Abstract
Background
Sediment deposition constitutes a major disturbance having negative effects on aquatic ecosystems. The Chaitén Volcano eruption occurred on May 2008. As a consequence, broad areas along the Argentine Andes (40° S to 46° S) were covered with ash. This event provided an excellent opportunity to investigate how a natural and exceptional sedimentation episode affects Trichoptera communities.
Results
We assessed changes in caddisfly community attributes (composition, density and diversity) and 11 biological traits, by comparing pre-eruption (May 2007 to April 2008) and post-eruption (July 2008 to March 2010) data at two headwater streams. As a consequence of the event, total suspended solids increased and Trichoptera richness and density significantly diminished. By March 2010, two common species of Hydroptilidae (Metrichia patagonica and Metrichia neotropicalis) were no longer recorded at one site; while species richness and density values were still low indicating that the community had not recovered. Scrapers, shredders, and predators were among the most affected functional feeding groups and changes in their relative abundance were tracked in subsequent years after the ashfall event.
Conclusions
In this study, species tolerance to sedimentation was related to certain traits such as poorly synchronized life history, filter-feeding habits, rounded body shape, tegument respiration mode, and poorly sclerotized life forms.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Biological and functional traits are variables describing characters of organisms (including morphological characters with biological implications), either on a continuous scale or through categories or trait-states (Statzner and Bêche [2010]). The biological trait profile of a community offers an alternative approach for assessing the response of a stream community to disturbance, and can be expected to reflect the functional relationships between biota and environmental characteristics (Townsend et al. [1997]; Dolédec et al. [2011]).
Based on Southwood's habitat template model, trait approaches propose that habitat selects for characteristic life history traits through natural selection, and species are expected to respond to environmental gradients (Southwood [1977]; Poff et al. [2006]; Buendia et al. [2013]). In this context, trait patterns can be indicators of the source of impairment, and species possessing relevant adaptive traits are likely to remain (Statzner et al. [2004]; Buendia et al. [2013]). Therefore, changes in trait profiles reflect changes in the ability of invertebrate communities to cope with disturbances, as only those traits conferring resistance and resilience are selected (Statzner and Bêche [2010]; Vandewalle et al. [2010]). Facing an environmental disturbance, populations of species with traits conferring resilience or resistance are predicted to increase (Townsend et al. [1997]).
Among natural disturbances, explosive volcanic eruptions can exert major changes in the surrounding landscape (Jones and Gislason [2008]; Ruggieri et al. [2011]). The magnitude and extent of this kind of phenomena as well as their ecological consequences vary widely depending on the type of eruption and distance to the volcano (Óskarsson [1980]; Annen and Wagner [2003]; Witham et al. [2005]). Fine volcanic ash deposition is often responsible for the more widespread impact of a volcanic eruption, affecting hundreds of square kilometers (km2) by covering the soil with dense layers of volcanic material, leading to significant changes in both terrestrial and aquatic environments (McDowall [1996]; Del Moral and Grishin [1999]; Martin et al. [2009]). While eruptions may be ephemeral, ashfall sediments can remain in the local environment for many years to decades, and in arid conditions, they could be preserved for millions of years (Ruggieri et al. [2011]). Additionally, strong wind storms may result in ash resuspension, causing similar effects to those of the original eruption (Inbar et al. [1995]; Ruggieri et al. [2011]).
The effect of sedimentation processes of volcanic origin on benthic fauna are comparable to those caused by certain anthropogenic activities such as those derived from dredging, land movement, and deforestation (Miserendino et al. [2012]). According to Paul and Meyer ([2001]), a strong decrease in density and diversity of benthic macroinvertebrates can be expected as a response to siltation. Increased turbidity has been associated with higher insect drifts (Doeg and Milledge [1991]), and generally, the shift in bed sediments from stable boulder-cobble to finer sediment sizes favors species adapted to unstable habitats, such as chironomids and oligochaetes (Pedersen and Perkins [1986]; Collier [1995]). Studies of sedimentation processes related to urban streams, have shown a decrease in available refugial space, making invertebrates highly susceptible to drift during floods (Borchardt and Statzner [1990]). It has also been confirmed that high levels of suspended solids alter benthic primary production in streams. Wantzen ([1998]) observed a significant scouring effect by silt (‘sand jet effect’) that reduced or removed epilithic algae and biofilms, which play a key role as a food source for grazing invertebrates. Additionally, high loads of suspended solids may increase turbidity, reducing stream primary production and interfering predator-prey relationships (e.g., optical orientation of dragonfly larvae). Moreover, allochtonous material, such as leaves and detritus may be covered by slow sand movement and become inaccessible for epibenthic detritivorous animals, consequently modifying the trophic structure (Wantzen [1998], [2006]).
Following a natural disturbance like an ashfall phenomenon, the recolonization process will depend on species showing selected traits that maximize their survival rates, arrival of new colonizers, and the presence of resources as well as appropriate habitat for organisms to establish. As shown by Anderson and Wisseman ([1987]), 5 years after Mt. Saint Helen's eruption, Trichoptera species were still uncommon in benthic samples taken in a stream 15 km northeast of the mountain. However, light traps showed an unexpected species richness of adult Trichoptera, suggesting that recovery of fauna was not limited by adult colonists, but by changes in the physical environment that prevented larvae from developing appropriately. Dolédec et al. ([2011]) proposed that after sediment deposition episodes, those species displaying egg protection, being burrowers and/or fine detritus consumers might be favored.
The eruption of Chaitén volcano (42° 50′ S; 72° 39′ W) is considered to be the largest one at a global scale since that of the Hudson (Chile) in 1991 (Nillni and Bitschene [1995]), and was the first large eruption of high-silica rhyolite since that of Alaska's Novarupta volcano in 1912 (Pallister et al. [2010]). On May of 2008, its explosion caused a strong emission of volcanic ashes of predominantly rhyolitic composition, which covered broad areas ranging from 0.1 to 15 mm of thickness in the Argentine side of the Andes Mountains (Watt et al. [2009]). As a result of the prevailing western winds, the lighter material was transported eastward in a few hours, affecting broad areas of northwestern Chubut province (Argentina), between 42° S and 46° S (Ovdas [2008]).
