Abstract
Background
Drought stress is one of the major abiotic stresses that adversely affect rice production. Four rice genotypes, Giza177, IR64 (as sensitive genotypes) and Vandana, Orabi3 (as tolerant genotypes) were used to screen and characterize the soil microbes associated with each genotype under drought stress.
Results
The soil microbes associated with the tolerant genotypes showed high drought tolerance and high levels of enzyme activity. The most drought-tolerant isolates were inoculated with the sensitive genotype Giza177 under drought conditions. Some morphological, biochemical and molecular responses of inoculated plants were estimated. Inoculated plants showed regulation of some growth and stress-related genes (COX1, AP2-EREBP, GRAM, NRAMP6, NAM, GST, DHN and three genes of expansin (EXP1, EXP2 and EXP3) under drought conditions. Expression profiling of these genes were highly induced in plants inoculated with 4E11 and were correlated with improved growth status under drought stress.
Conclusion
Based on this, drought-tolerant plant growth-promoting rhizobacteria (PGPRs) were associated with the drought-tolerant genotype (Orabi 3). They were related to the significant increase in soil enzymes activities (dehydrogenase, nitrogenase, urease and alkaline phosphatase) in the rhizosphere of tolerant genotype. Inoculation the drought-sensitive genotype (Giza 177) with the most drought-tolerant isolates improved the tolerance status of the sensitive rice genotype and induced the expression of some growth and stress-responsive genes. AP2-EREBP, NRAMP6, DHN and all expansin genes (EXP1, EXP2 and EXP3) were the highly induced genes in inoculated plants with 4E11 strain and the consortium of three selected strains under drought condition.
Graphic abstract

Similar content being viewed by others
Background
Rice is the main food for the majority of the population in developing countries. Water scarcity and drought are imminent threats to food security [1]. Water is considered as main factor in a rice plantation, subsequently food production requires this highly limited resource [2]. Water deficit causes great loss to crop production globally, thus being a major risk to sustainable agriculture [3]. Over the last three decades, water resources have been severely affected by climate changes and that in turn has an influence on rice yield [4]. Plant growth-promoting rhizosphere (PGPR) is beneficial microbiomes associated with plants in their natural environment as endocellular and intracellular microorganisms [5]. Association of PGPR with plant tissues can develop plant performance under different stresses by different mechanisms [6, 7] consequently, it can improve yield both directly and indirectly [8]. Planned usage of PGPR could be one of the developing strategies used for water conservation [7]. It can directly or indirectly contribute to the growth and development of plants through the production and release of various regulatory chemicals near the rhizosphere [9]. PGPRs can also directly stimulate micro-nutrients uptake and influence phytohormones homeostasis, or indirectly enhance the plant defense system toward biotic and/or abiotic stress and improve soil structure and texture [7, 10, 11]. Plant species, growth stage, intensity of stress and duration are factors that can detect the role of PGPR in alleviation of drought stress tolerance [12]. Increasing the nutrient availability in the rhizosphere is the direct way of these microorganisms improving plant growth where, it has the ability to produce phytohormones, exopolysaccharides and siderophore. It also can help indirectly by protecting plants from pathogen attack and improve soil texture and structure [12].
The aim of this study was to screen isolated microbes associated with both tolerant and sensitive rice genotypes under normal and drought stress conditions in order to state the association of drought-tolerant PGPRs and drought-tolerant genotypes. Recent study planned to investigate the role of inoculation with the most tolerant isolated PGPRs in the acquisition of drought tolerance in sensitive genotype. Consideration of the enzyme activity of PGPRs and their role in inducing gene expression of some growth and stress-related genes will be helpful in understanding the role of PGRPs in improving plant growth under water stress condition.
Methods
Genotypes and experimental design
Four rice (Oryza sativa L.) genotypes were used in this investigation. Orabi3 and Vandana genotypes were used as tolerant genotypes while IR64 and Giza177 genotypes as sensitive genotypes. Seeds were received from Rice Research and Training Center (RRTC) at Kafr El sheikh-Egypt, the genotype Orabi3 seeds obtained by Prof. Dr. Said Soliman, Genetics Dept., Fac. of Agric., Zagazig University, Egypt. The experiment was conducted under the farm condition at the Faculty of Agriculture, Tanta University, Egypt, at latitude 30.826401977769848, and longitude 30.99560238465692. The mean of temperature and humidity were ranged from 25 to 31 ℃ and 49 to 59%, respectively, with 0 mm precipitation during experiment period. The germinated seeds were then placed in pots (25 cm diameter and 35 cm height). Four seedlings per pot were maintained after seven days from planting, the pots were irrigated up to water holding capacity of soil. The water stress treatment began 10 days after planting. Soil water content (SWC) was calculated according to Cha-um et al. [13]. Irrigation water had pH 7.41 and EC 0.42 ds/m. The soil used for our experiments had pH 7.94, EC 3.73 ds/m, 2.28% organic matter, nitrogen of 477 mg/kg, phosphorus of 8.31 mg/kg and potassium of 604.53 mg/kg.
Collection of rhizosphere from soil and plant root samples
Samples of soil rhizosphere were collected from the soil adhering to the roots. After 75 days from the time of planting, two plants were carefully uprooted individually and shaken into a sterile polythene bag. In another sterile polythene bag, the free roots of the rice plants were cut off at the base of each stem to obtain the root sample. Microbiological activity and distribution were stabilized during soil sampling and handling by keeping soil samples at 4 ℃. Plate count technique was applied using a nutrient agar medium [14] and dextrose–rose Bengal agar [15] to enumerate total bacteria and fungi count in respective order. Total actinomycetes were determined by the standard protocol [16]. Isolation and characterization of endophytic bacteria were carried out according to Woomer [17]. Different serial dilutions of sterilized macerated tissue were made up to 10–6 dilution and appropriate dilutions (100µL) were used for plated in triplicate and placed on different medium, Tryptic soy broth (TSB), yeast extract mannitol agar (YMA) [18], nutrient agar (NA) and starch casein agar media (SCA) [16]. After 2–3 days of incubation at 29 ± 1 °C, the independent colonies showed distinct colony morphology were picked and sampled again over the surface of their specific media to ensure purity of the culture. Further, bacterial isolates were also preserved on specific media slants at 4 °C for future use.
Enzymes activities
Measuring the activities of enzymes related to microbial activities were carried out in 50 g of soil. These enzymes included dehydrogenase, N cycling enzymes. Urease and nitrogenase, as well as P cycling enzyme: alkaline phosphatase. Each experiment was performed in triplicates.
Dehydrogenase activity was estimated as illustrated by Casida et al. [19], where dehydrogenase activity was expressed as (µg TPF.g−1 DW. h−1).
Urease activity was calculated by determination of the NH4+ released in the hydrolysis reaction after incubation of samples with urea (1%) as described by Tabatabai [20]. and expressed as (µmol NH4+g−1 h−1).
Nitrogen fixation: Nitrogenase activity in the rhizosphere was evaluated by the acetylene reduction as described by Somasegaran and Hoben [21]. Nitrogenase activity was expressed as (nmol C2H4 g−1 h−1).
Alkaline phosphatase was assessed as described in Tabatabai and Bremner [22] and its activity was expressed as (mg pNP g soil−1 h −1).
Characterization of endophytic bacteria
Screening of drought-tolerant bacteria
Tryptic soya broth (TSB) with different water potentials (−0.73, −1.0, −1.5, −2.0, MPa) was used to screen the isolates for drought stress tolerance. Appropriate concentrations of polyethylene glycol (PEG) 6000 were added to induce the different water potentials [23] Media were inoculated with the overnight-grown broth cultures with a population of around 1.5 × 107 CFU. ml−1 and incubated at 28 °C for 24 h, under shaking conditions. Optical Density (OD) values at 600 nm were used to determine the growth state of the isolates at various stress levels. Isolates that showed high optical density, were designated as more drought tolerant. Values of drought tolerance OD was determined as: highly sensitive OD < 0.3; sensitive OD = 0.3−0.4; tolerant OD = 0.4−0.5; highly tolerant OD > 0.5 [24].
Characterization of highly tolerant endophytic bacteria isolates for plant growth promotional (PGP) traits
IAA production
Salkowski’s reagent was used for quantity detection of IAA by colorimetric measurement at 530 nm as described by Acuña et al. [25]. Uninoculated broth was used as negative controls. The measurements were run in triplicate for each individual bacterium. Values are expressed in μg mL−1.
Test of exopolysaccharides (EPS) production
Three-day-old cultures of the highly tolerant isolates were analyzed for their ability to produce EPS. Optical density of each EPS sample was measured at a wavelength of 490 nm according to Dubois et al. [26]. The EPS production was expressed as (mg total carbohydrate. mg−1 protein).
Phosphate solubilization test
Test of phosphate solubilization of the selected isolates was performed by method of [27].
