechanical study in rabbits. Cell J 16:263–270
Behfar M, Sarrafzadeh-Rezaei F, Hobbenaghi R, Delirezh N, Dalir-Naghadeh B (2011) Adipose-derived stromal vascular fraction improves tendon healing in rabbits. Chin J Traumatol 14:329–335
Bora P, Majumdar AS (2017) Adipose tissue-derived stromal vascular fraction in regenerative medicine: a brief review on biology and translation. Stem Cell Res Ther 8:145
Bosch G, Moleman M, Barneveld A, van Weeren PR, van Schie HTM (2011) The effect of platelet-rich plasma on the neovascularization of surgically created equine superficial digital flexor tendon lesions. Scand J Med Sci Sports 21:554–561
Boswell SG, Schnabel LV, Mohammed HO, Sundman EA, Minas T, Fortier LA (2014) Increasing platelet concentrations in leukocyte-reduced platelet-rich plasma decrease collagen gene synthesis in tendons. Am J Sports Med 42:42–49
Burk J, Gittel C, Heller S, Pfeiffer B, Paebst F, Ahrberg AB, Brehm W (2014) Gene expression of tendon markers in mesenchymal stromal cells derived from different sources. BMC Res Notes 7:826
Caplan AI, Correa D (2011) The MSC: an injury drugstore. Cell Stem Cell 9:11–15
de Carvalho PK, Silveira L, Barbosa D, Munin E, Salgado MAC, Villaverde AB (2016) Analysis of experimental tendinitis in rats treated with laser and platelet-rich plasma therapies by Raman spectroscopy and histometry. Lasers Med Sci 31:19–26
Chen C, Zhang T, Liu F, Qu J, Chen Y, Fan S, Chen H, Sun L, Zhao C, Hu J, Lu H (2019) Effect of low-intensity pulsed ultrasound after autologous adipose-derived stromal cell transplantation for bone-tendon healing in a rabbit model. Am J Sports Med 47:942–953
Chen H-S, Su Y-T, Chan T-M, Su Y-J, Syu W-S, Harn H-J, Lin S-Z, Chiu S-C (2015) Human adipose-derived stem cells accelerate the restoration of tensile strength of tendon and alleviate the progression of rotator cuff injury in a rat model. Cell Transplant 24:509–520
Chen L, Liu J-P, Tang K-L, Wang Q, Wang G-D, Cai X-H, Liu X-M (2014) Tendon derived stem cells promote platelet-rich plasma healing in collagenase-induced rat achilles tendinopathy. Cell Physiol Biochem 34:2153–2168
Chen Q-J, Chen L, Wu S-K, Wu Y-J, Pang Q-J (2018) rhPDGF-BB combined with ADSCs in the treatment of Achilles tendinitis via miR-363/PI3 K/Akt pathway. Mol Cell Biochem 438:175–182
Chung SW, Song BW, Kim YH, Park KU, Oh JH (2013) Effect of platelet-rich plasma and porcine dermal collagen graft augmentation for rotator cuff healing in a rabbit model. Am J Sports Med 41:2909–2918
Ciccocioppo R, Cantore A, Chaimov D, Orlando G (2019) Regenerative medicine: the red planet for clinicians. Intern Emerg Med 14:911–921
Çirci E, Akman YE, Şükür E, Bozkurt ER, Tüzüner T, Öztürkmen Y (2016) Impact of platelet-rich plasma injection timing on healing of Achilles tendon injury in a rat model. Acta Orthop Traumatol Turc 50:366–372
Clarke G, Harley P, Hubber E-L, Manea T, Manuelli L, Read E, Watt FM (2018) Bench to bedside: current advances in regenerative medicine. Curr Opin Cell Biol 55:59–66
Dallaudière B, Lempicki M, Pesquer L, Louedec L, Preux PM, Meyer P, Hummel V, Larbi A, Deschamps L, Journe C, Hess A, Silvestre A, Sargos P, Loriaut P, Boyer P, Schouman-Claeys E, Michel JB, Serfaty JM (2013) Efficacy of intra-tendinous injection of platelet-rich plasma in treating tendinosis: comprehensive assessment of a rat model. Eur Radiol 23:2830–2837