Despite that many studies having attempted to describe the effects of volcanic ash deposition on aquatic environments, few of them support their comparisons with pre-eruption data (Anderson [1992]; Antos and Zobel [2005]). During 2007 to 2008, we were conducting a research on the life history of Trichoptera species inhabiting Nant y Fall and Chiquito Streams when the eruption of the Chaitén volcano occurred (May 2008). This provided a unique opportunity to investigate how a natural and exceptional (i.e., non-cyclic) episode affected the Trichoptera community in terms of structure, species richness, and biological traits. We hypothesize that traits conferring resistance to sediment deposition effects might be related to greater mobility or good swimming abilities, preference for depositional habitats and poorly synchronized life cycles.
In this study, we attempt to 1) assess the changes in attributes of the Trichoptera community in two streams affected by heavy ash deposition, 2) evaluate the persistence of these changes, and 3) discuss which biological and functional traits improved the survival chances of caddisfly species.
Methods
Study site
The study was undertaken in a transitional mountain and piedemont area, with a marked altitudinal gradient, located in the ecotone between the Sub-antarctic Forests and Patagonian Steppe (Paruelo et al. [1998]) of northwestern Chubut province (Argentina).
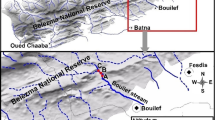
The samples were taken from two streams (Nant y Fall and Chiquito), on the Argentine side of the Andes Mountains. Ashfall at both sites was comprised between 5 and 10 mm (total catchment average) (Watt et al. [2009]) (Figure 1). However, values observed in the margins were between 15 and 25 mm.
Map of the study area. Shows location of the two sampling sites, Nant y Fall and Chiquito (filled circles), volcano and main Argentine cities (stars), Isopachs (black contours) represents ash thickness in mm. Reproduced and adapted from Watt et al. ([2009]) (Patagonia, Argentina).
Nant y Fall Stream (71° 25′ 17″ W; 43° 13′ 24″ S; 690 meters above sea level (m.a.s.l.); Futaleufú-Yelcho basin), is a third-order stream located downstream a small lake (Rosario Lake), and drains a catchment of 162 km2. Chiquito Stream (71° 30′ 22″ W; 43° 21′ 43″ S; 670 m.a.s.l.) is a permanent second-order stream in the Río Frío basin, draining a small catchment of 11 km2 (Figure 1).
Rainfall in the area, as estimated from a study by Jobbágy et al. ([1995]), is 600 mm/year. The discharge pattern is bimodal, with peaks concurrently with winter rainfalls, where 46% of the annual precipitation occurs (Paruelo et al. [1998]), and in spring with snowmelt (Coronato and del Valle [1988]).
At both sites, riparian forest is composed by the deciduous tree Ñire (Nothofagus antarctica), several native shrub species (e.g., Schinus patagonicus, Berberis buxifolia, and Berberis heterophylla) (León et al. [1998]), while 60% to 70% of the total coverage consists of herbaceous species. At both catchments, main agricultural activities are cattle grazing and forest practices such as wood extraction, no change in land use practices or intensity was recorded during any of the visits to the sites.
Aquatic vegetation covers one quarter of the studied reach at Nant y Fall and is mainly represented by the submersed Isoetes savatieri, Myriophyllum quitense, Limosella australis, Ranunculus flagelliformis, Callitriche lechleri, and Lilaeopsis macloviana; and the emergent macrophytes Mimulus glabratus, Verónica anagallis-aquatica, Eleocharis albibracteata, Juncus burkartii, Juncus diemii and Juncus microcephalus. At Chiquito Stream, bryophytes cover 50% to 80% of the reach.
Environmental characterization and sampling procedure
Both sites were sampled monthly during the year previous to the eruption of Chaitén volcano (from May 2007 to April 2008) and were revisited after the episode on six occasions (June and October 2008; February, June, and October 2009; and March 2010). This sequence of the post-eruption sampling was coordinated with another regional survey that involved several aquatic environments in the area (Miserendino et al. [2012]).
Substrate size composition was estimated as percentage of boulder (>25 cm), cobble (6.4 to 25 cm), pebble (1.6 to 6.4 cm), gravel (2 to 16 mm), and sand (0.25 to 2 mm) in the reach, using a 1-m2 grid (Ward [1992]). At post-eruption dates, an assessment of ash deposition was performed at each stream, depth of the stream bank ash deposits were measured with a caliper (mm). Additionally, percentage of riverbed covered by ash was estimated visually for riffles and pools separately.
Average depth (cm) was calculated from five measurements on three transects across the channel with a calibrated stick; wet width (m) was also measured. Current speed (m/s) was estimated in mid-channel by timing a float (average of three trials) as it moved over a distance of 10 m (Gordon et al. [2004]). Discharge (m3/s) was obtained by combining depth, wet width, and current speed as in the study by Gordon et al. ([2004]). On each sampling occasion, water temperature, pH, specific conductance (μS20/cm) and dissolved oxygen (mg O2/l) were measured with a multi-parameter probe (Hach SensION 156, Hach, Loveland, CO, USA).
Total suspended solids (TSS) were measured gravimetrically from separate water samples (2,000-ml plastic bottles) taken from the mid channel. In the laboratory, differences between the final and initial weights of dried filters were obtained (APHA [1999]). Since no TSS data were taken during pre-eruption dates, data obtained in a previous study at the same sampling sites (2005 to 2006 period, Miserendino et al. [2012]) were used to compare with post-eruption values. Since none of the sites revisited experienced major changes in land-use practices before and after the ashfall event, we assumed that TSS variation was exclusively related to the volcanic episode.
