Abstract
This article presents a thorough overview of gel-based cleaning methods used in art conservation. It covers the evolution of traditional approaches and the development of advanced gel systems. The paper examines the structure, characterization, and classification of gels, as well as their mechanical properties, which are crucial in art conservation. Various types of gels, including hydrogels, organogels, xerogels, semi-IPNs, and microgels, are discussed in detail, highlighting their unique properties and suitability for specific conservation applications. The advantages, limitations, and applications of both natural and synthesized polymers that form the basis for these gels are also analyzed. Case studies are presented to demonstrate the practicality and effectiveness of gels in cleaning different materials such as paper, paintings, metals, and textiles. These case studies showcase successful removal of stains, pollutants, and unwanted layers while preserving the integrity and aesthetic value of the artworks. By contributing to the existing knowledge on gel-based cleaning approaches in art conservation, this comprehensive review establishes a foundation for future research and development in this field. The review concludes with a discussion on the challenges and potential future directions in the development and optimization of gel-based cleaning methods for art conservation. Overall, this article is a valuable resource for researchers, conservators, and students in the field of art conservation, providing essential information and insights into the use of gels as effective and safe cleaning agents.
Similar content being viewed by others
Introduction
Historical objects degrade over time due to natural aging, primarily at the surface of the artifacts. Pollution and environmental sediments accumulate on the object’s surface, leading to deterioration and changes to the aesthetics. It is essential to clean the accumulated contaminants from the main substrate of these objects through the conservation process. Conservators play a crucial role in conserving artifacts by performing this cleaning process, recovering readability, and preventing the degradation of the original substrate [1].
The cleaning action consists of removing diverse groups of materials such as proteins, lipids, polysaccharides as natural polymers, hydrophobic particles, and synthetic resins such as acrylics and vinyls as synthetic polymers [2], and unwanted mineral compounds on metallic surfaces. This diversity in materials can make the cleaning process for cultural heritage very challenging. On the other hand, certain unstable materials utilized by contemporary artists in their pieces have posed a significant challenge for conservators [3].
Cleaning can be divided into two main categories of chemical and mechanical approaches. Laser, vibrating tools, ultra-high-pressure water, etc. are placed in mechanical approaches while using chemical compounds such as acids and bases and solvents belong to the chemical approaches [4]. Since cleaning the artistic substrates is so sensitive and must be carried out carefully without altering the original components, using a wide range of solvents can pose unwanted risks due to their several drawbacks [5]. One of the primary unintended consequences of using organic solvents in cleaning processes is the re-deposition of the dissolved macromolecules within the porous substrate as the solvent evaporates. This can highly affect the intrinsic properties of the original artwork materials [6]. For example in an original painting, due to the capillary forces, and the penetration rate of different solvents, many of them can simultaneously cause swelling and softening of the paint layer and leaching the binding medium in the artwork along with all those unwanted materials [7]. Additionally, sensitive materials can be easily affected and altered by the mechanical pressure of a cotton swab morphologically [8]. Moreover, it should be noted that solvent clearing, without the aid of mechanical swabbing, may not be effective enough to eliminate cleaning system residues from the innermost crevasses and fractures within the painting surface [9].
In recent decades, conservation scientists have been focusing on developing cleaning methods that minimize the problems associated with traditional cleaning methods. To this end, researchers have explored a wide range of materials such as modified natural products (e.g., Klucel®, Tylose) and synthetic polymers (e.g., Carbopol®). These materials are used as thickeners and are capable of controlling solvent penetration rate [5, 8]. More recently, the application of material science and nanoscience in art conservation has led to the development of advanced cleaning systems and approaches that are forging change in the conservation of cultural heritage. These include nanostructured fluids (NSFs), gels, and polymer networks [2]. In recent years, the use of gels as a cleaning method has gained popularity in the field of art conservation. This approach was first introduced by Wolbers in the early 1990s and has since shown suitable performance in cleaning various materials [10,11,12]. Gels help control solvent evaporation, limit penetration into the artworks through capillary action, and provide more precise control over the treatment application [13].
In science, gels are a form of matter that represent a specific behavior between a solid and a liquid. They can be cross-linked polymers, that create a tangled polymer network and most of the time immersed in a liquid medium. Interactions between polymer and liquid phase build up the significant properties of the gel. The polymer structure and the liquid phase interact with each other to stop the collapsing and flowing away respectively. As a result, making the structure of the gel to be somehow stable [14]. Some of the gels are water-based formulations thickened with high molecular weight materials such as polymers and are used as carriers for “active” cleaning components to the surface [15]. In most gels, two continuous phases interpenetrate at a macroscopic level: there is a liquid phase (e.g., water, alcohol) that has penetrated into the network structure made up by the gelator [16]. Gels can be made using natural polymers such as collagen, gelatin, hyaluronic acid, fibrin, sodium alginate, agarose, chitosan, dextran, and cyclodextrin [17], and synthetic polymers such as PVA, PMMA, etc.
Baglioni et al. [7, 18] categorized gelled systems in the art conservation field into physical gels, which were introduced by Wolbers in the 1990s, and innovative nanostructured gels or gel-like systems, including responsive gels and peelable systems, according to Gueidão et al. [19]. However, Zhang et al. [16] suggest that there are several types of gels, such as supramolecular gels, metal-organic gels, dynamic covalent gels, polymer gels, and inorganic gels. These can be classified based on their response to different stimulant, including temperature, pH, and magnetic fields and according to Baglioni et al. [20], and Carretti et al. [21] are called responsive gels. Gels can also be categorized based on their gellant nature and preparation methods, such as polymer gellant, monomer cross-linker, and low mass organo gelator (LMOG) gellant. In addition, Passaretti et al. [22] also indicate beside all those classifications, another way to categorize gels is based on the fluid entrapped in the polymer matrix, where hydrogels contain water, while organogels or solvent gels contain organic solvents.
Using gelled systems offers several advantages. Firstly, gels control organic solvent evaporation rate and the flow of the solution into surrounding areas and underlying layers. Secondly, gels provide control over the surface cleaning time. Lastly, the use of gels minimizes human exposure to toxic organic solvents [15]. To achieve these benefits, two essential features of gels are required. Firstly, they must possess high viscoelasticity (the property of materials that exhibit both viscous and elastic characteristics when undergoing deformation), and secondly, they must be capable of retaining acceptable amounts of water or solvents and releasing them at a controlled rate, as noted by Baglioni et al. [2]. However, limitations arise when gels are used for cleaning painted surfaces, as residual stains can remain, which may have adverse effects on works of art [10]. This can occur while using specific type of gels such as solvent gels consisting of Carbopol and other thickeners. Therefore, in cultural heritage conservation, chemical gels are more effective than traditional solvent thickeners, according to [1] due to their specific features such as solvent release control, selective cleaning, and leaving less residues (either solvent residues or polymer residues) than physical gels. Moreover, rigid gels provide a useful delivery system because of their compact consistency which makes them easier to remove [22]. Hydrogels can be used to remove dirt, varnish, and the rest of the undesirable contaminations from the artworks [23].
The study of gels and their various types has proven to be a valuable tool in cleaning, offering an effective and less invasive approach that allows conservators to better preserve the integrity of the artwork. As the field of art conservation progresses, gel technologies are getting more widely utilized, with potential for the development of new and improved gels that can address a wider range of cleaning needs. Given their versatility and effectiveness, the use of gels is a promising approach to the conservation of artistic works for generations to come. This article reviews recent studies on this issue, clarifying current practice and offer future perspectives of gel use in cultural heritage materials cleaning. Through a comprehensive analysis of publications from the last few years, this review paper provides insights into the benefits and limitations of some gel-based cleaning methods and presents identifies areas for future research.
Gels
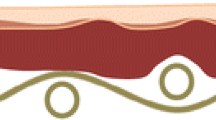
The introduction has mentioned the advantages of gels. Some of these benefits, such as controlling the rate of solvent evaporation and the flow of the solution, are a result of the three-dimensional structure of gels. Gels have a porous structure that allows them to trap contaminants within their pores. Figure 1 illustrates the interaction between a gel and a contaminated surface of a substance. As shown in Fig. 1, the porous structure of the gel traps contaminants and keeps them within its pores. Therefore, the more porous a gel is, the more unwanted materials it can hold. To fully understand how a gel interacts with contamination on a substance, it is important to consider certain chemical and mechanical properties. The following section provides a brief overview of these parameters, but they should be discussed in greater detail.
Composition and structure
In polymer-based gels, the major class of gel with a wide range of variety, the combination of a radical initiator, for activating the cross-linker, and specific monomers can build up a 3-D like network through polymerization process. However, other types of gels are made by heating and cooling down the distinctive compounds that leads to achieving a polymeric network [24].
Interacting of molecules with each other and with the solvent is the result of the gelation processes, causing them to become localized in space and resulting in macroscopic rigidity [25]. This process involves gelator molecules self-associating to form long, polymer-like fibrous aggregates that entangle during the aggregation process, building a matrix that through surface tension entraps the solvent [26]. And to this far, the important mechanism in gel formation is related to the bonds between the polymer molecule chains [25], which can be either physical or chemical (Fig. 2) [25]. Bonds of a physical gel (hydrophobic, electrostatic (ionic), van der Waals interactions, or hydrogen bonds) enables it to shape easily and resulting in the desirable interaction with a specific substrate. However, this ability can lead to leaving residues on the surface which is in need of extensive rinsing [24]. However, some physical hydrogels form more stable bonds, such as lamellar microcrystals, glassy nodules, and double and triple helices [27, 28]. Physical gels can be divided into two main categories of strong (e.g., agar, gelatin, gellan gum) and weak (e.g., xanthan gum, cellulose ethers, polyacrylics) physical gels. In strong physical gels the double or triple helix structure enables the gel to form a semi-rigid, peelable film, while weak physical gels only form a viscous paste [29].