Identification of isolated strains by 16S rRNA gene sequencing
DNA of the bacterial isolates was extracted by Sarkosyl method [28]. Spectrophotometric examination was conducted for the determination of DNA concentration. 1% agarose gels stained with RedSafe DNA Stain was also used for the optical examination of DNA. The PCR amplification of the 16S rRNA gene was accomplished using Applied Biosystems 2720 thermal cycler (ABI, Foster City, USA). For 16S rRNA gene amplification, forward primer (27F: 5′-AGAGTTTGATCMTGGCTCAG-3′) and reverse primer 1492R: 5′-TACGGYTACCTTGTTACGACTT-3′ were used [29]. The PCR reaction mixture (25 μL) was composed of: 50 ng of isolated DNA, 2 μL of primers mix (10 μM of each primer), 1.5 μL of (25 mM) MgCl2, 5 μL of (5X) PCR buffer, 0.5 μL of (10 mM) dNTPs, 0.5 μL of (50 unit/μL) GoTaq® Flexi DNA Polymerase, Promega, USA, Cat. No. M8297 and the final volume was brought to 25 μL by using nuclease free water. The protocol for PCR amplification started with initial denaturation for 3 min at 95 °C, then 35 cycles of (denaturation for 30 s at 95 °C, annealing for 30 s at 53 °C, extension for 90 s at 72 °C), followed by a final extension step for 7 min at 72 °C. 1% agarose gels stained with RedSafe DNA Stain was used to resolve PCR products. QIAquick PCR purification kit (QIAgen Inc., USA) was used to purify PCR products following manufacturer's protocol. Purified PCR products were sequenced with forward 16S rRNA primer using ABI 3730XL genetic analyzer (Applied Biosystems, Foster City, USA) at Macrogene, Inc., (Seoul, South Korea). The Partial 16S rDNA gene sequences of 16S rRNA were BLAST searched with NCBI database [30]. Phylogenetic analysis of partial gene sequences was carried out using the MEGA7.0 software [31].
Bacterial inoculation experiment
Three plant growth-promoting bacterial strains: Bacillus megaterium (4E3), Pseudomonas azotoformans (3E9) and Rhizobium sp (4E11) were isolated from roots of drought-tolerant rice genotypes. Isolated strains were grown individually in a nutrient broth medium in flasks incubated at 28 ºC with shaking for 48 h until the late exponential phase. Isolated strains were used to inoculate drought-sensitive genotype (Giza177). Giza 177 is a popular rice genotype in Egypt, but showed high sensitivity under drought conditions. The germinated seeds separated into treated and non-treated. The treated seeds were soaked with bacterial strains individually using 4E3, 3E9 and 4E11 and with the mixture of (4E3 + 3E9 + 4E11) in Petri dish for 6 h. The germinated seeds were placed in pots content about 2 kg soil and watered to field capacity before planting the seeds. At time of planting seeds in pots the bacterial inoculation were given 5 mL per pot. After 10 days, the first pots without inoculated were irrigated up to water holding capacity of soil (control treatment), the second pots without inoculated was subjected for drought stress by withholding (stress treatment). All other pots were subjected to drought stress by withholding and inoculated with isolated strains individually or in consortium stat.
Determination the responses of sensitive genotype under inoculation conditions
Morphological and biochemical responses
Growth state of sensitive genotype was determined as changes in shoot and root length, fresh and dry weights, Membrane stability as rates of lipid peroxidation and membrane leakage. The concentration of thiobarbituric acid (TBA) reactive products equated with malondialdehyde (MDA) was evaluated as indicator to lipid peroxidation, as described by Zedan and Omar [32]. The leakage rate of leaves (EL) was determined as described by [33]. Photosynthesis pigments (total chlorophyll and carotenoids) content were determined as described by Arnon [34]. Changes in protein profiling pattern were detected using SDS-polyacrelymide gel electrophoreses according to Laemmli [35]. Crude total soluble proteins were extracted from seedling leaves according to Dure et al. [36] and the concentration of extracted protein was determined as described by Bradford [37]. Samples were standardized on protein amount depending on protein concentration (15 μg proteins were loaded per lane on the gel. BLUeye pre-stained molecular protein Ladder (GeneDirex) was used.
Semi-quantitative RT-PCR
To determine changes in gene expression, EZ-10 Spin Column Plant RNA Mini-Preps Kit (BIO BASIC CANADA INC) was used for total RNA extraction from rice seedlings. Three µg of DNase free total RNA was used for cDNA synthesis in 20 µL reaction mix using GoScript ™ reverse transcription Kit using Oligo (dT) 15primer. Actin cDNA (accession no. X16280) used as an internal constitutively expressed control (reference gene) using gene specific primers in PCR (Table 1). PCR reaction was carried out using 1 μL cDNA as a template in a 25 µL reaction volume according to the instructions supporting the GoTaq® Green master Mix, 2X (Promega USA). The general PCR program was; 94 °C for 5 min, followed by cycle of 94 °C for 1 min, 54−56 °C for 1 min, and 72 °C for 1 min, and last extension step of 72 °C for 7 min.
Real-time quantitative of gene expression with real-time PCR
Real-time PCR (RT-qPCR) analysis was carried out to confirm the induced changes in gene expression. Total RNA was extracted from 0.1 g of ground rice seedlings using IQeasy™ plus plant extraction kit according to the attached protocol. RNA quantity and purity were determined using Nano drop spectrophotometer (BioDrop µLITE.UK). RNA samples with 260/280 nm ratio more than 1.9 was considered as acceptable for RT-qPCR reactions. RNA quality and integrity were confirmed via electrophoresis on a 1.0% agarose gel. One μg of total RNA were used for the single-stranded cDNAs synthesis in a 20-µL reaction mix using oligo (dT) primer and the HiSenScript™ RH cDNA synthesis kit (iNtRON Biotechnology). RT-qPCR reactions were conducted using Topreal™ qPCR 2X pre Mix SYPER Green with low ROX (enzynomics- Korea) in 20 μL reaction volume. The reactions were run on a (Applied Biosystem™ Step One Plus™ Real Time PCR system) using rice actin gene (X16280) as an internal control. All tests on samples were conducted in three biological replicates using the same genes specific primers as sq-RTPCR (Table 1).
Statistical analysis
The results are presented as means ± standard errors (n = 3). The data were analyzed by two-way ANOVA using IBM® SPSS® Statistics program version 22 (SPSS inc., il, USA) for windows at P < 0.05 level to test the effects of irrigation and dry regime and genotype, as well as their interactions according to the following model:
where u is the overall mean, Ii is the fixed effect of ith irrigation and dry regime, Gj is the fixed effect of jth plant genotype, IGij is the interaction effect and Eijk is the random error. Mean were tested for significant differences using Duncan’s multiple range test.
Results
Soil biological activities
Population density
Characterization of isolated microbes indicated that drought stress might have both positive and negative effects on the diversity of microorganisms in the short term. Bacterial abundance in the rhizosphere varied according to the rice genotype. Data shown in Table 2 revealed that the highest abundance of bacteria was found in the rhizosphere of tolerant genotype (Orabi3) with 20.47 ± 1.97 × 106 CFU g–1 FW) while the lowest bacterial count was recorded in a sensitive one (IR64) with 17.00 ± 0.62 × 106 (cells g–1FW). All the rhizosphere soil samples consistently showed a significantly decrease in bacterial counts after they were subjected to drought stress. The percentage of that decrease ranged from 9.3% to 20% depending on rice genotype. The highest reduction rate was recorded in IR64 genotype. Rhizosphere fungal counts showed the same pattern of growth response to drought stress. The lowest fungal count was recorded with IR64 genotype with 2.67 ± 0.81 × 106 CFU g–1 FW). In contrast, drought has both positive and negative effect on actinomycetes counts. Under stress conditions significant increase in actinomycetes populations was recorded with Vandana genotype with 0.743 ± 0.085 × 106 CFU g−1 FW, while the lowest population number was recorded with IR64 genotype 0.330 ± 0.076 × 106 CFU g−1 FW. A significant decrease in bacterial and fungal counts was observed in the rhizosphere of all genotype after they were subjected to drought stress. Actinomycetes were the most resistance group to drought stress.
Soil enzymatic activities
Dehydrogenase activity (DHA)
Obtained results showed the effect of water deficit on soil DHA activity (Table 3) where significant differences (P ≤ 0.05) existed as the effects of drought on soil DHA activity. Under stressed conditions Orabi3 genotype recorded the highest mean value for soil DHA activity (98.21 ± 5.43 µg TPF g−1 DW h−1). This was followed by G177 genotype with a mean value of 83.06 ± 9.41 µg TPF g−1 DW h−1, which was not significantly different from Vandana genotype with a mean value of 77.30 ± 11.43 µg TPF g−1DW h−1. For IR64 genotype, the soil DHA activity value was recorded as the lowest mean value (59.85 ± 5 µg TPF g−1 DW.h−1) for soil DHA activity which differed significantly (P ≤ 0.05) from other genotypes.