Caddisfly sampling
At each site and at all dates (pre-eruption: n = 12, post-eruption n = 6), larvae were collected from three riffles using a Surber net (0.09 m2; 250-μm mesh size). Samples (n = 108) were fixed with formaldehyde, in the field.
Larvae were sorted manually under × 5 magnification, counted and preserved in 70% alcohol. Caddisfly larvae were identified to the lowest possible taxonomic level using available keys (Angrisano [1997]; Sganga and Fontanarrosa [2006]; Angrisano and Sganga [2009]). Taxa were assigned to functional feeding groups based upon knowledge of feeding modes (mouthpart morphology and behavior), by gut content analysis and using available references (Merritt et al. [2008]; Miserendino and Masi [2010]; Brand and Miserendino [2011a], [b], [2012]).
Caddisfly community data were analyzed through the following attributes: species/taxa richness, density (ind/m2), Shannon diversity index (H′), and density and relative abundance of trophic guilds (Merritt et al. [2008]).
Caddisfly trait data
Information on 11 traits related to life history, morphology, and ecology was obtained for all caddisfly species (27 species). Traits included in the life history category were duration (<10 months and >10 months) and synchronicity (poorly synchronized and well synchronized) of the life cycle (Poff et al. [2006]; Brand and Miserendino [2011a], [2012]). Morphological traits include a spectrum of five larval attributes concerning: attachment (free living, non-attached forms vs. sedentary or attached forms), armoring (three states, ranging from non or poorly sclerotized larvae, heavily sclerotized larvae and larvae that construct cases out of mineral or inorganic material), body shape (fusiform and non-fusiform), body size (ranges: small <9 mm; medium 9 to 16 mm; and large >16 mm) and respiration modes (tegumentary and gills) (Poff et al. [2006]). Ecological larval traits included information related to hydraulic condition preferences (exclusively depositional, depositional and erosional, and erosional only), substratum and habitat selectivity (coarse, gravel, sand, macrophyte and organic detritus litter), food preferences (detritus, plant detritus, living macrophytes, living microphytes, and living macroinvertebrates), and feeding behavior (deposit feeder, shredder, scraper, filter-feeder, piercer, predator) (Poff et al. [2006]; Brand and Miserendino [2011a], [b]; Dolédec et al. [2011]; Brand et al. [2012]). Only traits with information available for all species were selected; and trait assignment was performed using regional databases and available publications (Brand and Miserendino [2011a]; Brand and Miserendino [2011b]; Brand and Miserendino [2012]; Brand et al. [2012]). Species trait information was then rescaled according to the number of states (from 1 to x, with x being the total number of states in each trait). Values then, were rescaled so that the sum of all states equalled one. This information was used to build the species-traits matrix for each stream.
Statistical procedure
One-way ANOVA model was employed in order to detect significant differences between pre- and post-eruption values of physicochemical variables. Prior to analyses variables were log (x + 1) transformed. Student's t test for dependent samples comparisons were used in order to identify the main changes between pre- and post-eruption dates in the caddisfly community attributes. Significant differences in species richness, total density, Shannon diversity, and the main density of functional feeding groups were assessed (Sokal and Rohlf [1995]).
Principal Component Analyses (PCA) was used to determine patterns of functional trait composition in the caddisfly assemblage at each stream. This ordination method (linear model) provided information enabling a more detailed examination of the relationship between the biologically defined traits at pre- and post-eruption dates. A matrix of the relative prevalence of each trait state (within each of the 11 traits) on each sampling occasion at both sites (n = 36) was created by log-transformed abundance weighting the taxon scores for each trait state for a given sample. The sums of weighted scores (one per trait state) were then expressed as the relative abundance distribution (within a trait), giving the sample trait profile (Larsen and Ormerod [2010]; Feio and Dolédec [2012]). The final matrix employed in the ordination (Sampling dates × Traits states) was obtained by adding the values of the trait states at each month. Ordinations were performed using CANOCO package version 4.0 (ter Braak and Smilauer [1998]).
Results
Physicochemical variables
Substrate size composition at Nant y Fall site was dominated by gravel (30%), cobbles (25%), and boulders (20%), and the remaining 25% was distributed among smaller fractions; Chiquito Stream dominant fractions were cobbles (30%), pebbles (25%), and boulders (15%), with the remaining 30% distributed among smaller fractions. Post-eruption substrate characterization did not vary from that of pre-eruption.
Immediately after the first eruption, ash deposition at the margins of Chiquito sampling site averaged 20 mm deep. Moreover, substrate occurring within a riffle were 50% covered in ash, while at deposition areas, up to 70% was covered with ash. At Nant y Fall site, ash deposition at the margins averaged 17.5 mm deep, while riffles and pools presented a 15% and 80% of the substrate covered with ash, respectively. During the study period ash deposit remained in the river banks, pool coverage decreased to 10%, and riffles presented no evidence of ash deposition.
The environmental characterization of study sites at pre- and post-eruption dates is shown in Table 1. Water temperature, wet and dry channel widths, depth, current velocity, and discharge showed no significant changes when comparing pre- and post-eruption datasets. Conductivity values were between 72.3 and 119.4 μS/cm (Nant y Fall), and 29.4 and 104.7 μS/cm (Chiquito). However, mean values did not significantly increase after the eruption. Dissolved oxygen ranged from 6.6 to 15.4 mg/l, with the highest mean values on post-eruption dates at both sites. Post-eruption pH values were comparable to pre-eruption values.
Mean values of TSS were significantly higher on post-eruption dates at the two study sites (ANOVA p < 0.05) (Table 1), and reflected ash deposition and remobilization episodes. Post-eruption values at Nant y Fall Stream (between 7 and 13.5 mg/l) were four times higher than before the eruption (1.7 to 3.6 mg/l) while at Chiquito Stream, post-eruption values (between 1.3 and 24.1 mg/l) were about three times higher than pre-eruption ones (between 2 to 6.1 mg/l).