In contrast, in a chemical gel, the bonds between gellant molecules are almost covalent bonds and represent these gels as one integrated molecule [24]. Chemical gels can be synthetized through different polymerization procedures such as; addition, condensation, free radical polymerization, and electromagnetic radiation [25]. Since the solvent retention and being residue-free are desirable features of a cleaning gel, several “chemical gels” have been proposed in few past years [8], although a gel might be residue-free but the employed solvent might not be unfavorably. One of these enhanced gels are “magnetic chemical gels”. Magnetic gels are made by accommodating ferrite nanoparticles in a polyacrylamide matrix. These nanoparticles need to be coated with dicarboxylic derivatives [21].
Gels that respond to external stimuli, such as changes in pH, temperature, or magnetic fields, are referred to as responsive gels [21]. Such gel properties, including swelling degree and elastic modulus, can significantly change with small variations in environmental conditions, rendering them stimuli-responsive [25]. The response of a responsive gel to the chemical and physical stimulants enables them to remove the unwanted materials completely and properly without any theoretical penetration into the lower layers [19].
To fully understand a gel structurally, considering three key parameters would be essential - the polymer volume fraction in the swollen state (v2,s), the molecular weight of the polymer chain between two adjacent cross-linking points almost found in chemical gels (Mc), and the characteristic mesh size (ζ). These interrelated parameters can be determined either through theoretical estimation or various experimental techniques [24].
Mechanical properties of gels
Young’s modulus besides viscoelastic properties, are major parameters to investigate mechanical properties of a gel [16]. Viscoelasticity pertains to the flow and deformational behavior of materials and can be studied through rheology, which examines the deformation and flow of matter under applied stress [30]. Stress and strain are measured parameters in appraising the viscoelastic behavior of gels. To be able to characterize gels in a standard way, the gel has to be subjected to a small oscillation and then the stress response to this turbulence is evaluated [25].
The elastic storage modulus or “elastic modulus” (G′) and the loss modulus or “viscous modulus” (G″) are two key parameters in rheological experiments that reflect the behavior and structure of gel materials [16]. Therefore, totally viscous material has an elastic modulus equal to zero, and reversely totally elastic material represent a viscous modulus equal to zero [24].
Generally strong gels (A type of physical gel with stronger bonds than ordinary physical gels, and is different from chemical gels [31]. Examples of strong physical gels include elastomers, block copolymers, and gelatin [32]) involve greater elastic modulus than viscous modulus, and both moduli behave almost frequency-independent upon a large frequency range. This is the result of the localized particles or molecular structure of these material which is able to store the deformation energy over a long time. Conversely, weak gels are classified by having strongly frequency-dependent of storage and loss moduli and are often named as “gel-like”. High-viscosity polymeric dispersions (HVPDs) may even represent crossover point frequencies above which viscous modulus is greater than elastic modulus (G” > G’) [33].
Classification of gels used in cleaning artworks
Art conservation often employs gels as a cleaning agent that can selectively remove unwanted substances from an object’s surface without causing unacceptable harm to the underlying material. The classification of gels used in art conservation can be based on their composition, structure, and properties.
According to the literature on the types of gels used in art conservation, two main approaches can be identified for classifying the cleaning gels employed in art conservation practices. One approach, which is more common, categorizes gels based on the type of fluid used in their preparation, while the other approach considers the nature of the polymer within the gel network. Based on the type of fluid, gels can be broadly classified into hydrogels and organogels. In terms of the polymer used in the network, gels fall into two general classes: natural and synthetic polymers.
In addition to these standard classifications, other gels have also been used in art cleaning. These gels, while they could fit into the previously mentioned categories, exhibit distinct physical properties and different gels networking approaches. Examples include microgels, xerogels, and semi-IPNs gels (Semi interpenetrated networks). Each type of gel possesses unique characteristics and properties that make it suitable for specific conservation applications. Figure 3 provides a brief overview of these gel classifications.
Classification based on the type of fluid
-
Hydrogels
Hydrogels can be introduced as useful hydrophilic systems with three-dimensional structure and remarkable features such as biocompatibility, adaptable mechanical properties, and controlled swelling behavior. They are able to retain water and resisting against deformation of their structure (from different types of stresses and mechanical forces) [34]. It was Wichterle and Lim whom introduced hydrogels in 1960, and described their ability to absorb and keep water within their structure [35, 36]. Generally, hydrogels have natural or synthetic origins. Synthetic-based hydrogels in compare to bio-based hydrogels show less hydrophilicity and are mechanically more robust [37]. These materials exhibit both solid and liquid-like properties due to their elastic and osmotic nature. Their unique characteristics have garnered significant attention in various fields, including art conservation, where preserving cultural heritage materials is crucial [38].
Hydrogels can attain a state of equilibrium when they absorb water and maintain their original shape, with an ideal water content of at least 10% by weight [39]. The hydrophilic properties of hydrogels are related to the presence of functional groups like -COOH, -NH2, -OH, -CONH, -CONH2, and -SO3H [35]. Hydrogels can be formed from copolymers or homopolymers. According to the different kinds of chemical and physical crosslinks such as covalent bonds and entanglements, hydrogels are insoluble in water [40, 41]. 3D polymerization is mostly used in chemical hydrogel synthesis. Hydrogels are polymerized through either polymerization of hydrophilic monomers or direct linking of water-soluble polymers. Polyacrylic acid, polyvinyl alcohol, polyvinyl pyrrolidone, polyethylene glycol, polyacrylamide, and some non-toxic polysaccharides are commonly used as water-soluble polymers in hydrogel formation [42].
The characteristics of the hydrogel matrix, including pore size, distribution, and interconnections, play a crucial role in their performance. Pores in a hydrogel can either form during phase separation and synthesis or within the network structure [43, 44]. Hydrogels exhibit regions of less swelled in water dispersed in areas of high swelled in water. This subject results in heterogeneous appearance in these gels [45]. They can be classified based on their physical network structure as amorphous, semi-crystalline, hydrogen-bonded structures, supermolecular structures, and hydro colloidal aggregates, and are considered physiologically-responsive hydrogels [41].
In the field of art conservation, hydrogels can be classified into two main categories: natural and synthetic. Natural hydrogels have natural origin, such as polysaccharides (e.g., cellulose, chitosan, and alginate) and proteins (e.g., gelatin). Due to their biocompatibility and biodegradability, they are suitable for use in art conservation [46]. On the other hand, synthetic hydrogels are made from synthetic polymers like polyvinyl alcohol [PVA], polyacrylic acid [PAA], polyacrylamide [PAAm], poly (2-hydroxyethyl methacrylate) [p(HEMA)], and polyethylene glycol [PEG] [47]. They offer greater control over properties such as mechanical strength, swelling behavior, and porosity [42].
The swelling behavior of hydrogels allows for the effective removal of contaminants without causing unacceptable damage to the underlying paint layers or other delicate materials [23]. In addition to cleaning and consolidation, hydrogels are useful in the protection of cultural heritage materials. For instance, they can be used as coatings or films to provide a barrier against environmental factors such as moisture, pollutants, and UV radiation [48].
The breakthrough in hydrogel technology have led to introduce the stimuli-responsive, smart, and nanocomposite hydrogels. Different types of stimulants such as pH or temperature can affect reversibly the swelling behaviors of a responsive hydrogel [38]. These smart hydrogels can be tailored to provide controlled release of cleaning agents, consolidants, or protective materials in response to specific environmental conditions. Nanocomposite hydrogels incorporate nanoparticles such as clay, silica, or metal oxides to enhance their mechanical properties, swelling behavior, and responsiveness [49]. These hydrogels can offer improved performance in art conservation applications, such as more efficient cleaning, enhanced consolidation, or better protection of cultural heritage materials.
Although hydrogels offer numerous benefits in art conservation, there are still challenges that need to be addressed. In this field, one major challenge could be the development of hydrogels that can be employed to clean a wide range of cultural heritage materials, since every single of these objects includes unique combinations of pigments, binders, and substrates.
Future research should focus on developing hydrogels with tailored properties that can address the specific needs of different cultural heritage materials. Advances in stimuli-responsive, smart, and nanocomposite hydrogels offer promising avenues for enhancing the effectiveness and versatility of hydrogels in art conservation. Furthermore, interdisciplinary collaborations between conservators, scientists, and engineers help ensure that hydrogel technologies are optimized for the complex challenges faced in preserving our shared cultural heritage.
-
Organogels
Organogels are three-dimensional networks of a dispersed organic solvent within a structuring agent. These kinds of gels alike the other types of gels own both natural and synthesis origins [50, 51]. Molecular gels or organogels are physical gels and semi-solid systems that include organic solvent as the dominant liquid within crosslinked 3D networks. They are the result of the self-association in different solvents of low molecular weight molecules called organo-gelators or LMWG (low molecular weight gelators) and are generally thermoreversible which means they are capable of reversing to their former state along with temperature changes. In an organic solvent media LMWGs interact with each other and create a 3D supramolecular network to entrap the solvent [52, 53]. These gels embody organic liquids as solvents and have the ability to eliminate the organic contaminations without a little impact on hydrophilic or hydrophobic components [50, 51]. Organogels are becoming increasingly popular due to their versatile properties, which can be easily adjusted to suit different needs. The adaptable properties of organogels are a significant advantage. These include the ability to adjust the freezing and boiling points of the organic liquid phase within the gel. This allows organogels to maintain their gel-like characteristics over a wide range of temperatures, by utilizing a variety of solvents with different boiling points. This versatility makes organogels highly desirable materials [52]. The use of organogels as a cleaning agent in art conservation has become increasingly popular in recent years, since their ability to selectively remove unwanted surface dirt from sensitive materials without damaging the underlying substrate is significant. This selectivity is achieved through the careful choice of the organic solvent and the structure of the agent, which is able to be adjusted for different needs of cultural heritage objects cleaned.