Del Buono A, Oliva F, Osti L, Maffulli N (2013) Metalloproteases and tendinopathy. Muscles Ligaments Tendons J 3:51–57
Deng S, Sun Z, Zhang C, Chen G, Li J (2017) Surgical treatment versus conservative Management for Acute Achilles Tendon Rupture: a systematic review and Meta-analysis of randomized controlled trials. J Foot Ankle Surg 56:1236–1243
Devana SK, Kelley BV, McBride OJ, Kabir N, Jensen AR, Park SJ, Eliasberg CD, Dar A, Mosich GM, Kowalski TJ, Péault B, Petrigliano FA, SooHoo NF (2018) Adipose-derived human perivascular stem cells may improve Achilles tendon healing in rats. Clin Orthop Relat Res 476:2091–2100
Docheva D, Müller SA, Majewski M, Evans CH (2015) Biologics for tendon repair. Adv Drug Deliv Rev 84:222–239
Dolkart O, Chechik O, Zarfati Y, Brosh T, Alhajajra F, Maman E (2014) A single dose of platelet-rich plasma improves the organization and strength of a surgically repaired rotator cuff tendon in rats. Arch Orthop Trauma Surg 134:1271–1277
Dyrna F, Zakko P, Pauzenberger L, McCarthy MB, Mazzocca AD, Dyment NA (2018) Human subacromial Bursal cells display superior engraftment versus bone marrow stromal cells in murine tendon repair. Am J Sports Med 46:3511–3520
Ersen A, Demirhan M, Atalar AC, Kapicioğlu M, Baysal G (2014) Platelet-rich plasma for enhancing surgical rotator cuff repair: evaluation and comparison of two application methods in a rat model. Arch Orthop Trauma Surg 134:405–411
Everts P, Onishi K, Jayaram P, Lana JF, Mautner K (2020) Platelet-rich plasma: new performance understandings and therapeutic considerations in 2020. Int J Mol Sci 21:E7794
Facon-Poroszewska M, Kiełbowicz Z, Prządka P (2019) Systemic inflammatory response to the radial pressure wave therapy (RPWT) in collagenase-induced Achilles tendinopathy treated with adipose derived stem cells or platelet rich plasma. Pol J Vet Sci 22:735–742
Faisal T, Asjid R, Qamar K, Akhtar N, Moeed K, Hussain T (2019) Effect of autologous platelet-rich plasma on appearance of Tenocytes at injured Achilles tendon Entheses in rabbits. J Coll Physicians Surg Pak 29:1029–1033
Fernández-Sarmiento JA, Domínguez JM, Granados MM, Morgaz J, Navarrete R, Carrillo JM, Gómez-Villamandos RJ, Muñoz-Rascón P, de Las M, Mulas J, Millán Y, García-Balletbó M, Cugat R (2013) Histological study of the influence of plasma rich in growth factors (PRGF) on the healing of divided Achilles tendons in sheep. J Bone Joint Surg Am 95:246–255
Frangogiannis N (2020) Transforming growth factor-β in tissue fibrosis. J Exp Med 217:e20190103
Fu X, Liu G, Halim A, Ju Y, Luo Q, Song AG (2019) Mesenchymal stem cell migration and tissue repair. Cells 8:E784
Fukawa T, Yamaguchi S, Watanabe A, Sasho T, Akagi R, Muramatsu Y, Akatsu Y, Katsuragi J, Endo J, Osone F, Sato Y, Okubo T, Takahashi K (2015) Quantitative assessment of tendon healing by using MR T2 mapping in a rabbit Achilles tendon transection model treated with platelet-rich plasma. Radiology 276:748–755