Community composition
Nant y Fall community attributes changed after the ashfall episode. Cumulative species richness on pre-eruption dates (16 species from 8 families), decreased on post-eruption samples (12 species from 6 families) (Table 2, Figure 2). Mean species richness decrease significantly in post-eruption samples, from 8 to 6 species (Table 3, Figure 2). Caddisfly density was also lower at post-eruption dates; however, these values were not significantly different from those recorded before the eruption (Tables 2 and 3, Figure 2). At this site, two caddisfly species accounted for more than 90% of the abundance during pre- and post-eruption surveys; Parasericostoma ovale (Sericostomatidae), with a density of 3,916 and 2,072 ind/m2 and Smicridea frequens (Hydropsychidae), with a density of 1,187 and 1,751 ind/m2, respectively. In contrast, Shannon diversity increased significantly post-eruption (Tables 2 and 3). This was probably explained by a drop in density of the dominant P. ovale, representing a decrease of about 20% in its relative contribution to the total density.
Caddisfly community composition at Chiquito Stream also changed after the volcanic eruption (Figure 2, Table 2). Cumulative species richness values were comparable between pre- and post-eruption periods (17 species in 9 families, Table 2). However, mean species richness (from 8 to 5 species), density (984 to 204 ind/m2), and diversity (from 1.5 to 1.2) all significantly declined after the eruption (Tables 2 and 3, Figure 2). Relative abundance of species changed considerably (Table 2). The dominant species at this site were Myotrichia murina (Sericostomatidae) and Scotiotrichia ocreata (Glossosomatidae); however, both species density showed a post-eruption decline. Neoatopsyche brevispina (Hydrobiosidae) also decreased by 50% in abundance after the eruption (Table 2).
Functional feeding groups
At Nant y Fall Stream, only shredder density significantly decreased after the volcano eruption (t test p < 0.05, Table 3). No significant differences were observed for the other groups. Collector-filterers' relative proportion increased in years following the eruption and this was clearly displayed in seasonal comparison during spring (October) and summer (February) (Figure 3, Table 3).
Relative abundance of the main functional feeding groups for Nant y Fall and Chiquito Streams (Patagonia, Argentina). Comparing three seasons (June: winter; October: spring, and February: summer) in consecutive years (2007 to 2009). Pre-eruption data in first column (marked with arrow) and post-eruption data in second and third column.
At Chiquito Stream, the density of scrapers, shredders, collector-filterers, and predators significantly decreased after the eruption (Table 3). A strong reduction in scrapers' contribution at all seasons was evidenced for the first and second years when comparing consecutive years. The relative contribution of shredders increased in spring (October) on following years after the ashfall (Figure 3).
Caddisfly trait ordination
All traits and trait states employed in the ordination are detailed in Table 4; trait state assignment for each caddisfly species is shown in Table 5. PCA identified major patterns in biological traits, for the two studied streams. The first two axes explained 84.2% and 96% of the variance in trait data for Nant y Fall and Chiquito Streams, respectively (Figure 4b, d). In both PCA ordinations, traits were ordered along the first axis according to their sensitivity to ashfall (Figure 4b, d), with pre-eruption sampling dates located toward negative values, and post-eruption dates grouped toward the positive end (Figure 4a, c). Therefore, in both ordination models, PCA1 highlighted a disturbance gradient (Figure 4).
PCA ordination. Shows the position of sampling dates (May 2007-March 2010) for (a) Nant y Fall and (c) Chiquito streams; and trait composition at (b) Nant y Fall and (d) Chiquito Streams (Patagonia, Argentina), for the same coordinates. Open circles correspond to pre-eruption sampling dates, and filled circles indicate post-eruption sampling dates. Codes for trait states are described in Table 4.
Accordingly, trait states sensitive to disturbance were well synchronized cycles, non-streamlined body shape, life-cycle longer than 10 months and the presence of gills for respiration (Figure 4b, d). Other features such as larvae with mineral or inorganic cases, sedentary/attached life forms, and a preference for erosional habitats were also, to a lesser extent, sensitive to sediment deposition. In contrast, trait states associated with the post-eruption period were detritus feeding, filter-feeders, and poorly sclerotized larvae which were grouped toward the positive end of PCA1 (Figure 4b, d).
Discussion
According to predictions of the habitat templet model (Southwood [1977]), the representation of biological traits are expected to reflect the functional relationships between biota and environmental characteristics (Townsend et al. [1997]; Lamoroux et al. [2004]; Dolédec et al. [2011]). Our study suggests that changes in environmental conditions due to the volcanic eruption, an intense and non-cyclical event, could favor those caddisfly species with trait profiles that allow them to tolerate heavy sediment deposition. In general, traits related with life history and morphological features are clearly the most responsive to sediment impairment (Vandewalle et al. [2010]). Species with long (>10 months) and well-synchronized life cycles were severely affected. Species displaying short or poorly synchronized life cycles did not disappear after the episode; these attributes seem to be common at frequently disturbed systems including sedimentation and flooding episodes (Mellado Díaz et al. [2008]).
Sedentary, non-streamlined, and gill respiration larvae, as well as those bearing mineral refugia were negatively affected by the successive ash remobilization and sedimentation, this is consistent with that reported by Townsend et al. ([1997]).
Traits hypothesized as resilient increased in frequency on post-eruption dates. Poorly sclerotized and free-living larvae appeared to be less sensitive to impairment; this may be related to increased mobility. Consistent with these observations, Buendia et al. ([2013]) found that mobile and streamlined forms were able to tolerate sedimentation by moving out of the impaired areas, while less mobile species were negatively affected.