Organogels are able to dissolve a wide range of undesirable materials such as wax, and diverse types of coatings from various cultural heritage objects like paper and canvas [19]. Studies have shown that organogels based on methyl methacrylate [MMA] are desirable for cleaning canvas paintings due to their selective removal ability [51]. Hydrogels and chemical organogels could be complementary approaches in cleaning artworks, since both of them together would exhibit greater retention and better mechanical features [54]. Organogels have a thermodynamic stable nature, which allows them to maintain their structural integrity for extended periods of time. Additionally, they are resistant to moisture and microbial contamination due to their organic composition. Furthermore, the use of biocompatible, biodegradable, and non-immunogenic materials ensures their safety for long-term applications [55].
The use of organogels in art conservation suggests numerous benefits over traditional cleaning methods. For instance, they sometimes are non-toxic, non-flammable, and often environmentally friendly. Furthermore, they can be applied in a controlled and precise manner, reducing the risk of accidental damage to the artworks.
However, despite the many advantages of organogels in art conservation, there are still some challenges that need to be addressed, such as selecting solvents with less unwanted impacts and being quite adaptable to the original material of artwork. One of the main concerns is their long-term stability and compatibility with the substrate. Organogels must not leave any residues or alter the physical and chemical properties of the original materials over time. Furthermore, there is still a need for further research into the potential toxicology and environmental impact of these gels, especially with regard to their disposal.
Classification based on the nature of the polymer
Based on the literature review, sources of gels used in art conservation can be classified into two categories: synthetic and natural. Synthetic gels are developed in the laboratory through chemical synthesis, while natural gels are derived from natural sources such as plants and animals. This classification is summarized in Fig. 3. In the following sections, the different types of gels used in art conservation based on their source will be discussed.
Natural polymer-based gels
-
*
Cellulose-based gels
The most abundant natural polymer derived from glucose is cellulose. It is the major component of the different kinds of plants and fibers [56]. Certain bacteria, such as Acetobacter xylinum, are also capable of synthesizing cellulose. Thus, there are two main classes of cellulose, bacterial cellulose (BC) and plant cellulose (PC). These two have similar chemical structure and are built up from glucose units by 1,4-β-glucosidic, however include various properties and macromolecular structure. Typically, PC exhibits crystallinity in the range of 40–60%, while BC demonstrates a higher crystallinity exceeding 60%. As a result, both types of cellulose are insoluble in water and other solvents [56].
Cellulose derivatives are commonly categorized as esters or ethers. Examples of esters include cellulose acetate [CA], cellulose acetate phthalate [CAP], cellulose acetate butyrate [CAB], cellulose acetate trimellitate [CAT], and hydroxypropyl methylcellulose phthalate [HPMCP]. Meanwhile, ethers include methylcellulose [MC], ethyl cellulose [EC], hydroxyethyl cellulose [HEC], carboxymethyl cellulose [CMC], sodium carboxymethyl cellulose [NaCMC], hydroxypropyl cellulose [HPC], and hydroxypropyl methylcellulose [HPMC] [57].
According to the ecological, safety, economical, biological advantages of cellulose, it would be a desirable choice for producing hydrogels. This natural polymer contains a porous structure and a great number of OH functional groups [37].
There are several groups of cellulose-based gels like MC, and HEC that are produced via covalent and non-covalent bindings. These gels contain three dimensional hydrophilic polymer networks [58]. Cellulose based gels can be divided into three main categories including Hydrogels, Xerogels, and Aerogels. This classification is based on the used solvent and the drying approach. [58]. Cellulose-based gels have been widely used in art conservation due to their biocompatibility and tunable rheological properties [59, 60]. These gels are primarily employed for cleaning surfaces, removing varnishes, and consolidating fragile materials.
-
*
Agar and agarose-based gels
Agar is a polysaccharide extracted from different types of red algae, Gelidiales and Gracilariales red seaweed [61], consisting primarily of D- and L-galactose units. There are some considerable parameters that have a lot of impact on ratio of main constituents, the gelling procedure of Agar, and its rheological properties. These parameters include the type of seaweed, the environment in which the algae had been growing, the physiological factors, and the extraction procedure [62]. To gain a semi-rigid, hydrophilic, thermoreversible gel out of agar, it should be exposed to heat and then cooled down immediately [63]. It is composed of a mixture of agarose and agaropectin in variable proportions depending on the type of algae used and the manufacturing process.
Gels made out of agar are thermoreversible and easily change their state upon cooling or heating. [27]. When lowering the heat, the polysaccharide molecular chains form a double-helices order via hydrogen bonds and create a three-dimensional structure with water in its pores [61]. Rigidity of agar gels is adaptable and as a result conservator can customize it to well fit the surface and remove the unwanted layers [27]. From a microscopic point of view, the porous structure of agar elevates its retention [64]. Generally, the best proportion of this gel is prepared in a concentration of 2 to 6% w/v [22].
Using agar gels as a cleaning medium includes some advantages such as; the ability to clean soil, controlling the solvent release, the less effect on the historical materials, being accessible, and green [65]. Amongst other gelling agents, agar is much more noteworthy due to its great adaptable chemical and physical properties, and its ability to adjust itself to various status [66].
Agarose resembled to Agar is a neutral polysaccharide derived from Rhodophyta (marine red algae). Its structural form contains repeating 1,3-binding β-D-galactose and 1,4-binding 3,6-anhydro α-L-galactose units, that has a high capacity to absorb water [67, 68]. Although agarose and agar are the same base on their origin and capabilities, thus due to the smaller pores of agar, rigid agarose gel sheets release their inner water faster than agar gels [69] and this is more expensive than agar, but offers greater transparency.
-
*
Gellan Gum and Xanthan Gum
Gellan gum is a linear, anionic heteropolysaccharide. It is obtained from a microorganism called Sphingomonas elodea [27]. It consists of repeating units of (1,3)-b-D-Glucose, (1,4)-b-D-Glucuronic acid, (1,4)-b-D-Glucose, and (1,4)-a-L-Rhamnose [70,71,72]. When cations, particularly Ca2+, are present, gellan gum forms hard and fragile gels that have a stable and ordered structure, a crystalline-like structure [27]. This gel is usually homogenous, clear and stable against pH changes. This characteristic makes it less thermo-reversible. The pH stability of gellan gum makes it suitable for use on paper samples with varying levels of acidity or alkalinity [73]. Gellan gum is found in two statuses, with high and low amount of acyl which produces soft and hard gels in return [74]. It has a wide range of use in different fields like biomedical and pharmacology as thickener. Similar to other natural-based material mentioned above, gellan gum is also safe and biodegradable. This material is capable of retaining water and then gradually release it and in return absorbing the undesired contaminations within itself. So it can be a useful choice in conservation of paper works [75]. When placed in water, gellan gels release polymer chains and can recover from mechanical disruption or the expulsion of water through slow compression [70]. These gels are sensitive to shear stress, which can alter their structure and viscosity. Gellan gum solutions have a high viscosity, making them useful as thickeners for liquids and suspensions [72]. Moreover, to enhance its microbial resistance and cytocidal action against bio-deteriogens, researchers have been using hydrolates [76].
On the other hand, when the bacterium Xanthomonas Campestris ferments the glucose or sucrose, the Xanthan gum is synthetized. The more it is effective in aqueous solutions, the less effective is in gelling basic solutions [22]. When combined with gluco- or galactomannans, xanthan has the ability to create physical hydrogels. These hydrogels are commonly referred to as “synergistic gels” due to the specific interactions that occur between the two types of polysaccharides. However, when xanthan is mixed with other polysaccharides at comparable concentrations, it does not result in a gel phase, according to Paradossi et al. [77].
-
*
Chitosan
Chitosan (CT) is a polysaccharide that can be obtained through the deacetylation of chitin or the enzymatic treatment of chitin deacetylase. Chitosan has many advantages and biological functions including; being abundant, biodegradable, anti-inflammatory, anti-microbial, etc. [47]. It is produced through the deacetylation of chitin under alkaline conditions. Chitin, a structural component found in mushrooms, fungi, the cell wall of fungi, and the skeletal structure of crustaceans and insects, is subjected to the deacetylation reaction to form chitosan. This material is a linear polysaccharide comprising of β-(1–4)-linked D-glucosamine and N-acetyl-D-glucosamine units [78]. With abundant amino groups, chitosan is hydrophilic, cationic, and reactive, rendering it a versatile polymer. Additionally, chitosan is characterized by its biodegradability, abundance, biocompatibility, non-toxicity, and versatility, making it an antibacterial, antioxidant, antifungal, and prebiotic compound with minor side effects [79].
These properties render chitosan an ideal material for use in art preservation. Furthermore, the amino groups of chitosan enable the creation of hydrogels. Hydrogels of chitosan are produced either via covalent cross linking or forming polyelectrolyte complexes [80]. Hydrogels derived from chitosan are capable to form in both alkaline and acidic solutions. The cleaning potential of these hydrogels have been investigated on metallic substrates [22]. Although CT hydrogels are often weak in terms of mechanical properties, thus to enhance the mechanical properties of Chitosan hydrogels, combining them with different copolymers such as PVA is suggested. Such modifications can be particularly useful for applications involving art conservation [47].
Synthetized polymer-based gels
-
*
PVA based gels
Poly(vinyl alcohol) (PVA) is one of the useful synthetic polymer with a relatively simple chemical structure, featuring a pendant hydroxyl group [81]. The ability of PVA to create hydrogel systems has made it a popular choice as a gelling agent [82]. It is highly biocompatible with low toxicity and excellent mechanical properties [83]. PVA-based chemical and physical hydrogels have been extensively studied for practical applications due to their low toxicity and high biocompatibility.