Geburek F, Mundle K, Conrad S, Hellige M, Walliser U, van Schie HTM, van Weeren R, Skutella T, Stadler PM (2016) Tracking of autologous adipose tissue-derived mesenchymal stromal cells with in vivo magnetic resonance imaging and histology after intralesional treatment of artificial equine tendon lesions--a pilot study. Stem Cell Res Ther 7:21
Geburek F, Roggel F, van Schie HTM, Beineke A, Estrada R, Weber K, Hellige M, Rohn K, Jagodzinski M, Welke B, Hurschler C, Conrad S, Skutella T, van de Lest C, van Weeren R, Stadler PM (2017) Effect of single intralesional treatment of surgically induced equine superficial digital flexor tendon core lesions with adipose-derived mesenchymal stromal cells: a controlled experimental trial. Stem Cell Res Ther 8:129
Genç E, Yüksel S, Çağlar A, Beytemur O, Güleç MA (2020) Comparison on effects of platelet-rich plasma versus autologous conditioned serum on Achilles tendon healing in a rat model. Acta Orthop Traumatol Turc 54:438–444
de Girolamo L, Morlin Ambra LF, Perucca Orfei C, McQuilling JP, Kimmerling KA, Mowry KC, Johnson KA, Phan AT, Whited JL, Gomoll AH (2019) Treatment with human amniotic suspension allograft improves tendon healing in a rat model of collagenase-induced Tendinopathy. Cells 8:E1411
Gong D, Zhao M, Su W, Dong C, Deng Y, Zhen P (2019) Experimental study of platelet-rich plasma in treatment of Achilles tendinopathy in rabbits. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 33:871–876
González JC, López C, Álvarez ME, Pérez JE, Carmona JU (2016) Autologous leukocyte-reduced platelet-rich plasma therapy for Achilles tendinopathy induced by collagenase in a rabbit model. Sci Rep 6:19623
Greimers L, Drion PV, Colige A, Libertiaux V, Denoël V, Lecut C, Gothot A, Kaux J-F (2018) Effects of allogeneic platelet-rich plasma (PRP) on the healing process of sectioned Achilles tendons of rats: a methodological description. J Vis Exp 133:55759
Gurger M, Once G, Yilmaz E, Demir S, Calik I, Say Y, Kavakli A, Key S, Gurbuz MU, Bingollu O (2021) The effect of the platelet-rich plasma and ozone therapy on tendon-to-bone healing in the rabbit rotator cuff repair model. J Orthop Surg Res 16:202
Hapa O, Cakıcı H, Kükner A, Aygün H, Sarkalan N, Baysal G (2012) Effect of platelet-rich plasma on tendon-to-bone healing after rotator cuff repair in rats: an in vivo experimental study. Acta Orthop Traumatol Turc 46:301–307
Hasan S, Weinberg M, Khatib O, Jazrawi L, Strauss EJ (2016) The effect of platelet-rich fibrin matrix on rotator cuff healing in a rat model. Int J Sports Med 37:36–42
Ho LK, Baltzer WI, Nemanic S, Stieger-Vanegas SM (2015) Single ultrasound-guided platelet-rich plasma injection for treatment of supraspinatus tendinopathy in dogs. Can Vet J 56:845–849
Hooijmans CR, Rovers MM, de Vries RBM, Leenaars M, Ritskes-Hoitinga M, Langendam MW (2014) SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol 14:43
Huang Z, Yin Z, Xu J, Fei Y, Heng BC, Jiang X, Chen W, Shen W (2021) Tendon stem/progenitor cell subpopulations and their implications in tendon biology. Front Cell Dev Biol 9:631272
Inoue N, Ikeda K, Aro HT, Frassica FJ, Sim FH, Chao EYS (2002) Biologic tendon fixation to metallic implant augmented with autogenous cancellous bone graft and bone marrow in a canine model. J Orthop Res 20:957–966
James R, Kesturu G, Balian G, Chhabra AB (2008) Tendon: biology, biomechanics, repair, growth factors, and evolving treatment options. J Hand Surg Am 33:102–112