Life history traits represent an important factor contributing to resilience following a disturbance (Wallace [1990]). Species with shorter life cycles seem to be better colonizers than those having longer ones (Collier et al. [2002]). Brand and Miserendino ([2011a], [2012]) showed that most of the dominant caddisfly species in the study area were univoltine, with relatively well-synchronized life cycles, a pattern that seems to be the most common for Patagonian Trichoptera. Our study suggests that species with highly synchronized life cycles and egg hatching coinciding with the ashfall period (e.g., Mastigoptila longicornuta, Scotiotrichia ocreata (Glossosomatidae); Brachysetodes quadrifidus (Leptoceridae); and Eosericostoma aequispina (Helicophidae) were those most strongly affected. These species showed an important decrease in density, probably due to the disturbance that took place during their larval recruitment and to a decrease in the quality of their main food supply (i.e., epilithon).
In line with a recent study (Miserendino et al. [2012]), a clear distinction in Trichoptera communities between pre- and post-eruption sampling dates was observed, with a significantly decreased density and species richness at both streams. This also has been reported for other volcanically impaired streams (Anderson and Wisseman [1987]; Anderson [1992]; Dorava and Milner [1999]; Collier et al. [2002]). Moreover, significant changes in Trichoptera richness and density of some functional feeding groups were associated with the ashfall event. In this sense, a decrease in overall scrapers density, and absence of some scrapers species was detected, probably due to a reduction in food resources. During the study timeframe (22 months after the eruption) the scrapers Metrichia patagonica and Metrichia neotropicalis were completely eradicated from Chiquito site, while other species showed a strong decrease in density. The large amounts of sediment deposited in the streams could have diminished the quality of algal food supply in epilithic communities as demonstrated by Izagirre et al. ([2009]). This in turn could have a negative influence on the density of some invertebrate species (Graham [1990]; Collier [2002]).
Shredders were significantly less abundant in post-eruption samples, suggesting that either habitat and/or leaf litter became unsuitable after the sedimentation process. Kreutzweiser et al. ([2005]) encountered similar effects on shredders at sites where sediment input from logging activities increased. They argue that this event may be derived from the physical interference of sediments on the species' feeding behavior or by reducing palatability of particulate organic matter as a consequence of a poor microbial colonization and conditioning. It has been suggested that filtering devices, such as the nets built by the Hydropsychidae species, may be obstructed by inorganic suspended sediments transported by the water column (Wood and Armitage [1997]; Wantzen [2006]; Buendia et al. [2013]). Nevertheless, species at Nant y Fall Stream (i.e., Smicridea annulicornis and S. frequens, Hydropsychidae) appeared to be unaffected by ashfall, since pre- and post-eruption density values were nearly the same. Moreover, their recruitment period occurred mostly in February (Brand and Miserendino [2012]), attaining a mixed size structure (larval instars 3 and 4 at the same time) at the time of the ashfall. Other features that probably contributed to the survival of these species were their active behavior, their ability to move and clean the net used for filtering, or their capacity to abandon an old net and build a new one. According to Brand and Miserendino ([2011a], [2012]) predator species (N. brevispina and Neopsilochorema tricarinatum, Hydrobiosidae), have poorly synchronized life cycles, with an extended recruitment and co-occurrence of many size classes during several months of the year. Volcanic ash deposition had a minor effect on them, as shown in the present study. Traits displayed by the two species (e.g., poorly synchronized life cycles, high mobility, no mineral case, no gill respiration system) as well as in other hydrobiosids, could result in resilience to disturbances such as high inputs of fine sediment.
The overall success of diverse benthic macroinvertebrate communities is strongly dependent on their physical integrity of their environment (Karr et al. [1986]). Since many macroinvertebrates require specific substrates for oviposition and the completion of other life cycle stages (Merritt and Cummins [1996]), sedimentation can ultimately result in the local extinction of certain species (Waters [1995]). Sediment deposition affects macroinvertebrate communities by reducing the number of suitable habitats (Chutter [1968]), decreasing substrate stability which in turn increases drift rates (Suren and Jowett [2001]), reducing food resources and food quality (Broekhuizen et al. [2011]), or by affecting aquatic respiration due to deposition of silt on respiratory structures (Lemly [1982]). In all cases, the timing of the ashfall and the population features such as size structure, at that moment seemed to play a determining role on the ultimate effect on species.
Nearly 2 years after the first Chaitén ashfall, the Trichoptera community was not yet fully recovered at Chiquito Stream. Nant y Fall Stream, however, showed a lower impact than Chiquito and by March 2010 caddisfly richness and density reached similar values to those before the ashfall. At Chiquito Stream, the Trichoptera community showed no signs of recovery at the end of the study period, with some species being absent from the samples and density values of other species consistently lower than pre-eruption ones. Similar results were documented by Lallement et al. ([2014]), for other Patagonian streams affected by heavy ashfall produced by Puyehue-Cordón Caulle eruption (2011). After the Chaitén eruption, Miserendino et al. ([2012]) found that the effects were more dramatic in small rivers than in larger ones, which are characterized by a larger discharge. Anderson ([1992]) also observed that the increases in density, biomass, and diversity after a volcanic episode (Mt. Saint Helen) were related with the stabilization of stream bed. Five years after the Redoubt eruption, Dorava and Milner ([1999]) documented a recovery in the macroinvertebrate community, with unaffected upstream sites acting as sources for drifting organisms toward disturbed downstream areas.
While the presence of an epicenter or source of organisms for recolonization is vital, the survival chances of larvae in the impacted environment are related to how ashfall affected the provision of food, the composition of suitable substrates, and the amount of suspended solids. These factors would affect the colonization and reestablishment of the community structure to its reference values, within a particular habitat (Anderson and Wisseman [1987]; Wallace [1990]; Lallement et al. [2014]). In the case of Chaitén ashfall, it is likely that periodic bankside and upstream sediment inputs due to wind and rainfall could act as a bottleneck to successful recolonization, delaying the reestablishment of original conditions and therefore preventing the caddisfly community from recovering.