Chemical PVA gels can be obtained by crosslinking PVA in solution using methods such as γ-ray or electron beam irradiation, or through chemical reactions with crosslinking agents [84]. Physically cross-linked PVA gels, on the other hand, are created with hydrogen bonds using two methods that do not require a cross-linking agent: repeated freeze-thawing (FT), and cast-drying (CD) of aqueous PVA solutions [83]. However, CD and FT gel structure resembles to each other, but there are some macroscopic differences such as transparency, and elasticity between them [36]. After a PVA gel solution freezes the polymer phase and water separate from each other. This action leads to creating crystalline spots which link to each other through hydrogen bonds. These crystalline spots function as joints and build a three dimensional network of a hydrogel with remarkable properties of flexibility and high viscoelasticity [33].
The reason in flexibility of physical PVA gel sheets is the great viscoelasticity of the networks. This allows the gel to exhibit a good resistance against mechanical stresses [85]. Another advanced cleaning system is based on twin-chain polymer networks (TC-PNs) comprised of PVA chains, semi-interpenetrated (semi-IPN) with lower molecular weight PVA (L-PVA). The moment the L-PVA is added, it converts the gels into a new system of semi-IPNs with disordered porous structure with different dynamical behaviors. Nevertheless, despite of non-covalent links in the network structure, the new gel system acts equivalent to chemical networks mechanically [85]. In TC-PNs, two types of polyvinyl alcohol with varying molecular weights and bond breakability by water are blended. This interpenetration leads to a more effective gel system for dirt capture, transport, and controlled wetting at the gel–artifact interface, enabling selective dirt detachment [86]. Since the L-PVA contains plasticizing properties, and morphological alterations, TC-PNs represent various properties in contrast with pure PVA including interconnected macroporosity, surface roughness, higher storage modulus, and improved stress relief [85].
PVA-based hydrogels can be filled with aqueous nanofluids (NSFs) [2]. PVA based gels has been used not only in TC way, but also in the semi-IPN system with linear PVP. The preparing procedure includes crosslinking the PVA via FT cycles and using PVP as a hydrophilic porogen to provide the needed equilibrium water contents [33].
Although PVA/borate-based systems appear macroscopically similar to gels from a rheological perspective and are suitable for cleaning painted surfaces, they cannot be classified as gels. Instead, these fluids belong to the category of highly viscous polymeric dispersions (HVPDs) [7]. To produce HVPDs, PVA or semi-hydrolyzed PVAc polymer chains need to be cross-linked using borax [5].
-
*
PAA p(HEMA) and PMMA based gels
PAA, p(HEMA), and poly(methyl methacrylate) [PMMA] are synthetic polymers that have been used to create gels with tunable properties for various applications in art conservation. One of the main features of p(HEMA)/PVP gels is their high water retentiveness, which in the case of sensitive materials it would be a remarkable characteristic [5]. The ability of getting loaded with both aqueous formulations and some polar solvents makes them adequate to clean the both hydrophilic and adhesives from canvas at the same time [51]. Because of the chemical structure of the Polyacrylic acid (linking the carboxylic groups of its monomers via cross-links), water and organic solvents can create a gel out of PAA. These gels are extensively utilized in the conservation of cultural heritage scope as cleaning medium, particularly for the conservation of easel paintings, instead of pure solvents [87].
PMMA is a hydrophobic polymer. PMMA based organogels can be synthetized via copolymerization of free radicals of MMA and the monomers of diacrylate in various solvents [59]. Since this polymer is transparent it could be a great aid through cleaning process. It should be noted that these organogels can be applied directly onto the cultural heritage objects and easily removed by a cotton swab without leaving any residues behind [19].
Gels with special properties
-
Xerogels
Xerogel is a gel without solvents, also known as a sol-gel, which can be used in the preservation of cultural heritage. They are gel systems with specific characteristics, such as a large surface area, a highly porous structure, and small pore size. By allowing it to dry slowly, the formation of potential cracks and fractures inside its structure can be prevented. This type of gel can be produced using various natural polymer structures like chitosan, alginate, pectin, and cellulose [88]. Actually, if a gel system is dried without managing its shrinkage, xerogels will be produced. This results in a partial or full collapse of the gel’s framework and alters its porosity [24]. During the process of evaporative drying, the wet gels lose their moisture, resulting in xerogels with greater permeability, a high specific pore size, and a structure that closely resembles aerogels obtained through supercritical solvent drying [58, 89]. Owing to their significant properties such as low density, high surface area, adsorption properties, [90, 91] they are desirably used in different fields of art conservation. According to Mosquera et al. [92], xerogels also can be used as stone consolidants. Unlike cleaning, in this consolidating method the stone samples are first fully saturated with a solution, and then polymerization occurs within the stone.
The main advantage of using xerogels in art conservation, similar to other gel-based methods compared to traditional cleaning techniques, is their ability to gently remove surface contaminants from cultural heritage materials without damaging the underlying layers. This is especially important when dealing with fragile and historically significant pieces, as traditional cleaning methods may not be appropriate due to the risk of damaging the original materials. Additionally, xerogels can be synthesized using a variety of materials, such as silica, metal oxides, and organic polymers, allowing their properties to be tailored to meet specific conservation requirements [24]. For example, xerogels with controlled pore sizes can be synthesized to target specific contaminants or to allow for the penetration of specific cleaning agents. Furthermore, the use of functionalized xerogels can provide enhanced selectivity and effectiveness in the removal of specific types of contaminants, such as organic materials or metal ions [89].
Despite the numerous benefits of xerogels in art conservation and cleaning, some challenges must be addressed. The potential for xerogels to leave residues on surfaces of cultural heritage objects is a primary concern that could negatively impact the appearance and long-term stability of treated works of art. Additionally, the synthesis and handling of xerogels can be complex and require specialized knowledge and equipment, limiting their widespread adoption in the field of art conservation.
In conclusion, xerogels offer a promising and versatile approach to art conservation and cleaning, with the ability to address a wide range of contamination challenges while preserving the integrity of the artworks. Continued research and development in this area will likely lead to further advancements in the synthesis, functionalization, and application of xerogels, enabling conservators to more effectively protect and restore our shared cultural heritage.
-
Semi-interpenetrating polymer networks gels
Semi-interpenetrating polymer networks are a kind of gels that can be placed in both hydrogels and organogels simultaneously. Advanced semi-IPNs are created without any chemical cross-linking (resembled to chemical gels) via accommodating a linear or branched polymer into another polymer network with varied properties [1]. The linear polymers that function as a cross-link within the other polymer network, result in a smoother and softer gel system that are capable of eliminate hydrophobic contaminations from highly sensitive substrates [93]. The properties of these semi-IPN hydrogels alike the other mentioned gels are adaptable. It means that by changing the composition, the amount of the constituents, and the synthesis process a customized gel would be developed [94]. One of the highly retentive semi-IPN hydrogels that has been used to clean hydrophobic layers properly is semi-IPNs of polyhydroxyethylmethacrylate/polyvinylpyrrolidone [95]. These semi-IPNs, typically synthesized as 2-mm-thick sheets, are easily handled and peeled-off after application [2]. One of the semi-INP hydrogels effective in cleaning hydrophilic dirt from water sensitive substrates is the combination of HEMA/N, N’-Methylenebisacrylamide [MBA] polymers with accommodated linear polyrvinylpyrrolidone [PVP] [96].
-
Microgels
Microgels are going to be useful as a cleaning agent due to their unique properties. Microgels are colloidal particles with a size range of 10–1000 nm, which can swell or shrink depending on the pH, temperature, or solvent polarity [97]. This property makes them ideal for selective cleaning of historic objects without damaging the underlying layers, such as cleaning two different paper samples: ancient paper from “Breviarium Romanum ad usum fratrum” (1738) and modern paper (office paper from 1965) [98].
Microgels are a kind of particles with the features of being soft, penetrable, and deformable capable of swelling in dispersing solvent [99]. Due to their hybrid nature, microgels are classified as soft colloids and consist of a mesoscopic crosslinked polymer network. These particles can undergo deformation, shrinkage, or interpenetration when interacting with other microgels. Microgels the same as other mentioned gels and depending on the type of the polymer used, can be responsive to different stimuli’s such as pH and temperature, this makes them capable of altering their size and other associated properties like polarizability or elasticity [100, 101]. The responsiveness of microgels, combined with their versatility and relatively simple synthesis methods, has led to their attractiveness for numerous applications [102]. Microgels exhibit stable structures as their polymer network is stabilized through covalent bonds or strong physical forces. However, similar to other colloidal dispersions, microgel particles have the potential to aggregate through flocculation or coagulation processes [103].
The smaller size of constituents in microgels and their great softness, causes microgels to be superior to hydrogels in some special cases such as cleaning in depth contaminations in paper works and adaptability of these gels to uneven irregular substrates [98]. The use of microgels in art conservation and cleaning allows for precise control of the cleaning process, minimizing potential damage to the historic works. Moreover, microgels can be synthesized with various functional groups, which can be useful for the targeted cleaning of specific contaminants or materials. Recent studies have explored the potential of using microgels with tailored properties for cleaning applications in art conservation, showing promising results in terms of both efficiency and safety [98]. However, it is crucial to conduct further investigations into the ability to effectively remove microgels from surfaces after the cleaning process, as well as the absence of any residual gel traces. As the field of art conservation continues to evolve, microgels and semi-IPN gels are expected to play a significant role in the development of advanced cleaning methodologies and materials for preserving our cultural heritage.
Case studies
The use of gels in paper cleaning
Using gels in cleaning paper works has become common among conservators. There has been a lot of case studies in which the cleaning medium is a gel (Table 1). Rigid aqueous Gellan gels, for instance, were utilized in the removal of auxiliary supports, substances left on the surface, and adhesives [104]. The combination of Agar-Agar and semiIPN p(HEMA)/PVP hydrogel has been tested on a paper with a sensitive ink [93].