Jiang G, Wu Y, Meng J, Wu F, Li S, Lin M, Gao X, Hong J, Chen W, Yan S, Yan R, Feng G, Cheng Z (2020) Comparison of leukocyte-rich platelet-rich plasma and leukocyte-poor platelet-rich plasma on Achilles Tendinopathy at an early stage in a rabbit model. Am J Sports Med 48:1189–1199
Jun J-I, Lau LF (2010) Cellular senescence controls fibrosis in wound healing. Aging (Albany NY) 2:627–631
Kane SF, Olewinski LH, Tamminga KS (2019) Management of Chronic Tendon Injuries. Am Fam Physician 100:147–157
Kang K-K, Lee E-J, Kim Y-D, Chung M-J, Kim J-Y, Kim S-Y, Hwang S-K, Jeong K-S (2017) Vitamin C improves therapeutic effects of adipose-derived stem cell transplantation in mouse tendonitis model. In Vivo 31:343–348
Kaux J-F, Drion PV, Colige A, Pascon F, Libertiaux V, Hoffmann A, Janssen L, Heyers A, Nusgens BV, Le Goff C, Gothot A, Cescotto S, Defraigne J-O, Rickert M, Crielaard J-M (2012) Effects of platelet-rich plasma (PRP) on the healing of Achilles tendons of rats. Wound Repair Regen 20:748–756
Kaux J-F, Libertiaux V, Dupont L, Colige A, Denoël V, Lecut C, Hego A, Gustin M, Duwez L, Oury C, Gothot A, Greimers L, Drion P (2020) Platelet-rich plasma (PRP) and tendon healing: comparison between fresh and frozen-thawed PRP. Platelets 31:221–225
Kaux J-F, Libertiaux V, Leprince P, Fillet M, Denoel V, Wyss C, Lecut C, Gothot A, Le Goff C, Croisier J-L, Crielaard J-M, Drion P (2017) Eccentric training for tendon healing after acute lesion: a rat model. Am J Sports Med 45:1440–1446
Kim HJ, Nam H-W, Hur C-Y, Park M, Yang HS, Kim B-S, Park J-H (2011) The effect of platelet rich plasma from bone marrow aspirate with added bone morphogenetic protein-2 on the Achilles tendon-bone junction in rabbits. Clin Orthop Surg 3:325–331
Kim SJ, Lee SM, Kim JE, Kim SH, Jung Y (2017) Effect of platelet-rich plasma with self-assembled peptide on the rotator cuff tear model in rat. J Tissue Eng Regen Med 11:77–85
Lane JG, Healey RM, Chase DC, Amiel D (2013) Use of platelet-rich plasma to enhance tendon function and cellularity. Am J Orthop (Belle Mead NJ) 42:209–214
Lee SY, Kwon B, Lee K, Son YH, Chung SG (2017) Therapeutic mechanisms of human adipose-derived Mesenchymal stem cells in a rat tendon injury model. Am J Sports Med 45:1429–1439
Li ZJ, Yang QQ, Zhou YL (2021) Basic research on tendon repair: strategies, evaluation, and development. Front Med (Lausanne) 8:664909
Lipman K, Wang C, Ting K, Soo C, Zheng Z (2018) Tendinopathy: injury, repair, and current exploration. Drug Des Devel Ther 12:591–603
Liu XN, Yang C-J, Kim JE, Du ZW, Ren M, Zhang W, Zhao HY, Kim KO, Noh K-C (2018) Enhanced tendon-to-bone healing of chronic rotator cuff tears by bone marrow aspirate concentrate in a rabbit model. Clin Orthop Surg 10:99–110
Lu L-Y, Kuang C-Y, Yin F (2018) Magnetic resonance imaging and biomechanical analysis of adipose-derived stromal vascular fraction applied on rotator cuff repair in rabbits. Chin Med J 131:69–74
Lu L-Y, Ma M, Cai J-F, Yuan F, Zhou W, Luo S-L, Pan Z-Y, Zeng W, Zhong N, Yin F (2018) Effects of local application of adipose-derived stromal vascular fraction on tendon-bone healing after rotator cuff tear in rabbits. Chin Med J 131:2620–2622