Conclusions
Our study demonstrates that volcanic sedimentation causes high mortality on Trichoptera populations and has detectable ecological effects. The community recovery and successful reestablishment of original community values depend on the return to the suitable conditions of the studied streams. Moreover, certain traits such as poorly synchronized life cycle, filter feeder habits, tegumentary respiration, and good armoring (i.e., mineral case builders) were proved to be the most resilient to this kind of disturbance.
References
Anderson NH: Influence of disturbance on insect communities in Pacific Northwest streams. Hydrobiologia 1992, 248: 79–92. 10.1007/BF00008887
Anderson NH, Wisseman RW: Recovery of the Trichoptera fauna near Mt St Helens five years after eruption. In Proceedings of the 5th International Symposium on Trichoptera. Edited by: Bournaud M, Tachet H. Dr. W. Junk Publishers, Dordrecht/Boston/Lancaster; 1987:367–373. 10.1007/978-94-009-4043-7_65
Angrisano EB: Contribution to the knowledge of the larvae of Hydrobiosidae. I. Neopsilochorema tricarinatum and Australochorema rectispinum . In Proceedings of the 7th International Symposium on Trichoptera. Edited by: Otto C. Blackuys Publishers, Netherlands; 1997:15–17.
Angrisano EB, Sganga JV: Trichoptera. In Macroinvertebrados bentónicos sudamericanos. Edited by: Domínguez E, Fernández HR. Fundación Miguel Lillo, Tucumán, Argentina; 2009.
Annen C, Wagner JJ: The impact of volcanic eruptions during the 1990's. Nat Hazards 2003, 4: 169–175. 10.1061/(ASCE)1527-6988(2003)4:4(169)
Antos JA, Zobel DB: Plant responses in forests of the tephra-fall zone. In Ecological responses to the 1980 eruption of Mount St. Edited by: Dale VH, Swanson FJ, Crisafulli CM. Helens. Springer, New York; 2005:47–58. 10.1007/0-387-28150-9_4
APHA:Standard methods for the examination of water and wastewate. American Public Health Association, Hanover, MD; 1999.
Borchardt D, Statzner B: Ecological impact of urban stormwater runoff studied in experimental flumes: population loss by drift and availability of refugial space. Aquat Sci 1990, 52: 299–314. 10.1007/BF00879759
Brand C, Miserendino ML: Life history strategies and production of caddisflies in a perennial headwater stream in Patagonia. Hydrobiologia 2011,673(1):137–151. 10.1007/s10750-011-0768-3
Brand C, Miserendino ML: Characterizing Trichoptera trophic structure in rivers under contrasting land use in Patagonia, Argentina. In Proceedings of the 13th International Symposium on Trichoptera. Edited by: Majecka K, Majecki J, Morse J. Magnolia Press, New Zealand, Zoosymposia; 2011:29–40.
Brand C, Miserendino ML: Life cycle phenology, secondary production, and trophic guilds of caddisfly species in a lake-outlet stream of Patagonia. Limnologica 2012,42(2):108–117. 10.1016/j.limno.2011.09.004
Brand C, Miserendino ML, Epele LB: Spatial and temporal pattern of caddisfly distribution at a mesohabitat scale in two Patagonian mountain streams subjected to pastoral use. Int Rev Hydrobiol 2012,97(2):83–99. 10.1002/iroh.201111368
Broekhuizen N, Parkyn S, Miller D: Fine sediment effects on feeding and growth in the invertebrate grazers Potamopyrgus antipodarum (Gastropoda, Hydrobiidae) and Deleatidium sp. (Ephemeroptera, Leptophlebiidae). Hydrobiologia 2011, 457: 125–132. 10.1023/A:1012223332472
Buendia C, Gibbins CN, Vericat D, Batalla RJ, Douglas A: Detecting the structural and functional impacts of fine sediment on stream invertebrates. Ecol Ind 2013, 25: 184–196. 10.1016/j.ecolind.2012.09.027
Chutter FM: The effects of silt and sand on the invertebrate fauna of streams and rivers. Hydrobiologia 1968, 34: 57–76. 10.1007/BF00040323
Collier KJ: Environmental factors affecting the taxonomic composition of aquatic macro-invertebrate communities in lowland waterways of Northland New Zealand. New Zeal J Mar Freshwat Res 1995, 29: 453–465. 10.1080/00288330.1995.9516679
Collier KJ: Effects of flow regulation and sediment flushing on instream habitat and benthic invertebrates in a New Zealand river influenced by a volcanic eruption. River Res Applic 2002, 18: 213–226. 10.1002/rra.666
Collier KJ, Parkyn SM, Quinn JM, Scarsbrook MR: Bouncing back: how fast can stream invertebrates recolonise? Water Atmosph 2002,10(2):9–11.
Coronato FR, Del Valle HF: Caracterización hídrica de las cuencas hidrográficas de la provincia del Chubut. Cenpat-Conicet, Puerto Madryn, Chubut, Argentina; 1988.
Del Moral R, Grishin SY: Volcanic disturbances and ecosystem recovery. In Ecosystems of Disturbed Ground. Edited by: Walker LR. Ecosystems of the World 16. Vol. 2, Elsevier, New York, USA; 1999:137–155.