Nitrogen cycling enzyme (urease and nitrogenase)
Data shown in Table 3 indicated that the observed decrease in urease activities under drought stress in all studied genotypes was significant. Orabi3 recorded high values of urease activities under drought stress compared with other studied genotypes. The lowest urease activity was recorded with IR64 genotype (447.56 ± 14.48 µmol N-NH3 g−1 soil h−1) this value was not significantly different from other genotypes' values. On the other hand, under drought stress, low nitrogen-fixing activity was obtained. The highest nitrogenase activity was recorded with Orabi3 genotype (5.65 ± 0.41 n mole C2H4 g−1soil h−1) followed by Vandana (4.14 ± 0.50 n mole C2H4 g−1 soil h−1). The lowest values were recorded with G177 and IR64 genotypes with 3.09 ± 0.82, 2.20 ± 0.91 n mole C2H4 g−1soil h−1, respectively (Table 3). Therefore, it can be suggested that the rice genotype significantly (P ≤ 0.05) affected the soil nitrogenase activity.
Alkaline phosphatase activity
The results of soil alkaline phosphatase activity (Table 3) showed that the drought treatment did not affect soil phosphatase activity. The enzyme activity is clearly influenced by the rice genotype. Under stressed conditions, the highest alkaline phosphatase value was recorded with Orabi3 genotype (0.48 ± 0.01 mg pNP g−1 soil h−1) which did not differ significantly from Vandana genotype with 0.44 ± 0.04 mg pNP g−1 soil h−1, IR64 genotype recorded the lowest alkaline phosphatase value with 0.28 ± 0.03 mg pNP g−1 soil h−1 which was not significantly different from the G177 genotype (0.30 ± 0.01 mg pNP g−1soil h−1).
Isolation and screening of drought-tolerant endophytic bacteria
Results regarding drought tolerance examined at different drought levels revealed that the isolates had variable drought tolerance potential. The overall number of bacterial cells decreased with increased in osmotic pressure in growth media. Screening of endophytic bacteria revealed that a total 106 bacterial strains that are observed starting at −0.73 MPa pressure were classified into four grouped categories. The first category included 47 isolates (44.34%) that were listed as very sensitive because of the decrease in osmotic pressure. The OD value of this group was decreased to < 0.3 at the osmotic pressure − 2.0 MPa; whereas, 13 isolates (12.27%) that have OD value between 0.310 and 0.368 were included in the sensitive class. The third category including 22 isolates (20.75%) which have OD value between 0.412 and 0.492 at the osmotic pressure − 2.0 MPa were classified as a tolerant class. Out of 106 isolated strains only 24 isolates (22.64%) were able to grown on all PEG-6000 with OD value > 5.0 unless at − 2.0 MPa and were selected as the highly tolerant isolates. Data presented in (Fig. 1) showed that, sensitive isolates were the most common with both tolerant and sensitive genotypes. The IR64 genotype recorded the highest value in the number of sensitive isolates. While the high tolerant isolates were associated with both of Orabi3 and Vandana genotypes with a significant increase compared to IR64 and G177 genotypes. Only the highly tolerant selected strains were subjected for further characterization through in vitro plant growth-promoting test. On the basis of morphological studies and microscopic examination of the 24 highly tolerant isolates the main morphological character; shape, color, elevation, surface and pigmentation are shown in Additional file 1: Table S1, also other characters as cell shape, motility, gram reaction and spore formation are shown in Additional file 1: Table S2.
Analysis of drought-tolerant isolate for IAA and EPS production and phosphate solubilization
All 24 drought-tolerant endophytic bacterial isolates were tested for qualitative IAA and EPS production. Isolates producing a pink color reaction with Salkowski’s reagent indicated their ability to produce IAA. Data shown in (Table 4) indicated that out of 24 endophytic bacterial isolates, 16 isolates produced significant amounts of IAA. Isolates, 3E9 and 4E3 tended to produce high amounts of IAA in the range of 44.60–46.60 μg mL−1 In the presence of tryptophan, significantly high amounts of IAA production was observed in 3E9 (46.60 μg mL−1) isolated from Vandana genotype followed by 4E3 (44.9 μg mL−1) isolated from Orabi3 genotype. In contrast, 1E3 and 2E6 were found to be a medium producer of IAA (20.33 and 17.50 µg mL−1) in comparison to the weak producer isolates 1E4, 1E7, 1E8, 2E2, 2E3, 2E11, 3E5, 3E6, 3E8, 3E10, 3E11 and 3E20; whereas, in the absence of tryptophan, 1E3 isolated from G177 produced significantly high amount of IAA (5.8 μg ml−1) followed by 2E6 (4.8 μg mL−1) isolated from IR64 (Table 4).
IAA is generally considered to be the most important native auxin. It may also function as an important signal molecule in the regulation of plant development. In our results, only 16 isolates out of 24 isolates were positive for IAA production (Table 4).
Drought-tolerant isolates were examined for their ability to produce EPS under control and drought stress conditions. Among 24 endophytic bacterial isolates, 1E3, 1E7, 2E3, 2E6, 2E11, 3E9, 3E11, 4E1 and 4E11 tended to produce high amounts of EPS (Table 4). Isolate 4E11 from Orabi3 genotype was the highest EPS producer (324.30 mg mg−1 protein) followed by 1E7 (227.33 mg mg−1 protein), 1E3 (201.42 mg mg−1 protein isolated from G177 genotype and 3E9 (190.80 mg mg−1 protein) isolated from Vandana genotype (Table 4) under level of metric stress.
The test of relative effectiveness of isolates in phosphate solubilization according to Mehta and Nautiyal [78] revealed that, among 24 drought-tolerant isolates, only 9 isolates displayed the phosphate-solubilizing activity by inducing clear zones on Pikovskaya’s agar plates. Data in Table 5 also revealed that the isolates 1E3, 1E4, 1E7, 1E8, 2E6, 3E9, 4E3, 4E10 and 4E11 have a high efficiency to dissolve phosphate. Isolate 4E3, which was isolated from Orabi3 genotype, was the most effective isolate (Table 5).
Identification of endophytic isolates
The nine most drought-tolerant endophytic isolates were tested for their taxonomically related microorganisms based on the 16S r DNA gene sequences to get more information about their taxonomical state. The closely aligned organisms according to the sequenced fragment and their accession no. are shown in Table 6.
Inoculation experiment
The inoculation experiment was designed according to the characterization analysis of all isolates. Three plant growth-promoting bacterial strains: Bacillus megaterium (4E3), Pseudomonas azotoformans (3E9) and Rhizobium sp. (4E11) which are isolated from roots of drought-tolerant rice genotypes (Orabi3) were used. These strains showed good values in their PGP traits. Inoculation plants of the sensitive genotype (Giza 177) with these selected strains caused a series of changes in its performance under drought stress conditions. Figure 2 illustrates the general changes in growth state as shoot growth and root lengths, and spread.
Plant growth parameters
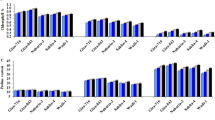
Changes in shoot and root growth we looked at as changes in shoots and roots lengths and fresh and dry weights of Giza 177 plants under three conditions: control, drought stress and inoculated with PGPRs under drought stress are shown in Fig. 3. Under control treatment, shoot length recorded the highest value. Other treatments showed a significant decrease in shoot length compared with the control and the most affected were drought stress treated and least affected were the treated inoculated with consortium strains (Fig. 3A).
Changes of shoot length (A), root length (B), shoot FW (C), shoot DW (D) root FW (E) and root DW (F) of Giza 177 genotype PGPRs under all experimental conditions: control, drought stress (stress) and inoculated with individual selected PGPRs (E3, E9 and E11) and consortium of three selected strains (Mix) under drought stress
Treatment with consortium strains showed the highest value in the length of root compared to the control (Fig. 3B). Other treatments showed significant decreased in the length of root, except 4E11 treatment which showed a non-significant decrease in root length. Determination of fresh and dry weights showed that plants under control treatment showed the highest value in shoot's FW and DW while other treatment showed a significant decreased in FW and DW value compared with the control.
The highest effect was noted under stress treatment, while inoculated treatments were less affected (Fig. 3C, D). Changes in root's FW and DW are shown in (Fig. 3E and F). Figure 3E shows changes in root FW, treatment with mix strains showing a non-significant increase in FW as compared to the control, while other treatments showed a significant decrease in FW as compared to the control. The most FW values affected were under stress treatment, while treatments inoculated with strains 4E3, 3E9 and 4E11 were less affected. The root DW shown in Fig. 3F revealed that treatment with mix strains showed highest values in DW with a non-significant increase compared with the control, while treatments inoculated with strain E11 showed non-significant decrease in DW compared with the control. On the other hand, both the stress treatments and inoculated treatments with strains 4E3 and 3E9 showed a significant decreased in DW compared with the control and the most affected were stress treatment while inoculated treatments with strains 4E3 and 3E9 were least affected. The inoculation effect of our isolated strains had a notably positive effect on growth measurements such as fresh and dry weights compared to uninoculated treatment.