Lyras D, Kazakos K, Verettas D, Polychronidis A, Simopoulos C, Botaitis S, Agrogiannis G, Kokka A, Patsouris E (2010) Immunohistochemical study of angiogenesis after local administration of platelet-rich plasma in a patellar tendon defect. Int Orthop 34:143–148
Lyras DN, Kazakos K, Agrogiannis G, Verettas D, Kokka A, Kiziridis G, Chronopoulos E, Tryfonidis M (2010) Experimental study of tendon healing early phase: is IGF-1 expression influenced by platelet rich plasma gel? Orthop Traumatol Surg Res 96:381–387
Lyras DN, Kazakos K, Georgiadis G, Mazis G, Middleton R, Richards S, O’Connor D, Agrogiannis G (2011) Does a single application of PRP alter the expression of IGF-I in the early phase of tendon healing? J Foot Ankle Surg 50:276–282
Lyras DN, Kazakos K, Tryfonidis M, Agrogiannis G, Botaitis S, Kokka A, Drosos G, Tilkeridis K, Verettas D (2010) Temporal and spatial expression of TGF-beta1 in an Achilles tendon section model after application of platelet-rich plasma. Foot Ankle Surg 16:137–141
Lyras DN, Kazakos K, Verettas D, Polychronidis A, Tryfonidis M, Botaitis S, Agrogiannis G, Simopoulos C, Kokka A, Patsouris E (2009) The influence of platelet-rich plasma on angiogenesis during the early phase of tendon healing. Foot Ankle Int 30:1101–1106
Maffulli N, Via AG, Oliva F (2015) Chronic Achilles tendon disorders. Clin Sports Med 34:607–624
Matsunaga D, Akizuki S, Takizawa T, Omae S, Kato H (2013) Compact platelet-rich fibrin scaffold to improve healing of patellar tendon defects and for medial collateral ligament reconstruction. Knee 20:545–550
McCarrel TM, Minas T, Fortier LA (2012) Optimization of leukocyte concentration in platelet-rich plasma for the treatment of tendinopathy. J Bone Joint Surg Am 94(1-8):e143
McGoldrick R, Chattopadhyay A, Crowe C, Chiou G, Hui K, Farnebo S, Davis C, Le Grand A, Jacobs M, Pham H, Chang J (2017) The Tissue-Engineered Tendon-Bone Interface: In Vitro and In Vivo Synergistic Effects of Adipose-Derived Stem Cells, Platelet-Rich Plasma, and Extracellular Matrix Hydrogel. Plast Reconstr Surg 140:1169–1184
Meadows MC, Levy DM, Ferry CM, Gardner TR, Teratani T, Ahmad CS (2017) Effects of platelet-rich plasma and indomethacin on biomechanics of rotator cuff repair. Am J Orthop (Belle Mead NJ) 46:E336–E343
Millar NL, Reilly JH, Kerr SC, Campbell AL, Little KJ, Leach WJ, Rooney BP, Murrell GAC, McInnes IB (2012) Hypoxia: a critical regulator of early human tendinopathy. Ann Rheum Dis 71:302–310
Millar NL, Silbernagel KG, Thorborg K, Kirwan PD, Galatz LM, Abrams GD, Murrell GAC, McInnes IB, Rodeo SA (2021) Tendinopathy. Nat Rev Dis Primers 7:1–21
Murphy MB, Moncivais K, Caplan AI (2013) Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med 45:e54
Oh JH, Chung SW, Kim SH, Chung JY, Kim JY (2014) 2013 Neer award: effect of the adipose-derived stem cell for the improvement of fatty degeneration and rotator cuff healing in rabbit model. J Shoulder Elb Surg 23:445–455
Oshita T, Tobita M, Tajima S, Mizuno H (2016) Adipose-derived stem cells improve collagenase-induced Tendinopathy in a rat model. Am J Sports Med 44:1983–1989
Ouyang HW, Goh JCH, Thambyah A, Teoh SH, Lee EH (2003) Knitted poly-lactide-co-glycolide scaffold loaded with bone marrow stromal cells in repair and regeneration of rabbit Achilles tendon. Tissue Eng 9:431–439