Doeg TJ, Milledge GA: Effects of experimentally increasing concentrations of suspended sediment on macroinvertebrate drift. Aust J Mar Freshwat Res 1991, 42: 519–526. 10.1071/MF9910519
Dolédec S, Phillips N, Townsend C: Invertebrate community responses to land use at a broad spatial scale: trait and taxonomic measures compared in New Zealand rivers. Freshwat Biol 2011,56(8):1670–1688. 10.1111/j.1365-2427.2011.02597.x
Dorava JM, Milner AM: Effects of recent volcanic eruptions on aquatic habitat in the Drift River, Alaska, USA: implications at other cook inlet region volcanoes. Environ Manag 1999,23(2):217–230. 10.1007/s002679900181
Feio MJ, Dolédec S: Integration of invertebrate traits into predictive models for indirect assessment of stream functional integrity: a case study in Portugal. Ecol Ind 2012, 15: 236–247. 10.1016/j.ecolind.2011.09.039
Gordon ND, McMahon TA, Finlayson BL, Gippel CJ, Nathan RJ: Stream hydrology: an introduction for ecologists. John Wiley & Sons, Ltd., Sussex, England; 2004.
Graham AA: Siltation of stone-surface periphyton in rivers by clay sized particles from low concentrations in suspension. Hydrobiologia 1990, 199: 107–115. 10.1007/BF00005603
Inbar M, Ostera HA, Parica CA, Remesal MB, Salani FM: Environmental assessment of 1991 Hudson volcano eruption ashfall effects on southern Patagonia region, Argentina. Env Geol 1995, 25: 119. 10.1007/BF00767868
Izagirre O, Serra A, Guasch H, Elosegi A: Effects of sediment deposition on periphytic biomass, photosynthetic activity and algal community structure. Sci Total Environ 2009,407(21):5694–5700. 10.1016/j.scitotenv.2009.06.049
Jobbágy EG, Paruelo JM, León JC: Estimación de la precipitación y de su variabilidad interanual a partir de información geográfica en el NW de Patagonia, Argentina. Ecol Austr 1995, 5: 47–53.
Jones MT, Gislason SR: Rapid releases of metal salts and nutrients following the deposition of volcanic ash into aqueous environments. Geochim Cosmochim Acta 2008, 72: 3661. 10.1016/j.gca.2008.05.030
Karr JR, Fausch JD, Yant PR, Schlosser IL: Assessing biological integrity in running waters: a method and its rationale. Illinois Natural History Survey Special Publication 5, Champaign, IL, USA; 1986.
Kreutzweiser DP, Capell SS, Good KP: Effects of fine sediment inputs from a logging road on stream insect communities: a large-scale experimental approach in a Canadian headwater stream. Aquat Ecol 2005,39(1):55–66. 10.1007/s10452-004-5066-y
Lallement ME, Juárez SM, Macchi PJ, Vigliano PH: Puyehue Cordón-Caulle: post-eruption analysis of changes in stream benthic fauna of Patagonia. Ecol Austr 2014, 24: 64–74.
Lamoroux N, Dolédec S, Gayraud S: Biological traits of stream macroinvertebrate communities: effect of microhabitat, reach and basin filters. J N Am Benthol Soc 2004, 23: 449–449. 10.1899/0887-3593(2004)023<0449:BTOSMC>2.0.CO;2
Larsen SE, Ormerod SJ: Combined effects of habitat modification on trait composition and species nestedness in river invertebrates. River Res Applic 2010, 143: 2638–2646.
Lemly AD: Modification of benthic insect communities in polluted streams: combined effects of sedimentation and nutrient enrichment. Hydrobiologia 1982, 87: 229–245. 10.1007/BF00007232
León R, Bran D, Collantes M, Paruelo JM, Soriano A: Grandes unidades de vegetación de la Patagonia extra andina. Ecol Austr 1998, 8: 125–144.
Martin RS, Watt SFL, Pyle DM, Mather TA, Matthews NE, Georg RB: Environmental effects of ashfall in Argentina from the 2008 Chaitén volcanic eruption. J Volcanol Geotherm Res 2009, 184: 462–472. 10.1016/j.jvolgeores.2009.04.010
McDowall RM: Volcanism and freshwater fish biogeography in the northeastern North Island of New Zealand. J Biogeogr 1996, 23: 139–148. 10.1046/j.1365-2699.1996.00960.x
Mellado Díaz A, Suárez Alonso ML, Vidal-Abarca Gutiérrez MR: Biological traits of stream macroinvertebrates from a semi-arid catchment: patterns along complex environmental gradients. Freshwat Biol 2008, 53: 1–21.
Merritt RM, Cummins KW (1996) An introduction to the aquatic insects of North America, 3rd edn. Kendall ⁄Hunt Publishing Company, Dubuque, Iowa
Merritt RW, Cummins KW, Berg MP: An introduction to the aquatic insects of North America. 4th edition. Kendall/Hunt Publishing Company, Dubuque, Iowa; 2008.
Miserendino ML, Masi CI: The effects of land use on environmental features and functional organization of macroinvertebrate communities in Patagonian low order streams. Ecol Ind 2010,10(2):311–319. 10.1016/j.ecolind.2009.06.008
Miserendino ML, Archangelsky M, Brand C, Epele LB: Environmental changes and macroinvertebrate responses in Patagonian streams (Argentina) to ashfall from the Chaitén Volcano (May 2008). Sci Total Environ 2012, 424: 202–212. 10.1016/j.scitotenv.2012.02.054
Nillni A, Bitschene PR: Sedimentología y procesos de sedimentación de la tefra caída de la erupción del volcán Hudson en agosto de 1991. In The august eruption of the Hudson Volcano (Patagonian Andes): a thousand days after. Edited by: Bitschene PR, Mendía J. Universidad Nacional de la Patagonia San Juan Bosco, Servicio Nacional de Geología, Comodoro Rivadavia, Argentina; 1995:116–134.
Óskarsson N: The interaction between volcanic gases and tephra: fluorine adhering to tephra of the 1970 Hekla eruption. J Volcanol Geotherm Res 1980, 8: 251–266. 10.1016/0377-0273(80)90107-9
Ovdas S: Erupción del volcán Chaitén Informes técnicos (mayo). Servicio Nacional de Geología y. Minería, Gobierno de Chile; 2008.