Changes in membrane stability and photosynthesis pigments
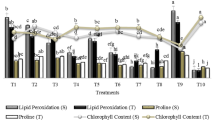
Changes in membrane stability as determined from the rate of electrolyte leakage (EL) and MDA content are shown in Fig. 4A, B. Stress treatment induced the highest values of MDA content and EL with a significant increase compared with the control. All inoculated treatments showed a significant increase at MDA (Fig. 4A) and EL (Fig. 4B) values comparing with control treatment but to a lesser extent than the stress treatment, except inoculated treatment with consortium strains which showed a non-significant increase in EL value compared with the control treatment. PGPR enhances the stability of cell membranes and improves plants performance under water-deficit condition.
Total chlorophyll and carotenoids of G177 genotype under three conditions: control, drought stress and inoculated with PGPRs under all experimental conditions: control, drought stress (stress) and inoculated with individual selected PGPRs (4E3, 3E9 and 4E11) and the consortium of three selected isolates (Mix) under drought stress
The effect of inoculating sensitive plants with drought-tolerant strains (4E3, 3E9 and 4E11) on photosynthesis pigments are estimated by changes in total chlorophyll and carotenoids contents (Fig. 4C). A significant reduction in total chlorophyll and carotenoid contents was found with stress treatment. Majority of inoculated treatments showed significant improvement in total chlorophyll and carotenoid contents over control treatment. The highest value of total chlorophyll was recorded in plants inoculated with 4E11 isolate with a significant increase over the control, while the lowest value for carotenoid was recorded in this treatment as it was significantly low (Fig. 4C).
Analysis of total protein
Electrophoresis analysis of total proteins fractions from leaves of all treated plants showed a series of changes in protein pattern under experimental conditions (Fig. 5). These changes included an increase in the expression (as increasing in bands volume and darkness) of some protein bands under stress and inoculated treatment compared with the control. Inoculated treatment with the three strains consortium showed a reduction in the expression of protein bands with low molecular weight of approximately 25–35 kDa; this change was similar to the control. Other changes in protein pattern were in the expression of new protein bands with low molecular weight of approximately (20–25 KDa) under stress treatment and both of the inoculated treatment with 4E3 and 3E9 while this new protein bands were less pronounced with the treatment of 4E11 and disappeared again under the three strains consortium treatment.
Semi-quantitative analysis (sqRT-PCR)
The applied sqRT-PCR analysis of some growth and stress-responsive genes: cytochrome c oxidase subunit 1 (COX1), glucosyltransferases, Rab-like GTPase activators, APETALA2-ethylene responsive element binding protein (AP2-EREBP), myotubularin (GRAM), natural resistance-associated macrophage protein 6 (NRAMP6), glutathione S-transferase (GST), no apical meristem (NAM), dehydrin (DHN), and Expansin genes (EXP1, EXP2 and EXP3) facilitates loosening of cell wall and hence root length. All studied genes showed differential expression under stress and inoculation conditions (Fig. 6). In general, inoculated plants with selected bacterial strains showed up regularly in most of the genes tested, compared with plants under stress without inoculation.
Semi-quantitative RT-PCR transcription pattern study of some stress-responsive genes in G177 plants under all experimental conditions: control, drought stress (stress) and inoculated with individual selected PGPRs (4E3, 3E9 and 4E11) and the consortium of the three selected strains (Mix) under drought stress
Real-time quantitative of gene expression with real-time PCR
Real-time PCR was conducted for precise detection of changes in relative expression of studied genes. Expression level was standardized on the actin gene (X16280) as the internal control. Relative expression of the genes studied is shown in (Fig. 7) where it is illustrated that the transcript amounts of COX1 gene (Fig. 7A) has decreased from 359 copies in the control treatment to 241.83 copies in plants under drought stress. The inhibition rate of drought condition on the expression level of COX1 gene was reduced in the inoculated plants. The lowest rate of inhibition was in the case of inoculated plants with 3E9 strain (341.07 copies).
Real-time PCR analysis changes in the expression level of some stress-responsive genes in G177 plants under all experimental conditions: control, drought stress (stress) and inoculated with individual selected PGPRs (4E3, 3E9 and 4E11) and the consortium of the three selected strains (Mix) under drought conditions. The expression level was standardized on the actin gene (X16280) as internal control. Data are given as means ± SE of relative expression for three biological replicates for each cDNA sample
Plants inoculated with each of 4E11 and the three strains consortium (Mix) strains showed a significant increase in regulation in the expression of AP2-EREBP (~453, 449 copies) compared to plants under control conditions (~300 copies) (Fig. 7B), while stressed uninoculated treatment and inoculated treatment with 4E3 and 3E9 strains showed a significant decreased in AP2-EREBP gene expression compared to plants under control condition. Drought stress induced slight increase in the expression of GRAM and NRAMP6 (Fig. 7C, D) genes compared to plants under control treatment. Gradual increase in the expression of these genes was recorded in inoculation treatments compared with both of control and uninoculated plants. The highest expression value of GRAM transcript was recorded in plants inoculated with 3E9 strain (~348 copies) (Fig. 7C). While the highest expression level of NRAMP6 transcript was noted in plants inoculated with mix isolates (~488 copies) almost threefold (Fig. 7D).
Expression of another studied gene, the NAM gene, showed a noticeable increase in its transcript amount in uninoculated and inoculated stressed plants compared with plants under the control conditions (Fig. 7E). The highest number of NAM transcript was recorded in plants inoculated with 4E11 strain.
The transcription level of GST as one of the main antioxidant genes involved in drought stress tolerance was analyzed (Fig. 7F). GST transcript amounts showed the highest value in plants inoculated with the 4E11 strain with a significant increase. Followed by uninoculated stressed plants while, inoculated treatment with 4E3 and mix strains showed a significant decreased in expression of GST gene compared to the control treatment. One of rice dehydrins (DHN) acts as protective protein family that maintains cell membrane stability and was involved in our investigation (Fig. 7G). DHN expression level in inoculated plants with mix strains was the highest (~331 copies) followed by plants treated with 4E11 strain (~326 copies).
Expansin genes are cell elongation proteins that facilitate loosening of cell wall and hence root length. The expression of EXP1 and EXP3 showed significant increase under both of uninoculated and inoculated stressed plants compared with the control (Fig. 7H, J). The highest expression of EXP1 was recorded in inoculated plants with mixed strains (almost twofold), while a maximum expression of EXP3 gene was found in 4E11-treated plants. In contrast, uninoculated stressed plants and inoculated plants with 3E9 strain decreased the expression of EXP2 gene compared with the control (Fig. 7I). This down regulation in EXP2 was accompanied with a decrease in root length in plants inoculated with 3E9 (Fig. 3). While inoculated plants with both of 4E3, 4E11 and mix strains recorded an increase of EXP2 gene expression and the highest value of expression was recorded in plants treated with a mixed strain.
Discussion
Responses of rice root microbiomes to drought stress were explored by [38]. Bacterial activity in drought soil continues to reduce as microbes die or fall into dormancy [39], this in turn affects crop production and sustainability of soil health. Increasing the antibiotic content of drought-treated soils is believed to be induced by drought-tolerant bacteria. Bacterial production of antibiotic is considered as one of bacterial mechanism to outcompete other bacteria under limited resources. It also could be signals to form biofilms as one of the drought-response pathways [40]. In addition, other compounds synthesized by bacteria under drought conditions effect on the aggregate stability of soil rhizosphere [41] and hydrophobicity [42]. Furthermore, bacteria differ in their ability to generate and accumulate osmolytes component that retain cellular turgid and save macromolecular structures [43]. Some of these compounds could be amino acids, such as glutamine, proline, glycine betaine and carbohydrates such as ectoine and trehalose [40, 44].
Water and nutrient availability are main elements that have a significant on the activities and survival of soil microbes [45, 46]. Positive relations between soil moisture content or water potential and microbial biomass or microbial activities have been demonstrated [47, 48].
Enzyme activity in the soil ecosystem is seen as a significant contributor to soil microbial activity [11, 49]. The enzymes activities of the soil were significantly influenced by drought stress and that effect was varied depending on rice genotype (Table 3). Soil enzyme activity is mainly restricted by availability of C, soil pH, the water profile, nutrients and the interactions between them [50]. For all living microbial cells, DHAs occur intracellular, and are linked to microbial respiratory processes. DHA activity in soil is also an indicator of overall soil microbial activity. Data offered in (Table 3) clearly indicated the negative effect of drought stress on soil DHA activity along with all rice genotypes. While plants of Orabi3 genotype showed the highest value of DHA activity under drought conditions compared with other genotypes, these results were linked with the results of tolerant and high tolerant microbes associated with Orabi3 genotype, which could maintain their activity under drought conditions; whereas, most microbes associated with IR64 genotype were a highly sensitive and sensitive microbe to drought stress, so IR64 genotype showed a lowest value of DHA activity under drought conditions. This is in accordance with many authors who reported that DHA is strongly influenced by water content and its activity reduced with the reduction of soil moisture [51, 52]. It was found that soil dehydrogenase activity increased threefold with increasing soil water content and was highly influenced by both redox potential and oxygen diffusion rate [53]. Soil water content suggested that it influenced the DHA by affecting the oxidation–reduction status of the soil.