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 88:105906
Perucca Orfei C, Bowles AC, Kouroupis D, Willman MA, Ragni E, Kaplan LD, Best TM, Correa D, de Girolamo L (2021) Human tendon stem/progenitor cell features and functionality are highly influenced by in vitro culture conditions. Front Bioeng Biotechnol 9:711964
Perucca Orfei C, Viganò M, Pearson JR, Colombini A, De Luca P, Ragni E, Santos-Ruiz L, de Girolamo L (2019) In vitro induction of tendon-specific markers in tendon cells, adipose- and bone marrow-derived stem cells is dependent on TGFβ3, BMP-12 and ascorbic acid stimulation. Int J Mol Sci 20:E149
Ruiz-Alonso S, Lafuente-Merchan M, Ciriza J, Saenz-Del-Burgo L, Pedraz JL (2021) Tendon tissue engineering: cells, growth factors, scaffolds and production techniques. J Control Release 333:448–486
Sarıkaya B, Yumuşak N, Yigin A, Sipahioğlu S, Yavuz Ü, Altay MA (2017) Comparison of the effects of human recombinant epidermal growth factor and platelet-rich plasma on healing of rabbit patellar tendon. Eklem Hastalik Cerrahisi 28:92–99
Sarrafian TL, Wang H, Hackett ES, Yao JQ, Shih M-S, Ramsay HL, Turner AS (2010) Comparison of Achilles tendon repair techniques in a sheep model using a cross-linked acellular porcine dermal patch and platelet-rich plasma fibrin matrix for augmentation. J Foot Ankle Surg 49:128–134
Schmidt CC, Jarrett CD, Brown BT (2015) Management of Rotator Cuff Tears. J Hand Surg 40:399–408
Schnabel LV, Mohammed HO, Miller BJ, McDermott WG, Jacobson MS, Santangelo KS, Fortier LA (2007) Platelet rich plasma (PRP) enhances anabolic gene expression patterns in flexor digitorum superficialis tendons. J Orthop Res 25:230–240
Schulz KS, Ash KJ, Cook JL (2019) Clinical outcomes after common calcanean tendon rupture repair in dogs with a loop-suture tenorrhaphy technique and autogenous leukoreduced platelet-rich plasma. Vet Surg 48:1262–1270
Shin MJ, Shim IK, Kim DM, Choi JH, Lee YN, Jeon I-H, Kim H, Park D, Kholinne E, Yang H-S, Koh KH (2020) Engineered cell sheets for the effective delivery of adipose-derived stem cells for tendon-to-bone healing. Am J Sports Med 48:3347–3358
Si Z, Wang X, Sun C, Kang Y, Xu J, Wang X, Hui Y (2019) Adipose-derived stem cells: sources, potency, and implications for regenerative therapies. Biomed Pharmacother 114:108765
Smith RKW, Werling NJ, Dakin SG, Alam R, Goodship AE, Dudhia J (2013) Beneficial effects of autologous bone marrow-derived mesenchymal stem cells in naturally occurring tendinopathy. PLoS One 8:e75697
Solchaga LA, Bendele A, Shah V, Snel LB, Kestler HK, Dines JS, Hee CK (2014) Comparison of the effect of intra-tendon applications of recombinant human platelet-derived growth factor-BB, platelet-rich plasma, steroids in a rat achilles tendon collagenase model. J Orthop Res 32:145–150
Spang JT, Tischer T, Salzmann GM, Winkler T, Burgkart R, Wexel G, Imhoff AB (2011) Platelet concentrate vs. saline in a rat patellar tendon healing model. Knee Surg Sports Traumatol Arthrosc 19:495–502
Takamura M, Yasuda T, Nakano A, Shima H, Neo M (2017) The effect of platelet-rich plasma on Achilles tendon healing in a rabbit model. Acta Orthop Traumatol Turc 51:65–72