The other studies are concentrated on using Gellan gum-based rigid hydrogel applied on XVI to XIX centuries samples [71], for removing unwanted paper layers from other substrates [75]. In another case, gellan gum microgels were utilized as innovative agents to preserve old and modern papers during the cleaning process [98] and for conserving xuan paper [105].
While these case studies have proven the efficiency of gellan gum-based hydrogels, but when the contamination contains glue or oil, it would be better to combine its properties with cleaning ability of different agents such as enzyme proteinase K, and α-cyclodextrin and Pluronic P-123 polymer [73].
Other researches discuss the effect of two semi-IPN hydrogels based on p(HEMA)/PVP on very fragile paper works [106], and to eliminate dirt and aged varnish from paper samples [96].
Alongside hydrogels, an organogel has been tested on an ancient inked paper [54], and to remove pressure sensitive tapes (PSTs) from a solvent sensitive paperwork [107]. Others have tested the efficiency of p(HEMA)/PVP loaded with EAPC fluid (a combination of water, sodium dodecylsulfate (SDS), 1-pentanol (PeOH), propylene carbonate (PC), and ethyl acetate (EA))on Michelangelo’s masterpiece [23] and Renato Bezerra de Mello’s sketchbook [108].
Another method to clean different types of tapes is two complementary approaches of using hydrogels and organogels [46, 108].
The use of gels in painting cleaning
Gels have been increasingly used for cleaning different kinds of paintings with various techniques, and acceptable results have been reported (Table 2). For instance, a kind of organogel has been assessed to clean an oil on wood painting belonging to XIX and XV centuries [10]. As it is essential for a gel to be residue-free, an acrylamide based chemical gel has been afforded to eliminate synthetic adhesives from artistic substrate [109]. Another study has explored the effect of HEMA monomers in combination with PVP and MBA (as cross linker) on water sensitive mock-ups [110]. P(HEMA)/PVP based hydrogels have been also tested to clean an aged varnish from an 18th century oil on canvas [111], and on highly sensitive tempera magra on canvas [5].
Owing to the ability of being loaded with various surfactants and cleaning agents, acrylamide/bisacrylamide based gels have been employed to clean synthetic adhesives from canvas [5]. Another organogels based on MMA-EA [51], and poly-3-hydroxybutyrate [PHB] [112] are examined on oil on canvas paintings. A mixture of various solvents in presence of borax and epoxy resin has been also tested on an Iranian oil painting belonging to the Qajar era [113]. In another case, organogels have been used to clean a gold-leaf coated painting along with an oxidized varnish [50]. Prati et al. studied the impact of PHB-GVL organogel to clean a tempera painting [8], and have obtained a new PHB-based organogel in combination with ethyl lactate and dimethyl carbonate [DMC] [114]. To remove an aged varnish, a TC-PN based gel of PVA and an oil-in-water nanostructured fluid has been examined [115]. Mastrangelo et al. have also utilized the TC-PN hydrogel to clean the contaminations of Jackson Pollock painting gradually [85]. To clean the carbonaceous dirt from a wall painting, Nanorestore Gel® Peggy 6 was employed [116]. Kevin et al. have prepared a new hydrogel system with dual crosslinks to eliminate the unwanted layers from modern paintings such as based on acrylic paints, however this new recipe needs further optimizations [117]. Other research has reported on using hydrogels loaded with nanostructured fluids to clean street art [118]. An Egyptian multilayered polychrome wooden sculpture has been cleaned by PVA based hydrogel [119]. Another case represents the combination of DES (deep eutectic solvents) with agar to eliminate the aged thin varnish from oil and tempera paintings [120]. A mixture of PVA alongside with rice starch has produced an effective gel to clean soil from modern paintings [121]. On the other hand, to properly clean water sensitive artistic surfaces, a new system prepared by castor oil has been examined on mock-ups [3]. One of the major challenges that conservators confront, is the inefficient former restorations done by other conservators. To deal with this, a new organogel has been obtain to clean the wax resins from those canvases that had been restored using the Dutch method (Fig. 4) [122]. Chelazzi and Baglioni [123] have reported that the aged varnish of a modern painting has been well cleaned using castor oil-based organogel.
Photos and optical microscopic images of canvas before the cleaning process and after the cleaning process lasting, respectively, 5, 10, 20, 30, 40, 50, and 60 min [122]
Overall, gels have proved to be effective and versatile cleaning agents for various types of paintings, including those with delicate and sensitive surfaces. The choice of gel composition depends on the specific cleaning needs and the nature of the painting materials. However, further research is still needed to optimize the gel formulations and ensure their long-term stability and compatibility with painted surfaces.
The use of gels in metal cleaning
According to the studies, to clean the copper stains from a metallic surface, using Agar gels containing additives is twice effective than the pure Agar [65]. In another case, to eliminate the contamination from a bronze artifact, a PVA based gel has been used [4]. Furthermore, using a PHB-based organogel would be an effective method to clean wax coatings from indoor bronze works [124].
Researchers have examined diverse combinations of various gels to clean some archaeological silver-plated copper alloy coins. They have reported that using physical peelable gels along with treatment solutions were acceptably efficient [29].
Other studies have proven that to clean natural corrosions of iron, using agar-based gel with siderophore DFO (deferoxamine B) will be effective [125]. Guaragnone et al. have developed a semi-IPN of pHEMA/PAA to clean copper ions and corrosion products [126].
In addition, polysaccharide-based hydrogels have been used for cleaning various metal objects, including copper alloys, lead artifacts, vermeil gold, and painted aluminum alloy on a gun from the Pierro Loti museum collection, an Armenian censer from Dobrée museum, and coins from Cléons treasure [27] (Fig. 5).
And to eliminate the corrosion products from a metallic substrate, Polysaccharide phases (agar and gellan gum) loaded with deferoxamine, as an active agent, were found to be effective [127]. Table 3 lists the different kinds of gels that have been utilized for the purpose of cleaning a diverse array of historical metals.
The use of gels in cleaning other materials
Gel is not only utilized in the conservation of metal, paper, and painting objects but has also been employed for various other objects as well (as shown in Table 4). In fact, different types of gels have been used with different purposes in the field of art conservation. For example, a kind of organogel which is prepared through combination of thiol or allyl monomers are reported for removing any dammar-based varnishes from artistic surfaces [128]. Various other types of gels have also been reported, and each of these has been employed for particular applications. A Dimethyl sulfoxide (DMSO) solvent gel is an effective and low-impact method for removing biologically patinated stone. It is easy to use and inexpensive [129]. AgarArt 1% with ammonium citrate tribasic (TAC) is an effective formulation for copper removal from built heritage through Lyophilization of hydrogels [130]. In a case study, to clean Mn rich black stains from a granite belonging to a church and a number of glass jars, chitosan based gel has been assessed [131]. In another case a HEMA-MBA/PVP hydrogel loaded with NSE is employed to clean polymeric coatings from built heritage and stone surface [132].
Gellan gum has proven to be an effective cleaning agent for damaged collages, silver gelatin photographs, and even a terrestrial globe (as a 3D object) [75]. In another study, 18th-century gilt leather samples were successfully cleaned using gellan gum hydrogels, which proved useful due to the minimal water release of gellan gum [133]. One prominent example of modern artwork treated with gels is Eva Hesse’s sculpture Addendum, where a modified gel from the Nanorestore Gel® Peggy 5 (PVA/PVP) was used for cleaning [134]. Additionally, hydrogels have been validated as effective systems for cleaning metal historic properties, with DFO-B ethanol gels based on 4% agarose performing better in cleaning water-sensitive substrates like wooden artworks [135].
Textile is another material that gel is used for cleaning. An archaeological Coptic textile contaminated by polyurethane adhesive is one of these textiles. To clean this cultural material, a Nano Restore Gel® Peggy 5 loaded with Nano Restore Cleaning® Polar fluid was assessed [136]. Moreover, a new frontier in textile cleaning has been investigated by Jedrusik, Krasnan, Rehakova and Rebros [137] through bio-cleaning with the aid of placed microorganisms in a gel system. In this case, cotton and silk models were stained using iron gall ink, and P. putida bacteria have been placed in an agar-based gel system to clean the undesired discoloration. In another report by Smets, De Vis and Ortega-Saez [138], an embroidered silk textile has been investigated. The researchers have utilized an agarose 5% base loaded with pH buffers and chelators to clean the yellowness caused by natural degradation. However, the cleaning system presented satisfactory results in dye bleeding prevention, but the stains could not be cleaned thoroughly.
Data analysis
In this section, a total of fifty-nine case studies were reviewed. These case studies were classified into four main categories: paper, painting, metal, and other materials. The distribution of these case studies among the categories is presented in Fig. 6. As can be seen from the figure, a considerable number of studies have focused on the application of gel for cleaning paintings, followed by paper conservation.
Based on the review of the use of gels in cleaning historical and artistic artifacts, it is evident that a variety of gels have been utilized in different studies. Organogels and hydrogels have been the main types of gels used, with gellan gum and p(HEMA/PVP) being the most frequently employed in paper cleaning. As shown in Table 1, there have been fourteen research studies conducted on paper cleaning. Out of these, three studies have utilized organogels, while the remaining eleven studies have involved the testing of hydrogels. The primary damage types observed during cleaning were unwanted layers and PSTs being effectively removed.
According to the information in Table 2, a total of twenty-three research studies have been reviewed about painting cleaning. These studies have explored a diverse range of gel combinations, with p(HEMA/PVP) and PHB/GVL being commonly used as base materials. Additionally, other materials such as PVA, castor oil, and acrylamide have also been employed in addressing the challenges associated with painting cleaning.