Nitrogen affects multiple features of plant development and metabolic pathways where it is considered as an essential plant macronutrient [54, 55]. It is well known that nitrogen types improve drought tolerance in rice by photosynthesis and root water uptake [56, 57]. Data presented in (Table 3) clearly indicate the inhibition effect of drought stress on urease activities in all studied genotypes. Urease is common in soil environments and a great range of organisms such as several bacterial species, algae, fungi, plants and vertebrates generate urease [58]. Soil urease has some significance in agriculture where it has been indicated that urease immobilization causes an increase in enzymatic stability against proteolytic enzymes and temperature [59, 60]. Regarding the soil nitrogenase activity as shown in Table 3, soil nitrogenase activity is significantly affected by drought stress and this effect was genotype dependent. Our results are in accordance with those of [61] who found that the presence of rice plants enhanced nitrogenase activity of heterotrophic bacteria. The genotype which had the bigger root weight and the minor shoot weight and enabled the larger production of methane had the greater activity of nitrogenase. Nitrogen fixation is extremely variable depending on the associated diazotroph and the plant genotype. On the other hand, the determinant factor is the host plant exerts which supply the carbon and energy source for bacterial growth and nitrogen fixation [62]. Nitrogen fixation is a high energy consuming process. Therefore, it can be suggested that the rice genotype significantly (P ≤ 0.05) affected the soil nitrogenase activity.
Phosphatase activity plays an essential role in soil P cycles, which is associated with P stress and plant development [63]. The results of soil alkaline phosphatase activity (Table 3) showed that the drought condition did not change soil phosphatase activity. The enzyme activity is clearly influenced by rice genotype. It was shown that soil phosphatase activities were influenced by drought and warming together; where the drought treatment alone did not induce any changes soil phosphatase activities or Pi availability [64]. Changes in phosphatases activities and phosphorus uptake among cereal are genotypic dependent [65, 66].
Drought-tolerant endophytic isolates were mostly associated with both of Orabi3 and Vandana (tolerant genotype) with significant increased compared to IR64 and G177 (sensitive genotypes) (Fig. 1). The high tolerant isolates are the most important, as they can remain active under stress conditions and supply the plant with some compounds that help the plant to alleviate stress. Characterization of highly tolerant selected strains through in vitro plant growth-promoting test pointed to the role of these isolates in IAA and EPS production and phosphate-solubilizing capacity. IAA produced by PGPR depending on culture conditions, growth stage and substrate availability and species or strain variation [67]. Isolates from the rhizosphere are more effective in auxin production than others from the bulk soil [68]. Inoculation of numerous plant species by IAA-producing bacteria induced an improvement in root growth and/or an enhancement in developing of lateral roots and roots hairs [8]. Under stress condition, the cells' energy flow is directed towards defensive mechanisms, which may influence their growth pattern [69]. EPS is generated by microorganisms under various environmental stresses such as salinity, heat, drought and heavy metal stress [70, 71]. Some PGPRs are able to synthesize EPS. These support plant development and growth by easing the circulation of nutrients and defending the plant from pathogen attack. EPS-generating microbes could also help in protection against drought constitute shielding from desiccation and the plant invasion defense response in plant–microbe interactions [72]. The high efficiency of some isolates in dissolving phosphate may be due to its ability to exert organic acid phosphorus as one of the main nutrients in demand for plants. In soil, most of phosphorus is present as insoluble phosphates and cannot be consumed by the plants [73]. Bacteria's ability to solubilize mineral phosphates can enhance phosphorus and iron availability for the plant, which is a possible mechanism for the promotion of plant growth under field conditions [74]. Solubilization can be achieved through a number of mechanisms as well as excretion of metabolites, such as proton extrusion, organic acids and chelating agent production. Phosphate solubilization by PSB strains is related to the release of low molecular weight gluconic acid and ketogluconic [75, 76] that chelates phosphate-bound cations through their carboxyl and hydroxyl groups changing them into soluble forms. Hydrochloric acid, as with other inorganic acids, is less effective in phosphate solubilization than organic acids at the same pH [77]. Inoculation of soil with phosphate-solubilizing bacteria resulted in increased uptake of phosphorus by the plant subsequently increasing the yield [78]. Bacterial isolates belonging to the genera Enterobacter, Pseudomonas, Serratia and Bacillus have been reported for their ability for solubilization of insoluble phosphate compounds [79, 80]. Similar PGPB isolates belonging to the Pseudomonas and Bacillus genera have recently been reported to improve plant growth and tolerance to drought stress in green gram plants [81].
Inoculation of the selected characterized strains to sensitive genotype had a marked positive effect on growth parameters such as fresh, dry weights and other biochemical and molecular parameters.
The enhancement in tissue mass and root number after inoculation treatment may promote the wider and deeper reach of plant roots for water further away and increase the area of nutrient absorption. This helps plants to survive under water-deficient conditions. Numerous studies indicated that PGPRs-inoculated plants showed an improvement in plant qualities and increased stress tolerance [11, 82]. Increasing root length was associated with the role of some bacteria in the enhancement of cell membrane elasticity in root cells subsequently enhancing the tolerance to drought stress [8]. Synthesis of phytohormones by PGPRs stimulates plant cell growth and division which support the increase in plant height and tissue mass and induce tolerance against environmental stresses [83]. Inoculation of plants with IAA-producing bacteria was associated with the improvement of root growth rate and/or formation of lateral roots and root hairs in various plant species [8, 84] which help plants to adapt to drought condition [85]. Considering the particular significance of phosphorous in rice growth and the development where it plays a key role in most physiological processes including photosynthesis, cell division and development of root system. This suggests the effective role of PGPRs in phosphate solubilization and its relation in improving plant performance under water-deficit conditions [11, 75, 82]. EPS produced by PGPR were reported with their thoughtful effects on plant growth and drought tolerance [86].
Treatment with microbial isolates improves the stability of the plant cell membranes as determined from the values of EL rate and MDA content. These effects could be related to the activation of antioxidant defense system in inoculated plants. Using PGPRs in some rice genotypes improved plant growth and induced some stress-related enzymes (SOD, CAT, POD, APX), this subsequently reduced the levels of H2O2 and MDA under drought stress compared to the control [87]. Inoculation of plans under field condition with Pseudomonas sp. caused a significant increase in the CAT, GPX and APX enzyme activities under drought stress. That explains the role of PGPR in adapting plants to drought stress [88]. Inoculation of sensitive genotype with isolated strains has an effect on photosynthesis process and induces changes in photosynthesis pigments. Increases in the chlorophyll content of inoculated plants reflect the improvement in the photosynthetic efficiency and could induce tolerance of drought stress. Increased total chlorophyll and carotenoid content in inoculated plants with PGPRs is supported by many previous studies [89, 90]. Increasing photosynthesis pigments in inoculated treatments support our obtained results related to the improvement in fresh and dry weights in inoculated treatments as a result of metabolism improvement [91].
Changes in protein pattern of separated bands on the gel under experimental conditions (Fig. 5) reveal the association of inoculation of microbial isolates with induced changes. These changes included increases in the expression (as increasing in bands volume and darkness) of some protein bands under stress and inoculated treatment compared with the control. Changes in protein content were reported with enhancement in root and shoot length as well as the biomass in plants inoculated with some PGPRs [90]. Six differentially expressed proteins associated with PGPRs inoculation were detected in pepper plants under water-deficit conditions, genes of sHSP, Cadhn, CaPR-10 and VA as stress-related proteins were identified and showed an increase in their transcript amount (1.5-fold) in inoculated plants compared with non-inoculated plants [92].