Tempfer H, Traweger A (2015) Tendon vasculature in health and disease. Front Physiol 6:330
Thangarajah T, Sanghani-Kerai A, Henshaw F, Lambert SM, Pendegrass CJ, Blunn GW (2018) Application of a demineralized cortical bone matrix and bone marrow-derived Mesenchymal stem cells in a model of chronic rotator cuff degeneration. Am J Sports Med 46:98–108
Torricelli P, Fini M, Filardo G, Tschon M, Pischedda M, Pacorini A, Kon E, Giardino R (2011) Regenerative medicine for the treatment of musculoskeletal overuse injuries in competition horses. Int Orthop 35:1569–1576
Trzyna A, Banaś-Ząbczyk A (2021) Adipose-Derived Stem Cells Secretome and Its Potential Application in “Stem Cell-Free Therapy”. Biomolecules 11:878
Uysal CA, Tobita M, Hyakusoku H, Mizuno H (2012) Adipose-derived stem cells enhance primary tendon repair: biomechanical and immunohistochemical evaluation. J Plast Reconstr Aesthet Surg 65:1712–1719
Valencia Mora M, Antuña Antuña S, García Arranz M, Carrascal MT, Barco R (2014) Application of adipose tissue-derived stem cells in a rat rotator cuff repair model. Injury 45(Suppl 4):S22–S27
Vasiliadis AV, Katakalos K (2020) The role of scaffolds in tendon tissue engineering. J Funct Biomater Multidisciplinary Digit Publishing Inst 11:78
Viganò M, Sansone V, d’Agostino MC, Romeo P, Perucca Orfei C, de Girolamo L (2016) Mesenchymal stem cells as therapeutic target of biophysical stimulation for the treatment of musculoskeletal disorders. J Orthop Surg Res 11:163
Walden G, Liao X, Donell S, Raxworthy MJ, Riley GP, Saeed A (2017) A clinical, biological, and biomaterials perspective into tendon injuries and regeneration. Tissue Eng Part B Rev 23:44–58
Wang JH-C, Guo Q, Li B (2012) Tendon biomechanics and mechanobiology--a minireview of basic concepts and recent advancements. J Hand Ther 25:133–140 quiz 141
Wang Y, Chen X, Cao W, Shi Y (2014) Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol 15:1009–1016
Wong C-C, Huang Y-M, Chen C-H, Lin F-H, Yeh Y-Y, Bai M-Y (2020) Cytokine and growth factor delivery from implanted platelet-rich fibrin enhances rabbit Achilles tendon healing. Int J Mol Sci 21:E3221
Wu Y, Dong Y, Chen S, Li Y (2014) Effect of platelet-rich plasma and bioactive glass powder for the improvement of rotator cuff tendon-to-bone healing in a rabbit model. Int J Mol Sci 15:21980–21991
Wynn T (2008) Cellular and molecular mechanisms of fibrosis. J Pathol 214:199–210
Xiong X, Wu L, Xiang D, Ni G, Zhao P, Yu B (2012) Effect of platelet-rich plasma injection on early healing of Achilles tendon rupture in rats. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 26:466–471
Xu K, Al-Ani MK, Sun Y, Xu W, Pan L, Song Y, Xu Z, Pan X, Yang L (2017) Platelet-rich plasma activates tendon-derived stem cells to promote regeneration of Achilles tendon rupture in rats. J Tissue Eng Regen Med 11:1173–1184
Yan R, Gu Y, Ran J, Hu Y, Zheng Z, Zeng M, Heng BC, Chen X, Yin Z, Chen W, Shen W, Ouyang H (2017) Intratendon delivery of leukocyte-poor platelet-rich plasma improves healing compared with leukocyte-rich platelet-rich plasma in a rabbit Achilles Tendinopathy model. Am J Sports Med 45:1909–1920