Pallister JS, Major JJ, Pierson TS, Hoblitt RP, Lowernstern JB, Eichelberger JC: Interdisciplinary studies of eruption at Chaitén Volcano, Chile. Eos Transactions AGU 2010, 91: 19. 10.1029/2010EO420001
Paruelo JM, Beltrán A, Jobbágy E, Sala OE, Golluscio RA: The climate of Patagonia : general patterns and controls on biotic processes. Ecol Austr 1998, 8: 85–101.
Paul MJ, Meyer JL: Streams in the urban landscape. Ann Rev Ecol System 2001, 32: 333–365. 10.1146/annurev.ecolsys.32.081501.114040
Pedersen ER, Perkins MA: The use of benthic macroinvertebrate data for evaluating impacts of urban runoff. Hydrobiologia 1986, 139: 13–22. 10.1007/BF00770238
Poff NL, Olden JD, Vieira NKM, Finn DS, Simmons MP, Kondratieff BC: Functional trait niches of North American lotic insects: traits-based ecological applications in light of phylogenetic relationships. J N Am Benthol Soc 2006,25(4):730–755. 10.1899/0887-3593(2006)025[0730:FTNONA]2.0.CO;2
Ruggieri F, Fernández-Turiel JL, Saavedra J, Gimeno D, Polanco E, Naranjo JA: Environmental geochemistry of recent volcanic ashes from the Southern Andes. Environ Chem 2011,8(3):236. 10.1071/EN10097
Sganga JV, Fontanarrosa MS: Contribution to the knowledge of the preimaginal stages of the genus Smicridea McLachlan in South America (Trichoptera: Hydropsychidae: Smicrideinae). Zootaxa 2006, 1258: 1–15.
Sokal RR, Rohlf FJ: Biometry. 3rd edition. W.H. Freeman and Company, New York, USA; 1995.
Southwood TRE: Habitat, the templet for ecological strategies? J Anim Ecol 1977,46(2):336–365. 10.2307/3817
Statzner B, Bêche L: Can biological invertebrate traits resolve effects of multiple stressors on running water ecosystems? Freshwat Biol 2010, 55: 80–119. 10.1111/j.1365-2427.2009.02369.x
Statzner B, Doledec S, Hugueny B: Biological trait composition of European stream invertebrate assemblages: assessing the effects of various trait filter types. Ecogeography 2004, 27: 470–488. 10.1111/j.0906-7590.2004.03836.x
Suren AM, Jowett IG: Effects of deposited sediment on macroinvertebrate drift: an experimental study. New Zeal J Mar Fresh Res 2001, 35: 725–738. 10.1080/00288330.2001.9517038
Ter Braak CJF, Smilauer P: CANOCO reference manual and user´s guide to Canoco for Windows. Software for Canonical Community Ordination (Version 4), Microcomputer power, Ithaca, N.Y; 1998.
Townsend CR, Scarsbrook MR, Dolédec S: The intermediate disturbance hypothesis, refugia, and biodiversity in streams. Limnol Oceanogr 1997,42(5):938–949. 10.4319/lo.1997.42.5.0938
Vandewalle M, Bello F, Berg MP, Bolger T, Dolédec S, Dubs F, Feld CK, Harrington R, Harrison P, Lavorel S, Martins P, Da Silva M, Moretti JN, Santos P, Sattler T, Sousa JP, Sykes MT, Vanbergen AJ, Woodcock BA: Functional traits as indicators of biodiversity response to land use changes across ecosystems and organisms. Biodivers Conserv 2010,19(10):2921–2947. 10.1007/s10531-010-9798-9
Wallace JB: Recovery of lotic macroinvertebrate communities from disturbance. Environ Manage 1990,14(5):605–620. 10.1007/BF02394712
Wantzen KM: Siltation effects on benthic communities in first order streams in Mato Grosso. Verh Int Verein Limnologie 1998, 26: 1155–1159.
Wantzen KM: Physical pollution: effects of gully erosion on benthic invertebrates in a tropical clear-water stream. Aquat Conserv 2006, 16: 733–749. 10.1002/aqc.813
Ward JV: Aquatic insect ecology. Wiley, New York-London; 1992.
Waters TF: Sediment in streams: sources, biological effects, and control. American Fisheries Society, Bethesda, USA; 1995.
Watt SFL, Pyle DM, Mather TA, Martin RS, Matthews NE: Fallout and distribution of volcanic ash over Argentina following May 2008 explosive eruption of Chaitén. Chile. J, Geophys Res; 2009.
Witham CS, Oppenheimer C, Horwell CJ: Volcanic ash-leachates: a review and recommendations for sampling methods. J Volcanol Geotherm Res 2005, 141: 299–326. 10.1016/j.jvolgeores.2004.11.010
Wood PJ, Armitage PD: Biological effects of fine sediment in the lotic environment. Environ Manage 1997,21(2):203–217. 10.1007/s002679900019
Acknowledgements
This paper was partially supported by CONICET (PIP 11220080101907). This is scientific contribution No. 96 from LIESA. The authors would like to thank Dr. Luis Epele, Dr. Miguel Archangelsky, Dr. Cecilia Di Prinzio, and Dr. Ricardo Casaux for the fieldtrip assistance, and Dr. Gabriel M. Martin for the comments. We also thank the two anonymous reviewers for valuable comments that greatly improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
CB participated in the design of the study; carried out the field work and sample processing; and drafted the manuscript. MLM participated in the design of the study and field work, collaborated with the statistical analysis, and helped to draft the manuscript. Both authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Brand, C., Miserendino, M.L. Biological traits and community patterns of Trichoptera at two Patagonian headwater streams affected by volcanic ash deposition. Zool. Stud. 53, 72 (2014). https://doi.org/10.1186/s40555-014-0072-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40555-014-0072-9