Semi-quantitative as well as real-time PCR analysis of some growth and stress-responsive genes confirmed the differential expression of all studied genes under stress and inoculation conditions (Fig. 6). In general, inoculated plants with selected bacterial strains showed upregulation of most tested genes compared with plants under stress without inoculation. Expression pattern of studied genes was considerable in association with our morphological and biochemical results. Alteration in the transcript amount of studied genes in this investigation was related to their role in protecting and improving the plant performance under stress conditions. COX1 gene is a mitochondrial DNA coding for subunit of respiratory complex IV which is a biological trigger in the mitochondrial oxidative phosphorylation electron transport chain. COX1 protein works to regulate the metabolism of carbohydrates, nitrogen and energy. The protein COX1 functions as a scavenging agent for ROS and is involved in mRNA and protein processing [93]. Our results are in accordance with those of [94] who reported that, the COX1 gene showed upregulation in the rice plant under drought stress conditions inoculated with Pseudomonas fluorescens. Upregulation in the expression of AP2-EREBP in inoculated plants with each of 4E11 and the mix is agreed with its role as a transcription factor. AP2-EREBP is a multigenes family of transcription factors. This transcription factor regulates several developmental processes throughout the plant’s life cycle and controls various stress-related responses [95]. Overexpression of AP2-EREBPs in Arabidopsis showed enhanced drought stress tolerance [96]. It was found that the expression of the AP2-EREBP gene was influenced by both inoculation treatment and water stress conditions [94]. Alteration in relative expression of GRAM transcript was associated with the improvement of membrane stability in inoculated plants. GRAM domain is likely to be implicated in membrane-related processes such as lipid-binding signals or intracellular protein [97]. Certain phytohormones and abiotic stresses are reactive to GRAM-domain containing genes [98]. Recent results are in agreement with [99] where the expression of GRAM was increased in both uninoculated and inoculated rice seedlings under drought stress. Interestingly, a more recent study pointed to the increase in GRAM expression as highly detectable in inoculated plants compared with uninoculated ones. In plants, NRAMP genes are known to encode intracellular metal transporters capable of transporting both the metal nutrient. According to our results, increased expression of NRAMP gene may indicate to the possible role of the NRAMP gene in regulation of stress alleviation in rice. NAM gene as another studied transcription factor showed a noticeable increase in uninoculated and inoculated stressed plants compared with plants under the control conditions. NAM is a transcription factor and has been confirmed to play a major role in abiotic stress, with tolerance in a range of crops [100]. Increased expression of the NAM gene with exposure to different stresses in rice was recorded by [90, 101]. Increased expression of NAM gene as a response to inoculation treatment was in line with [90, 102]. Changes in GST transcript amounts as an important antioxidant gene involved in drought stress tolerance could help to evaluate the role of microorganism's inoculation in acquisition of drought tolerance. Up regulation in GST expression in rice plants inoculated with P. fluorescens was reported by [103]. GSTs are antioxidant enzymes that help to detoxify by transforming oxidative form compounds into reduced glutathione and thus promote their elimination, sequestration or metabolism [104]. Our results pointed to the evidence that IAA was more highly produced in the tolerant used strains. Considering the role of IAA in upregulation of DHN as a protective protein family that maintains cell membrane stability [99] could explain the upregulation of DHN expression in inoculated rice seedlings rather than uninoculated ones. Dehydrin gene mainly contributes to stress tolerance and thus functions as a marker gene for plant stress response [90]. Overexpression of these genes in several crop plants has been reported to support tolerance to a variety of abiotic stresses [105].
Expansin genes are cell elongation proteins that facilitate loosening of cell wall and hence root length. The obtained results about the three studied expansin genes were in harmony with morphological analysis of root length (Fig. 3) where it was associated with the increasing in root growth and/or enhancement in formation of lateral roots and root hairs, thus guaranteeing the water and nutrient uptake and helping plants to adapt to drought stress [85]. Expansins, a class of family of pH-dependent proteins, play a part in the proliferation and development of cell walls. In general, the expansion of glucan-coated cellulose in the cell wall is believed to cause reversible disruption of the hydrogen bond between cellulose microfibrils and glucan matrix, resulting in cell elongation by increasing the extensibility of the cell wall [106]. Expansins are also implicated in cell wall changes and cell expansion stimulated by phytohormones and also some biotic and abiotic stresses [107]. The functions of expansin genes in plant development and stress tolerance have given plant breeding opportunities to control leaf size, fruit production, root formation and tolerance to various stresses [108].
Conclusion
Rice is one of the most water consuming crops. Reducing irrigation water and improving performance under water-deficit conditions is a major challenge. Some components produced by drought-tolerant microorganisms could play an important role in acquiring drought tolerance in plants. Interestingly, our data indicated a positive interaction between drought-tolerant genotypes and the increased number of drought-tolerant microorganisms, especially bacteria and actinomycetes. The most drought-tolerant rhizobacteria were isolated from the drought-tolerant genotype (Orabi3), characterized, and then used in the inoculation of the sensitive genotype (Giza177). This inoculation resulted in an improvement in drought tolerance of the inoculated plants under drought conditions. This improvement was related to the changes in the expression level of some stress and growth-related genes (COX1, AP2-EREBP, GRAM, NRAMP6, NAM, GST, DHN, EXP1, EXP2 and EXP3) in the inoculated plants. Our results clearly indicate that some mechanisms of drought stress tolerance in rice largely depend on the genotype of the microbes. We report that improvement of drought stress tolerance status in rice could be achieved by inoculating the soil with drought-tolerant PGPRs.
Availability of data and materials
Not applicable.
References
Molden D, Oweis T, Steduto P, Bindraban P, Hanjra MA, Kijne J. Improving agricultural water productivity: between optimism and caution. Agric Water Manag. 2010;97(4):528–35.
Wang JH, Geng LH, Zhang CM. Research on the weak signal detecting technique for crop water stress based on wavelet denoising. In: Advanced Materials Research: 2012. Trans Tech Publ; pp. 966–970.
Foley JA, Ramankutty N, Brauman KA, Cassidy ES, Gerber JS, Johnston M, Mueller ND, O’Connell C, Ray DK, West PC. Solutions for a cultivated planet. Nature. 2011;478(7369):337–42.
Ray DK, Gerber JS, MacDonald GK, West PC. Climate variation explains a third of global crop yield variability. Nat Commun. 2015;6(1):1–9.
Gray E, Smith D. Intracellular and extracellular PGPR: commonalities and distinctions in the plant–bacterium signaling processes. Soil Biol Biochem. 2005;37(3):395–412.
Hayat R, Ali S, Amara U, Khalid R, Ahmed I. Soil beneficial bacteria and their role in plant growth promotion: a review. Ann Microbiol. 2010;60(4):579–98.
Mapelli F, Marasco R, Rolli E, Barbato M, Cherif H, Guesmi A, Ouzari I, Daffonchio D, Borin S. Potential for plant growth promotion of rhizobacteria associated with salicornia growing in Tunisian hypersaline soils. BioMed Res Int. 2013;2013:1.
Dimkpa C, Weinand T, Asch F. Plant–rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Environ. 2009;32(12):1682–94.
Ahemad M, Kibret M. Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. J King Saud Univ Sci. 2014;26(1):1–20.
Balloi A, Rolli E, Marasco R, Mapelli F, Tamagnini I, Cappitelli F, Borin S, Daffonchio D. The role of microorganisms in bioremediation and phytoremediation of polluted and stressed soils. Agrochimica. 2010;54(6):353–69.
Mekonnen H, Kibret M. The roles of plant growth promoting rhizobacteria in sustainable vegetable production in Ethiopia. Chem Biol Technol Agric. 2021;8(1):1–11.
Mapelli F, Marasco R, Balloi A, Rolli E, Cappitelli F, Daffonchio D, Borin S. Mineral–microbe interactions: biotechnological potential of bioweathering. J Biotechnol. 2012;157(4):473–81.
Cha-um S, Yooyongwech S, Supaibulwatana K. Water-deficit tolerant classification in mutant lines of indica rice. Scientia Agricola. 2012;69(2):135–41.
Rodriguez-Valera F, Ventosa A, Juez G, Imhoff JF. Variation of environmental features and microbial populations with salt concentrations in a multi-pond saltern. Microb Ecol. 1985;11(2):107–15.
Martin JP, Harding RB. Comparative effects of two bacterial growth preventives, acid (pH 4) and rose bengal plus streptomycin, on the nature of soil fungi developing on dilution plates 1. Soil Sci Society Am J. 1951;15(2):159–62.
Küster E, Williams S. Selection of media for isolation of streptomycetes. Nature. 1964;202(4935):928–9.
Woomer PL. Most probable number counts. Methods of Soil Analysis: Part 2 Microbiological and Biochemical Properties 1994, 5:59–79.
Vincent JM. A manual for the practical study of the root-nodule bacteria. A manual for the practical study of the root-nodule bacteria 1970.
Casida L Jr, Klein D, Santoro T. Soil dehydrogenase activity. Soil Sci. 1964;98(6):371–6.
Tabatabai M. Soil enzymes. Methods of soil analysis: part 2 microbiological and biochemical properties 1994, 5:775–833.
Somasegaran P, Hoben HJ. Quantifying the growth of rhizobia. In: Handbook for rhizobia. Springer; 1994; pp. 47–57.
Tabatabai M, Bremner J. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol Biochem. 1969;1(4):301–7.
Busse MD, Bottomley PJ. Growth and nodulation responses of Rhizobium meliloti to water stress induced by permeating and nonpermeating solutes. Appl Environ Microbiol. 1989;55(10):2431–6.
Alikhani H, Mohamadi L. Assessing tolerance of rhizobial lentil symbiosis isolates to salinity and drought in dry land farming condition. In: 19th world congress of soil science, soil solutions for a changing world. 2010; pp. 1–6.