For metal cleaning, agar and polysaccharide-based gels have been the most commonly used systems, as demonstrated in eight case studies (Table 3). Similarly, hydrogels based on agar, gellan gum, and agarose have been frequently employed in cleaning different materials such as textile, leather, and stone (Table 4).
One overlooked aspect in most of these studies is the lack of discussion regarding the potential defects or drawbacks of the examined gels. Addressing these cases in future research endeavors will undoubtedly contribute significantly to guiding the direction and progress in the study of gels for the cleaning of artworks.
Conclusion and future perspective
The use of gel-based cleaning methodologies in art conservation has significantly evolved over time, providing more than safer, more effective solutions for the preservation of cultural heritage. The ability of gels to control solvent evaporation rates, surface contact time, and minimize human exposure to toxic solvents has made them a preferred choice for art conservators. Even by employing enzymes and different kinds of surfactants in a gel system, the operator would be able to enhance the cleaning efficiency. It would be noteworthy to address that in some cases, due to the nature of the artwork material and the contaminant, there could be a great need for combining a gel system with other cleaning approaches to gain satisfactory results. Despite all the mentioned advantages, there are still some challenging aspects that need to be discussed in detail, such as the long-term impacts of gels in various environmental conditions leading to alteration and deterioration, and of course, the process of natural aging of the artwork material contacted with this cleaning method. However, challenges remain, particularly when cleaning painted surfaces, necessitating further research and development.
Further research needs to concentrate on enhancing gel formulations while guaranteeing their compatibility with different surfaces. Moreover, the long-term impacts of these gels on the surfaces of the artifacts should be explored if they are not entirely eliminated. In addition, it seems that further study of advanced and responsive gels is important in determining the potential of this type of gel in art conservation due to their prominent features such as low level of residue remaining and Nano-scale mesh size for reversible and magnetic gels respectively. The potential to combine gels with nanotechnology or materials science could lead to more efficient and specific cleaning and conservation methodologies. Additionally, the exploration of new natural and synthesized polymers could expand the types of gels available for use in the field.
The cleaning method discussed primarily focuses on paintings, covering various painting techniques. Paper works, and metallic works are also considered, but there is a need for further research on materials like glass, stone, wood, building materials, and 3-D objects. The main contaminants that are addressed through cleaning are aged varnishes, various types of STP tapes, and corrosion products. However, there are additional contaminants, such as fungal stains, foxing, and greasy stains on uncoated surfaces, that require investigation.
Preserving our cultural heritage is a collective responsibility and the advancements in gel-based cleaning methodologies play an essential role. As we move forward, a multi-disciplinary approach incorporating nanoscience, materials science, and polymer chemistry will be instrumental in developing innovative solutions for the challenges faced in art conservation.
Data availability
No datasets were generated or analysed during the current study.
References
Chelazzi D, Giorgi R, Baglioni P. Microemulsions, micelles, and functional gels: how colloids and soft matter preserve works of art. Angew Chem Int Ed. 2018;57:7296–303. https://doi.org/10.1002/anie.201710711.
Baglioni M, Poggi G, Chelazzi D, Baglioni P. Advanced materials in cultural heritage conservation. Molecules. 2021;26:3967. https://doi.org/10.3390/molecules26133967.
Poggi G, Santan HD, Smets J, Chelazzi D, Noferini D, Petruzzellis ML, Buemi LP, Fratini E, Baglioni P. Nanostructured bio-based castor oil organogels for the cleaning of artworks. J Colloid Interface Sci. 2023;638:363–74. https://doi.org/10.1016/j.jcis.2023.01.119.
Parisi EI, Bonelli N, Carretti E, Giorgi R, Ingo GM, Baglioni P. Film forming PVA-based cleaning systems for the removal of corrosion products from historical bronzes. Pure Appl Chem. 2018;90:507–22. https://doi.org/10.1515/pac-2017-0204.
Baglioni P, Chelazzi D, Giorgi R. Nanotechnologies in the conservation of cultural heritage: a compendium of materials and techniques. Springer; 2015.
Baglioni M, Raudino M, Berti D, Keiderling U, Bordes R, Holmberg K, Baglioni P. Nanostructured fluids from degradable nonionic surfactants for the cleaning of works of art from Polymer contaminants. Soft Matter. 2014;10:6798–809. https://doi.org/10.1039/C4SM01084A.
Baglioni P, Berti D, Bonini M, Carretti E, Dei L, Fratini E, Giorgi R. Micelle, microemulsions, and gels for the conservation of cultural heritage. Adv Colloid Interface Sci. 2014;205:361–71. https://doi.org/10.1016/j.cis.2013.09.008.
Prati S, Volpi F, Fontana R, Galletti P, Giorgini L, Mazzeo R, Mazzocchetti L, Samorì C, Sciutto G, Tagliavini E. Sustainability in art conservation: a novel bio-based organogel for the cleaning of water sensitive works of art. Pure Appl Chem. 2018;90:239–51. https://doi.org/10.1515/pac-2017-0507.
Stulik DM. David. In: Pardo P, editor. Solvent gels for the cleaning of works of art. Los Angeles, California: Getty; 2004.
Carretti E, Dei L, Weiss RG, Baglioni P. A new class of gels for the conservation of painted surfaces. J Cult Herit. 2008;9:386–93.
Wolbers R. Cleaning painted surfaces: aqueous methods. Archetype; 2000.
Wolbers RC, Stavroudis C. Handbook for critical cleaning: applications, processes, and controls. Boca Raton, Florida, United States: CRC; 2011.
Smith DK. Applications of supramolecular gels. Mol Gels: Struct Dyn. 2018;300–71.
Tanaka, Gels, Scientific American 244 (1981) 124-S-117.
Khandekar N. Gelled systems: theory and early application, Solvent gels for the Cleaning of Works of Art: the Residue question. Los Angeles: Getty Conservation Institute; 2004. pp. 5–11.
Zhang J, Hu Y, Li Y. Gel chemistry: interactions, structures and properties. Berlin: Springer; 2018.
Chen Y. Hydrogels based on natural polymers. Berlin: Elsevier; 2020.
Baglioni P, Baglioni M, Bonelli N, Chelazzi D, Giorgi R. In: Lazzara G, editor. Nanotechnologies and nanomaterials for diagnostic, conservation and restoration of cultural heritage. R. Fakhrullin (Elsevier; 2019.
Gueidão M, Vieira E, Bordalo R, Moreira P. Available green conservation methodologies for the cleaning of cultural heritage: an overview, Estudos De Conservação E Restauro (2020) 22–44. https://doi.org/10.34632/ecr.2020.10679.
Baglioni M, Giorgi R, Berti D, Baglioni P. Smart cleaning of cultural heritage: a new challenge for soft nanoscience. Nanoscale. 2012;4:42–53. https://doi.org/10.1039/C1NR10911A.
Carretti E, Bonini M, Dei L, Berrie BH, Angelova LV, Baglioni P, Weiss RG. New frontiers in materials science for art conservation: responsive gels and beyond. Acc Chem Res. 2010;43:751–60. https://doi.org/10.1021/ar900282h.
Passaretti A, Cuvillier L, Sciutto G, Guilminot E, Joseph E. Biologically derived gels for the cleaning of historical and artistic metal heritage. Appl Sci. 2021;11:3405. https://doi.org/10.3390/app11083405.
Bonelli N, Montis C, Mirabile A, Berti D, Baglioni P. Restoration of paper artworks with microemulsions confined in hydrogels for safe and efficient removal of adhesive tapes, Proceedings of the National Academy of Sciences 115 (2018) 5932–5937. https://doi.org/10.1073/pnas.1801962115.
Fratini E, Carretti E, Cleaning IV. Gels and polymeric dispersions, nanoscience for the conservation of works of art 28 (2013) 252–68.
Horkay F, Douglas JF. Polymer gels: basics, challenges, and perspectives, gels and other soft amorphous solids (2018) 1–13.
Sangeetha NM, Maitra U. Supramolecular gels: functions and uses. Chem Soc Rev. 2005;34:821–36. https://doi.org/10.1039/B417081B.
Guilminot E. The Use of hydrogels in the treatment of Metal Cultural Heritage objects. Gels. 2023;9:191. https://doi.org/10.3390/gels9030191.
An Y, Solis FJ, Jiang H. A thermodynamic model of physical gels. J Mech Phys Solids. 2010;58:2083–99. https://doi.org/10.1016/j.jmps.2010.09.002.
Giraud T, Gomez A, Lemoine S, Pelé-Meziani C, Raimon A, Guilminot E. Use of gels for the cleaning of archaeological metals. Case study of silver-plated copper alloy coins. J Cult Herit. 2021;52:73–83. https://doi.org/10.1016/j.culher.2021.08.014.
Yu G, Yan X, Han C, Huang F. Characterization of supramolecular gels. Chem Soc Rev. 2013;42:6697–722. https://doi.org/10.1039/C3CS60080G.
Redaelli F, Sorbona M, Rossi F. In: Perale G, editor. Bioresorbable polymers for biomedical applications. J. Hilborn (Woodhead Publishing; 2017.
Syed KHG, Saphwan A-A, Glyn OP. In: Angelo C, editor. Progress in Molecular and Environmental Bioengineering. Rijeka: IntechOpen; 2011.
Casini A, Chelazzi D, Baglioni P. Advanced methodologies for the cleaning of works of art. Sci China Technological Sci. 2023;1–21. https://doi.org/10.1007/s11431-022-2348-7.
Lupu A, Gradinaru LM, Gradinaru VR, Bercea M. Diversity of bioinspired hydrogels: from structure to applications. Gels. 2023;9:376. https://doi.org/10.3390/gels9050376.
Kaliaraj GS, Shanmugam DK, Dasan A, Mosas KKA. Hydrogels—a promising materials for 3D printing technology. Gels. 2023;9:260. https://doi.org/10.3390/gels9030260.