Yang X, Meng H, Peng J, Xu L, Wang Y, Sun X, Zhao Y, Quan Q, Yu W, Chen M, Shi T, Du Y, Lu S, Wang A (2020) Construction of microunits by adipose-derived Mesenchymal stem cells laden with porous Microcryogels for repairing an acute Achilles tendon rupture in a rat model. Int J Nanomedicine 15:7155–7171
Yuan Z, Cao F, Gao C, Yang Z, Guo Q, Wang Y (2021) Decellularized human umbilical cord Wharton jelly scaffold improves tendon regeneration in a rabbit rotator cuff tendon defect model. Am J Sports Med 50(2):371-383
Yüksel S, Adanır O, Gültekin MZ, Çağlar A, Küçükyıldırım BO, Güleç MA, Alagöz E (2015) Effect of platelet-rich plasma for treatment of Achilles tendons in free-moving rats after surgical incision and treatment. Acta Orthop Traumatol Turc 49:544–551
Yuksel S, Guleç MA, Gultekin MZ, Adanır O, Caglar A, Beytemur O, Onur Küçükyıldırım B, Avcı A, Subaşı C, İnci Ç, Karaoz E (2016) Comparison of the early period effects of bone marrow-derived mesenchymal stem cells and platelet-rich plasma on the Achilles tendon ruptures in rats. Connect Tissue Res 57:360–373
Zhang C-H, Jiang Y-L, Ning L-J, Li Q, Fu W-L, Zhang Y-J, Zhang Y-J, Xia C-C, Li J, Qin T-W (2018) Evaluation of Decellularized bovine tendon sheets for Achilles tendon defect reconstruction in a rabbit model. Am J Sports Med 46:2687–2699
Zhang J, Middleton KK, Fu FH, Im H-J, Wang JH-C (2013) HGF mediates the anti-inflammatory effects of PRP on injured tendons. PLoS One 8:e67303
Zhang Y, Yu J, Zhang J, Hua Y (2019) Simvastatin with PRP promotes Chondrogenesis of bone marrow stem cells in vitro and wounded rat Achilles tendon-bone Interface healing in vivo. Am J Sports Med 47:729–739
Zheng C, Lu H, Tang Y, Wang Z, Ma H, Li H, Chen H, Chen Y, Chen C (2019) Autologous freeze-dried, platelet-rich plasma carrying Icariin enhances bone-tendon healing in a rabbit model. Am J Sports Med 47:1964–1974
Zhou Y, Wang JH-C (2016) PRP treatment efficacy for Tendinopathy: a review of basic science studies. BioMed Res Int Hindawi 2016:e9103792
Zong J-C, Mosca MJ, Degen RM, Lebaschi A, Carballo C, Carbone A, Cong G-T, Ying L, Deng X-H, Rodeo SA (2017) Involvement of Indian hedgehog signaling in mesenchymal stem cell-augmented rotator cuff tendon repair in an athymic rat model. J Shoulder Elb Surg 26:580–588
Acknowledgements
None.
Funding
The project 20–107 was supported by a literature grant from the ON Foundation, Switzerland and by the Italian Ministry of Health (Ricerca Corrente).
Author information
Authors and Affiliations
Contributions
MV and LdG conceived the project. MV and ER performed the database search. MV, AM and ER performed title/abstract screening. MV, ER and AM performed full text screening. LdG supervised study project and provided resources. MV, ER performed data collection. MV and ER prepared the first draft of the manuscript. AM and LdG revised and edited the manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
N/A.
Consent for publication
N/A.
Competing interests
MV, LdG paid scientific consultant for Lipogems SpA.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Viganò, M., Ragni, E., Marmotti, A. et al. The effects of orthobiologics in the treatment of tendon pathologies: a systematic review of preclinical evidence. J EXP ORTOP 9, 31 (2022). https://doi.org/10.1186/s40634-022-00468-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40634-022-00468-w