Acuña J, Jorquera M, Martínez O, Menezes-Blackburn D, Fernández M, Marschner P, Greiner R, Mora M. Indole acetic acid and phytase activity produced by rhizosphere bacilli as affected by pH and metals. J Soil Sci Plant Nutr. 2011;11(3):1–12.
Dubois M, Gilles KA, Hamilton JK, Rebers PT, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28(3):350–6.
Pikovskaya R. Mobilization of phosphorus in soil in connection with the vital activity of some microbial species. 1948.
Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harber Laboratory 1982.
Frank JA, Reich CI, Sharma S, Weisbaum JS, Wilson BA, Olsen GJ. Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Appl Environ Microbiol. 2008;74(8):2461.
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215(3):403–10.
Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–4.
Zedan A, Omar S. Nano selenium: reduction of severe hazards of Atrazine and promotion of changes in growth and gene expression patterns on Vicia faba seedlings. African J Biotechnol. 2019;18:502-510.
Omar SA, Elsheery NI, Kalaji HM, Xu Z-F, Song-Quan S, Carpentier R, Lee C-H, Allakhverdiev SI. Dehydroascorbate reductase and glutathione reductase play an important role in scavenging hydrogen peroxide during natural and artificial dehydration of Jatropha curcas seeds. J Plant Biol. 2012;55(6):469–80.
Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase Beta Vulgaris Plant Physiol. 1949;24(1):1.
Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–5.
Dure L III, Greenway SC, Galau GA. Developmental biochemistry of cottonseed embryogenesis and germination: changing messenger ribonucleic acid populations as shown by in vitro and in vivo protein synthesis. Biochemistry. 1981;20(14):4162–8.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–54.
Santos-Medellín C, Edwards J, Liechty Z, Nguyen B, Sundaresan V. Drought stress results in a compartment-specific restructuring of the rice root-associated microbiomes. MBio. 2017;8(4):e00764-e1717.
Alster CJ, German DP, Lu Y, Allison SD. Microbial enzymatic responses to drought and to nitrogen addition in a southern California grassland. Soil Biol Biochem. 2013;64:68–79.
Bouskill NJ, Wood TE, Baran R, Ye Z, Bowen BP, Lim H, Zhou J, Nostrand JDV, Nico P, Northen TR. Belowground response to drought in a tropical forest soil. I. Changes in microbial functional potential and metabolism. Front Microbiol. 2016;7:525.
Kohler J, Caravaca F, Roldán A. Effect of drought on the stability of rhizosphere soil aggregates of Lactuca sativa grown in a degraded soil inoculated with PGPR and AM fungi. Appl Soil Ecol. 2009;42(2):160–5.
Elbl J, Plošek L, Kintl A, Hynšt J, Javoreková S, Záhora J, Charousová I. Effects of drought on microbial activity in rhizosphere, soil hydrophobicity and leaching of mineral nitrogen from arable soil depending on method of fertilization. World Acad Sci Eng Technol. 2014;8(8):741–7.
Welsh DT. Ecological significance of compatible solute accumulation by micro-organisms: from single cells to global climate. FEMS Microbiol Rev. 2000;24(3):263–90.
Elsakhawy TA, Nashwa A, Ghazi AA. The potential use of ectoine produced by a moderately halophilic bacteria Chromohalobacter salexigens KT989776 for enhancing germination and primary seedling of flax “Linum usitatissimum L.” under salinity conditions. Biotechnol J Int. 2019. https://doi.org/10.9734/bji/2019/v23i330078.
Daniel R. The metagenomics of soil. Nat Rev Microbiol. 2005;3(6):470–8.
Libudzisz Z, Kowal K, Żakowska Z. Mikrobiologia techniczna. T. 1, Mikroorganizmy i środowiska ich występowania: Wydawnictwo Naukowe PWN; 2007.
Orchard VA, Cook F. Relationship between soil respiration and soil moisture. Soil Biol Biochem. 1983;15(4):447–53.
Skopp J, Jawson M, Doran J. Steady-state aerobic microbial activity as a function of soil water content. Soil Sci Soc Am J. 1990;54(6):1619–25.
DeForest JL, Smemo KA, Burke DJ, Elliott HL, Becker JC. Soil microbial responses to elevated phosphorus and pH in acidic temperate deciduous forests. Biogeochemistry. 2012;109(1–3):189–202.
Fernández-Calviño D, Soler-Rovira P, Polo A, Díaz-Raviña M, Arias-Estévez M, Plaza C. Enzyme activities in vineyard soils long-term treated with copper-based fungicides. Soil Biol Biochem. 2010;42(12):2119–27.
Gliński J, Stępniewski W, Stępniewska Z, Włodarczyk T, Brzezińska M. Characteristics of aeration properties of selected soil profiles from central Europe. Int Agrophys. 2000;14(1):17–31.
Pascual J, Garcia C, Hernandez T, Moreno J, Ros M. Soil microbial activity as a biomarker of degradation and remediation processes. Soil Biol Biochem. 2000;32(13):1877–83.
Brzezińska M, Stępniewska Z, Stępniewski W. Dehydrogenase and catalase activity of soil irrigated with municipal wastewater. Pol J Environ Stud. 2001;10(5):307–11.
Xu Z, Zhou G, Shimizu H. Plant responses to drought and rewatering. Plant Signal Behav. 2010;5(6):649–54.
Wang M, Shen Q, Xu G, Guo S. New insight into the strategy for nitrogen metabolism in plant cells. In: International review of cell and molecular biology, Vol 310. Elsevier; 2014; pp. 1–37.
Li Y, Ren B, Yang X, Xu G, Shen Q, Guo S. Chloroplast downsizing under nitrate nutrition restrained mesophyll conductance and photosynthesis in rice (Oryza sativa L.) under drought conditions. Plant Cell Physiol. 2012;53(5):892–900.
Ding L, Li Y, Wang Y, Gao L, Wang M, Chaumont F, Shen Q, Guo S. Root ABA accumulation enhances rice seedling drought tolerance under ammonium supply: interaction with aquaporins. Front Plant Sci. 2016;7:1206.
Booth JL, Vishniac H. Urease testing and yeast taxonomy. Can J Microbiol. 1987;33(5):396–404.
Ciurli S, Marzadori C, Benini S, Deiana S, Gessa C. Urease from the soil bacterium Bacillus pasteurii: immobilization on Ca-polygalacturonate. Soil Biol Biochem. 1996;28(6):811–7.
Zantua M, Bremner J. Stability of urease in soils. Soil Biol Biochem. 1977;9(2):135–40.
Habte M, Alexander M. Effect of rice plants on nitrogenase activity of flooded soils. Appl Environ Microbiol. 1980;40(3):507–10.
Rosenblueth M, Ormeño-Orrillo E, López-López A, Rogel MA, Reyes-Hernández BJ, Martínez-Romero JC, Reddy PM, Martínez-Romero E. Nitrogen fixation in cereals. Front Microbiol. 2018;9:1794.
Tao J, Griffiths B, Zhang S, Chen X, Liu M, Hu F, Li H. Effects of earthworms on soil enzyme activity in an organic residue amended rice–wheat rotation agro-ecosystem. Appl Soil Ecol. 2009;42(3):221–6.
Sardans J, Peñuelas J, Estiarte M. Warming and drought alter soil phosphatase activity and soil P availability in a Mediterranean shrubland. Plant Soil. 2006;289(1–2):227–38.
Osborne L, Rengel Z. Genotypic differences in wheat for uptake and utilisation of P from iron phosphate. Aust J Agric Res. 2002;53(7):837–44.
Liu W, Hou Y, Zhan X, Li G, Zhang S. Comparison of rhizosphere impacts of wheat (Triticum aestivum L.) genotypes differing in phosphorus efficiency on acidic and alkaline soils. Commun Soil Sci Plant Anal. 2012;43(6):905–11.
Mirza MS, Ahmad W, Latif F, Haurat J, Bally R, Normand P, Malik KA. Isolation, partial characterization, and the effect of plant growth-promoting bacteria (PGPB) on micro-propagated sugarcane in vitro. Plant Soil. 2001;237(1):47–54.
Sarwar M, Kremer R. Determination of bacterially derived auxins using a microplate method. Lett Appl Microbiol. 1995;20(5):282–5.
Räsänen LA, Saijets S, Jokinen K, Lindström K. Evaluation of the roles of two compatible solutes, glycine betaine and trehalose, for the Acacia senegal–Sinorhizobium symbiosis exposed to drought stress. Plant Soil. 2004;260(1–2):237–51.
Priester JH, Olson SG, Webb SM, Neu MP, Hersman LE, Holden PA. Enhanced exopolymer production and chromium stabilization in Pseudomonas putida unsaturated biofilms. Appl Environ Microbiol. 2006;72(3):1988–96.
Sheng G, Yu H, Yue Z. Factors influencing the production of extracellular polymeric substances by Rhodopseudomonas acidophila. Int Biodeterior Biodegradation. 2006;58(2):89–93.
Afrasayab S, Faisal M, Hasnain S. Comparative study of wild and transformed salt tolerant bacterial strains on Triticum aestivum growth under salt stress. Braz J Microbiol. 2010;41(4):946–55.