Suzuki A, Sasaki S. Swelling and mechanical properties of physically crosslinked poly (vinyl alcohol) hydrogels, Proceedings of the Institution of Mechanical Engineers, Part H: Journal of Engineering in Medicine 229 (2015) 828–844. https://doi.org/10.1177/0954411915615469.
Mikhailidi A, Volf I, Belosinschi D, Tofanica B-M, Ungureanu E. Cellulose-based metallogels—part 1: raw materials and preparation. Gels. 2023;9:390. https://doi.org/10.3390/gels9050390.
Ahmed EM. Hydrogel: Preparation, characterization, and applications: a review. J Adv Res. 2015;6:105–21. https://doi.org/10.1016/j.jare.2013.07.006.
Park KR, Nho YC. Synthesis of PVA/PVP hydrogels having two-layer by radiation and their physical properties. Radiat Phys Chem. 2003;67:361–5. https://doi.org/10.1016/S0969-806X(03)00067-7.
Ghorpade VS. Hydrogels based on natural polymers. Berlin: Elsevier; 2020.
Peppas NA, Bures P, Leobandung W, Ichikawa H. Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm. 2000;50:27–46. https://doi.org/10.1016/S0939-6411(00)00090-4.
Caló E, Khutoryanskiy VV. Biomedical applications of hydrogels: a review of patents and commercial products. Eur Polymer J. 2015;65:252–67. https://doi.org/10.1016/j.eurpolymj.2014.11.024.
Ammar NEB, Barbouche M, Hamzaoui AH. Hydrogels based on natural polymers. Berlin: Elsevier; 2020.
Hoffman AS. Hydrogels for biomedical applications, advanced drug delivery reviews 64 (2012) 18–23. https://doi.org/10.1016/j.addr.2012.09.010.
Rosiak JM, Yoshii F. Hydrogels and their medical applications, nuclear instruments and methods in physics research section b: beam interactions with materials and atoms 151 (1999) 56–64. https://doi.org/10.1016/S0168-583X(99)00118-4.
Baglioni P, Chelazzi D, Giorgi R. (Springer, 2021).
Soto-Bustamante F, Bassu G, Fratini E, Laurati M. Effect of composition and freeze-thaw on the network structure, porosity and mechanical properties of polyvinyl-alcohol/chitosan hydrogels. Gels. 2023;9:396. https://doi.org/10.3390/gels9050396.
Zhou S, Zhao Z, Mao H, Wang L, Chen J, Chen J, Huang X. Bronze preservation by using composite hydrogel coating-loaded corrosion inhibitors. Herit Sci. 2022;10:116. https://doi.org/10.1186/s40494-022-00747-w.
Wahid F, Zhao X-J, Jia S-R, Bai H, Zhong C. Nanocomposite hydrogels as multifunctional systems for biomedical applications: current state and perspectives. Compos Part B: Eng. 2020;200:108208. https://doi.org/10.1016/j.compositesb.2020.108208.
Duncan TT, Berrie BH, Weiss RG. Soft, peelable organogels from partially hydrolyzed poly (vinyl acetate) and benzene-1, 4-diboronic acid: applications to clean works of art. ACS Appl Mater Interfaces. 2017;9:28069–78. https://doi.org/10.1021/acsami.7b09473.
Baglioni P, Bonelli N, Chelazzi D, Chevalier A, Dei L, Domingues J, Fratini E, Giorgi R, Martin M. Organogel formulations for the cleaning of easel paintings. Appl Phys A. 2015;121:857–68. https://doi.org/10.1007/s00339-015-9364-0.
Zeng L, Lin X, Li P, Liu F-Q, Guo H, Li W-H. Recent advances of organogels: from fabrications and functions to applications. Prog Org Coat. 2021;159:106417. https://doi.org/10.1016/j.porgcoat.2021.106417.
Yilmazer S, Schwaller D, Mésini PJ. Beyond sol-gel: molecular gels with different transitions. Gels. 2023;9:273. https://doi.org/10.3390/gels9040273.
Pianorsi MD, Raudino M, Bonelli N, Chelazzi D, Giorgi R, Fratini E, Baglioni P. Organogels for the cleaning of artifacts. Pure Appl Chem. 2017;89:3–17. https://doi.org/10.1515/pac-2016-0908.
Reddy GSC, Anilreddy B, Jotish M, Pranitha CN, Suryadevara H. Online through Review Article www.ijptonline.com.
Sannino A, Demitri C, Madaghiele M. Biodegradable cellulose-based hydrogels: design and applications. Materials. 2009;2:353–73. https://doi.org/10.3390/ma2020353.
Kabir SF, Sikdar PP, Haque B, Bhuiyan MR, Ali A, Islam M. Cellulose-based hydrogel materials: Chemistry, properties and their prospective applications. Prog Biomater. 2018;7:153–74. https://doi.org/10.1007/s40204-018-0095-0.
Duceac IA, Stanciu M-C, Nechifor M, Tanasă F, Teacă C-A. Insights on some polysaccharide gel type materials and their structural peculiarities. Gels. 2022;8:771. https://doi.org/10.3390/gels8120771.
Chelazzi D, Fratini E, Giorgi R, Mastrangelo R, Rossi M, Baglioni P. Gels and other soft amorphous solids. Washington, D.C.: ACS Publications; 2018.
Casoli A, Di Diego Z, Isca C. Cleaning painted surfaces: evaluation of leaching phenomenon induced by solvents applied for the removal of gel residues. Environ Sci Pollut Res. 2014;21:13252–63. https://doi.org/10.1007/s11356-014-2658-5.
Bertasa M, Poli T, Riedo C, Di Tullio V, Capitani D, Proietti N, Canevali C, Sansonetti A, Scalarone D. A study of non-bounded/bounded water and water mobility in different agar gels. Microchem J. 2018;139:306–14. https://doi.org/10.1016/j.microc.2018.03.016.
Bertasa M, Dodero A, Alloisio M, Vicini S, Riedo C, Sansonetti A, Scalarone D, Castellano M. Agar gel strength: a correlation study between chemical composition and rheological properties. Eur Polymer J. 2020;123:109442. https://doi.org/10.1016/j.eurpolymj.2019.109442.
Bertasa M, Botteon A, Brambilla L, Riedo C, Chiantore O, Poli T, Sansonetti A, Scalarone D. Cleaning materials: a compositional multi-analytical characterization of commercial agar powders. J Anal Appl Pyrol. 2017;125:310–7. https://doi.org/10.1016/j.jaap.2017.03.011.
Bosch-Roig P, Lustrato G, Zanardini E, Ranalli G. Biocleaning of cultural heritage stone surfaces and frescoes: which delivery system can be the most appropriate? Ann Microbiol. 2015;65:1227–41. https://doi.org/10.1007/s13213-014-0938-4.
Canevali C, Fasoli M, Bertasa M, Botteon A, Colombo A, Di Tullio V, Capitani D, Proietti N, Scalarone D, Sansonetti A. A multi-analytical approach for the study of copper stain removal by agar gels. Microchem J. 2016;129:249–58. https://doi.org/10.1016/j.microc.2016.07.007.
Sansonetti A, Bertasa M, Canevali C, Rabbolini A, Anzani M, Scalarone D. A review in using agar gels for cleaning art surfaces. J Cult Herit. 2020;44:285–96. https://doi.org/10.1016/j.culher.2020.01.008.
Kaneda I. Effect of Sweeteners on the solvent transport behaviour of mechanically-constrained agarose gels. Gels. 2018. https://doi.org/10.3390/gels4010023.
Kaczmarek B, Nadolna K, Owczarek A. The physical and chemical properties of hydrogels based on natural polymers, hydrogels based on natural polymers (2020) 151–72. https://doi.org/10.1016/B978-0-12-816421-1.00006-9.
Cremonesi P. Surface cleaning? Yes, freshly grated Agar gel, please, studies in conservation 61 (2016) 362–7. https://doi.org/10.1179/2047058415Y.0000000026.
Morris ER, Nishinari K, Rinaudo M. Gelation of gellan–a review. Food Hydrocoll. 2012;28:373–411. https://doi.org/10.1016/j.foodhyd.2012.01.004.
Mazzuca C, Micheli L, Carbone M, Basoli F, Cervelli E, Iannuccelli S, Sotgiu S, Palleschi A. Gellan hydrogel as a powerful tool in paper cleaning process: a detailed study. J Colloid Interface Sci. 2014;416:205–11. https://doi.org/10.1016/j.jcis.2013.10.062.
Gomes D, Batista-Silva J, Sousa A, Passarinha L. Progress and opportunities in gellan gum-based materials: a review of preparation, characterization and emerging applications. Carbohydr Polym. 2023. https://doi.org/10.1016/j.carbpol.2023.120782.
Mazzuca C, Micheli L, Lettieri R, Cervelli E, Coviello T, Cencetti C, Sotgiu S, Iannuccelli S, Palleschi G, Palleschi A. How to tune a paper cleaning process by means of modified gellan hydrogels. Microchem J. 2016;126:359–67. https://doi.org/10.1016/j.microc.2015.12.016.
Hughes A, Sullivan M. Targeted cleaning of works on paper: rigid polysaccharide gels and conductivity in aqueous solutions. Book Paper Group Annual. 2016;25:30–41.
Maheux AF. Cross-disciplinary uses for gellan gum in conservation. Book Paper Group Annual. 2015;34:69–79.
Di Vito M, Bellardi MG, Colaizzi P, Ruggiero D, Mazzuca C, Micheli L, Sotgiu S, Iannuccelli S, Michelozzi M, Mondello F. Hydrolates and gellan: an eco-innovative synergy for safe cleaning of paper artworks. Stud Conserv. 2018;63:13–23. https://doi.org/10.1080/00393630.2017.1389442.