Pradhan N, Sukla L. Solubilization of inorganic phosphates by fungi isolated from agriculture soil. Afr J Biotechnol. 2006;5(10):850-854.
Verma SC, Ladha JK, Tripathi AK. Evaluation of plant growth promoting and colonization ability of endophytic diazotrophs from deep water rice. J Biotechnol. 2001;91(2–3):127–41.
Shahab S, Ahmed N. Effect of various parameters on the efficiency of zinc phosphate solubilization by indigenous bacterial isolates. Afr J Biotechnol. 2008;7(10):1543-1549.
Kim KY, Jordan D, Krishnan HB. Rahnella aquatilis, a bacterium isolated from soybean rhizosphere, can solubilize hydroxyapatite. FEMS Microbiol Lett. 1997;153(2):273–7.
Rodrı́guez H, Fraga R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv. 1999; 17(4–5): 319–339.
Mehta S, Nautiyal CS. An efficient method for qualitative screening of phosphate-solubilizing bacteria. Curr Microbiol. 2001;43(1):51–6.
Frey-Klett P, Chavatte M, Clausse ML, Courrier S, Roux CL, Raaijmakers J, Martinotti MG, Pierrat JC, Garbaye J. Ectomycorrhizal symbiosis affects functional diversity of rhizosphere fluorescent pseudomonads. New Phytol. 2005;165(1):317–28.
Hameeda B, Harini G, Rupela O, Wani S, Reddy G. Growth promotion of maize by phosphate-solubilizing bacteria isolated from composts and macrofauna. Microbiol Res. 2008;163(2):234–42.
Saravanakumar D, Kavino M, Raguchander T, Subbian P, Samiyappan R. Plant growth promoting bacteria enhance water stress resistance in green gram plants. Acta Physiol Plant. 2011;33(1):203–9.
Van Oosten MJ, Pepe O, De Pascale S, Silletti S, Maggio A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem Biol Technol Agric. 2017;4(1):1–12.
Glick BR. Plant growth-promoting bacteria: mechanisms and applications. Scientifica. 2012;2012:1-15.
Mantelin S, Touraine B. Plant growth-promoting bacteria and nitrate availability: impacts on root development and nitrate uptake. J Exp Bot. 2004;55(394):27–34.
Egamberdieva D, Kucharova Z. Selection for root colonising bacteria stimulating wheat growth in saline soils. Biol Fertil Soils. 2009;45(6):563–71.
Roberson EB, Firestone MK. Relationship between desiccation and exopolysaccharide production in a soil Pseudomonas sp. Appl Environ Microbiol. 1992;58(4):1284–91.
Gusain YS, Singh U, Sharma A. Bacterial mediated amelioration of drought stress in drought tolerant and susceptible cultivars of rice (Oryza sativa L.). Afr J Biotechnol. 2015;14(9):764–73.
Heidari M, Golpayegani A. Effects of water stress and inoculation with plant growth promoting rhizobacteria (PGPR) on antioxidant status and photosynthetic pigments in basil (Ocimum basilicum L.). J Saudi Society Agric Sci. 2012;11(1):57–61.
Gururani MA, Upadhyaya CP, Baskar V, Venkatesh J, Nookaraju A, Park SW. Plant growth-promoting rhizobacteria enhance abiotic stress tolerance in Solanum tuberosum through inducing changes in the expression of ROS-scavenging enzymes and improved photosynthetic performance. J Plant Growth Regul. 2013;32(2):245–58.
Tiwari S, Lata C, Chauhan PS, Nautiyal CS. Pseudomonas putida attunes morphophysiological, biochemical and molecular responses in Cicer arietinum L. during drought stress and recovery. Plant Physiol Biochem. 2016;99:108–17.
Nkebiwe PM, Weinmann M, Müller T. Improving fertilizer-depot exploitation and maize growth by inoculation with plant growth-promoting bacteria: from lab to field. Chem Biol Technol Agric. 2016;3(1):1–16.
Lim J-H, Kim S-D. Induction of drought stress resistance by multi-functional PGPR Bacillus licheniformis K11 in pepper. Plant Pathol J. 2013;29(2):201.
Yan S, Tang Z, Su W, Sun W. Proteomic analysis of salt stress-responsive proteins in rice root. Proteomics. 2005;5(1):235–44.
Saakre M, Baburao TM, Salim AP, Ffancies RM, Achuthan VP, Thomas G, Sivarajan SR. Identification and characterization of genes responsible for drought tolerance in rice mediated by Pseudomonas Fluorescens. Rice Sci. 2017;24(5):291–8.
Riechmann JL, Meyerowitz EM. The AP2/EREBP family of plant transcription factors. Biol Chem. 1998;379:633–46.
Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K, Shinozaki K. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought-and low-temperature-responsive gene expression, respectively. Arabidopsis Plant Cell. 1998;10(8):1391–406.
Jiang S-Y, Ramamoorthy R, Ramachandran S. Comparative transcriptional profiling and evolutionary analysis of the GRAM domain family in eukaryotes. Dev Biol. 2008;314(2):418–32.
Baron KN, Schroeder DF, Stasolla C. GEm-Related 5 (GER5), an ABA and stress-responsive GRAM domain protein regulating seed development and inflorescence architecture. Plant Sci. 2014;223:153–66.
Tiwari S, Lata C, Singh Chauhan P, Prasad V, Prasad M. A functional genomic perspective on drought signalling and its crosstalk with phytohormone-mediated signalling pathways in plants. Curr Genomics. 2017;18(6):469–82.
Nakashima K, Ito Y, Yamaguchi-Shinozaki K. Transcriptional regulatory networks in response to abiotic stresses in Arabidopsis and grasses. Plant Physiol. 2009;149(1):88–95.
Nguyen KH, Ha CV, Watanabe Y, Tran UT, Esfahani NM, Nguyen DV, Tran L-SP. Correlation between differential drought tolerability of two contrasting drought-responsive chickpea cultivars and differential expression of a subset of CaNAC genes under normal and dehydration conditions. Front Plant Sci. 2015;6:449.
Wang Y, Ohara Y, Nakayashiki H, Tosa Y, Mayama S. Microarray analysis of the gene expression profile induced by the endophytic plant growth-promoting rhizobacteria, Pseudomonas fluorescens FPT9601-T5 in Arabidopsis. Mol Plant Microbe Interact. 2005;18(5):385–96.
Kandasamy S, Loganathan K, Muthuraj R, Duraisamy S, Seetharaman S, Thiruvengadam R, Ponnusamy B, Ramasamy S. Understanding the molecular basis of plant growth promotional effect of Pseudomonas fluorescens on rice through protein profiling. Proteome Sci. 2009;7(1):47.
Dalton DA, Boniface C, Turner Z, Lindahl A, Kim HJ, Jelinek L, Govindarajulu M, Finger RE, Taylor CG. Physiological roles of glutathione S-transferases in soybean root nodules. Plant Physiol. 2009;150(1):521–30.
Kumar M, Lee S-C, Kim J-Y, Kim S-J, Kim S-R. Over-expression of dehydrin gene, OsDhn1, improves drought and salt stress tolerance through scavenging of reactive oxygen species in rice (Oryza sativa L.). J Plant Biol. 2014;57(6):383–93.
Cosgrove DJ. Loosening of plant cell walls by expansins. Nature. 2000;407(6802):321–6.
Ding A, Marowa P, Kong Y. Genome-wide identification of the expansin gene family in tobacco (Nicotiana tabacum). Mol Genet Genomics. 2016;291(5):1891–907.
Marowa P, Ding A, Kong Y. Expansins: roles in plant growth and potential applications in crop improvement. Plant Cell Rep. 2016;35(5):949–65.
Acknowledgements
We acknowledge Tanta university for the majority of finincial support for our research.
Funding
This work was supported and funded by graduate student research affairs sector of Tanta University, Egypt. The project code TU-03-13-06.
Author information
Authors and Affiliations
Contributions
SAO and MEE designed research. SAO and MHA conducted the experiment, collected and analyzed the data of physiological and molecular parts. NAF, HME and MHA conducted the experiment of microbes screening and characterization. SAO, MHA and NAF wrote the manuscript. MEE supervised the experiment. MEE, JW and HMK helped us to solve the problems during the experiment, revised the manuscript and gave critical comments and suggestions while preparing the manuscript. All authors read and approved the final version of manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: Table S1. Morphological characteristics of 3-day-old colony of PGP endophytic isolates. Table S2. Cell shape, motility and gram reaction of PGP endophytic isolates.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Omar, S.A., Fetyan, N.A.H., Eldenary, M.E. et al. Alteration in expression level of some growth and stress-related genes after rhizobacteria inoculation to alleviate drought tolerance in sensitive rice genotype. Chem. Biol. Technol. Agric. 8, 41 (2021). https://doi.org/10.1186/s40538-021-00237-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40538-021-00237-4