Paradossi G, Finelli I, Cerroni B, Chiessi E. Adding chemical cross-links to a physical hydrogel. Molecules. 2009;14:3662–75. https://doi.org/10.3390/molecules14093662.
Taokaew S, Kaewkong W, Kriangkrai W. Recent development of functional Chitosan-based hydrogels for pharmaceutical and biomedical applications. Gels. 2023;9:277. https://doi.org/10.3390/gels9040277.
Carpa R, Farkas A, Dobrota C, Butiuc-Keul A. Double-network Chitosan-based hydrogels with improved mechanical, conductive, antimicrobial, and antibiofouling properties. Gels. 2023;9:278. https://doi.org/10.3390/gels9040278.
Lai H, Liu S, Yan J, Xing F, Xiao P. Facile fabrication of biobased hydrogel from natural resources: l-cysteine, itaconic anhydride, and Chitosan. ACS Sustain Chem Eng. 2020;8:4941–7. https://doi.org/10.1021/acssuschemeng.0c00774.
Hassan CM, Peppas NA. Structure and applications of poly (vinyl alcohol) hydrogels produced by conventional crosslinking or by freezing/thawing methods, biopolymers· PVA hydrogels, anionic polymerisation nanocomposites (2000) 37–65. https://doi.org/10.1007/3-540-46414-X_2.
Riedo C, Rollo G, Chiantore O, Scalarone D. Detection and identification of possible gel residues on the surface of paintings after cleaning treatments. Heritage. 2021;4:304–15. https://doi.org/10.3390/heritage4010019.
Sasaki S, Omata S, Murakami T, Nagasawa N, Taguchi M, Suzuki A. Effect of gamma ray irradiation on friction property of poly (vinyl alcohol) cast-drying on freeze-thawed hybrid gel. Gels. 2018;4:30. https://doi.org/10.3390/gels4020030.
Kamemaru K, Usui S, Hirashima Y, Suzuki A. Irreversible swelling behavior and reversible hysteresis in chemically crosslinked poly (vinyl alcohol) gels. Gels. 2018;4:45. https://doi.org/10.3390/gels4020045.
Mastrangelo R, Chelazzi D, Poggi G, Fratini E, Pensabene Buemi L, Petruzzellis ML, Baglioni P. Twin-chain polymer hydrogels based on poly (vinyl alcohol) as new advanced tool for the cleaning of modern and contemporary art, Proceedings of the National Academy of Sciences 117 (2020) 7011–7020. https://doi.org/10.1073/pnas.1911811117.
Leona M, Fukunaga K, Liang H, Baglioni P, Festa G, Levchenko V. From physics to art and back. Nat Reviews Phys. 2021;3:681–4. https://doi.org/10.1038/s42254-021-00362-x.
Carretti E, Dei L, Baglioni P. Aqueous polyacrylic acid based gels: physicochemical properties and applications in cultural heritage conservation. Trends Colloid Interface Sci XVI. 2004. https://doi.org/10.1007/978-3-540-36462-7_60.
Mummaleti G, Kong F. Fabrication, properties and applications of xerogels in food processing. J Agric Food Res. 2023;11:100506. https://doi.org/10.1016/j.jafr.2023.100506.
Yamasaki S, Sakuma W, Yasui H, Daicho K, Saito T, Fujisawa S, Isogai A, Kanamori K. Nanocellulose xerogels with high porosities and large specific surface areas. Front Chem. 2019;7:316. https://doi.org/10.3389/fchem.2019.00316.
Bratovcic A, Petrinic I, in: New technologies, development and, application III. 6Springer, (2020). https://doi.org/10.1007/978-3-030-46817-0.
Torres CEI, Quezada TES, Kharissova OV, Kharisov BI, de la Fuente MIG. Carbon-based aerogels and xerogels: Synthesis, properties, oil sorption capacities, and DFT simulations. J Environ Chem Eng. 2021;9:104886.
Mosquera MaJ, Pozo J, Esquivias L, Rivas T, Silva B. Application of mercury porosimetry to the study of xerogels used as stone consolidants. J Non-cryst Solids. 2002;311:185–94. https://doi.org/10.1016/S0022-3093(02)01370-4.
Domingues JA, Bonelli N, Giorgi R, Fratini E, Gorel F, Baglioni P. Innovative hydrogels based on semi-interpenetrating p (HEMA)/PVP networks for the cleaning of water-sensitive cultural heritage artifacts. Langmuir. 2013;29:2746–55. https://doi.org/10.1021/la3048664.
Tamburini G, Canevali C, Ferrario S, Bianchi A, Sansonetti A, Simonutti R. Optimized semi-interpenetrated p (HEMA)/PVP hydrogels for artistic surface cleaning. Materials. 2022;15:6739. https://doi.org/10.3390/ma15196739.
Domingues J, Bonelli N, Giorgi R, Baglioni P. Chemical semi-IPN hydrogels for the removal of adhesives from canvas paintings. Appl Phys A. 2014;114:705–10. https://doi.org/10.1007/s00339-013-8150-0.
Baglioni M, Domingues JA, Carretti E, Fratini E, Chelazzi D, Giorgi R, Baglioni P. Complex fluids confined into semi-interpenetrated chemical hydrogels for the cleaning of classic art: a rheological and SAXS study, ACS applied materials & interfaces 10 (2018) 19162–72. https://doi.org/10.1021/acsami.8b01841.
Bonham J, Faers M, Van Duijneveldt J. Non-aqueous microgel particles: synthesis, properties and applications. Soft Matter. 2014;10:9384–98. https://doi.org/10.1039/C4SM01834F.
Di Napoli B, Franco S, Severini L, Tumiati M, Buratti E, Titubante M, Nigro V, Gnan N, Micheli L, Ruzicka B. Gellan gum microgels as effective agents for a rapid cleaning of paper, ACS applied polymer materials 2 (2020) 2791–801. https://doi.org/10.1021/acsapm.0c00342.
Karg M, Pich A, Hellweg T, Hoare T, Lyon LA, Crassous J, Suzuki D, Gumerov RA, Schneider S, Potemkin II. Nanogels and microgels: from model colloids to applications, recent developments, and future trends. Langmuir. 2019;35:6231–55. https://doi.org/10.1021/acs.langmuir.8b04304.
Nigro V, Angelini R, Bertoldo M, Bruni F, Ricci MA, Ruzicka B. Dynamical behavior of microgels of interpenetrated polymer networks. Soft Matter. 2017;13:5185–93. https://doi.org/10.1039/C7SM00739F.
Bergman MJ, Gnan N, Obiols-Rabasa M, Meijer J-M, Rovigatti L, Zaccarelli E, Schurtenberger P. A new look at effective interactions between microgel particles. Nat Commun. 2018;9:5039. https://doi.org/10.1038/s41467-018-07332-5.
Zanatta M, Tavagnacco L, Buratti E, Chiessi E, Natali F, Bertoldo M, Orecchini A, Zaccarelli E. Atomic scale investigation of the volume phase transition in concentrated PNIPAM microgels. J Chem Phys. 2020. https://doi.org/10.1063/5.0007112.
Pelton R, Hoare T. Microgels and their synthesis: An introduction, microgel suspensions: fundamentals and applications (2011) 1–32. https://doi.org/10.1002/9783527632992.ch1.
Iannuccelli S, Sotgiu S, Wet treatments of works of art on paper with rigid gellan gels, The book and paper group annual 29 (2010) 25–39
Li H, Severini L, Titubante M, Gong D, Micheli L, Mazzuca C, Gong Y. Gellan gum hydrogel as an aqueous treatment method for Xuan paper, restaurator. Int J Preservation Libr Arch Mater. 2021;42:37–54. https://doi.org/10.1515/res-2020-0010.
Mazzuca C, Poggi G, Bonelli N, Micheli L, Baglioni P, Palleschi A. Innovative chemical gels meet enzymes: a smart combination for cleaning paper artworks. J Colloid Interface Sci. 2017;502:153–64. https://doi.org/10.1016/j.jcis.2017.04.088.
Ferrari P, Chelazzi D, Bonelli N, Mirabile A, Giorgi R, Baglioni P. Alkyl carbonate solvents confined in poly (ethyl methacrylate) organogels for the removal of pressure sensitive tapes (PSTs) from contemporary drawings. J Cult Herit. 2018;34:227–36. https://doi.org/10.1016/j.culher.2018.05.009.
Mirabile A, Chelazzi D, Ferrari P, Montis C, Berti D, Bonelli N, Giorgi R, Baglioni P. Innovative methods for the removal, and occasionally care, of pressure sensitive adhesive tapes from contemporary drawings. Herit Sci. 2020;8:42. https://doi.org/10.1186/s40494-020-00387-y.
Pizzorusso G, Fratini E, Eiblmeier J, Giorgi R, Chelazzi D, Chevalier A, Baglioni P. Physicochemical characterization of acrylamide/bisacrylamide hydrogels and their application for the conservation of easel paintings. Langmuir. 2012;28:3952–61. https://doi.org/10.1021/la2044619.
Domingues J, Bonelli N, Giorgi R, Fratini E, Baglioni P, Innovative method for the cleaning of watersensitive artifacts. : synthesis and application of highly retentive chemical hydrogels, International Journal of Conservation Science (2013).
Baglioni P, Carretti E, Chelazzi D. Nanomaterials in art conservation. Nat Nanotechnol. 2015;10:287–90. https://doi.org/10.1038/nnano.2015.38.
Samorì C, Galletti P, Giorgini L, Mazzeo R, Mazzocchetti L, Prati S, Sciutto G, Volpi F, Tagliavini E. The green attitude in art conservation: polyhydroxybutyrate–based gels for the cleaning of oil paintings, ChemistrySelect 1 (2016) 4502–8. https://doi.org/10.1002/slct.201601180.
Alizadeh S. Cleaning and restoration of an oil painting with a polymer gel in Iran. Conserv Sci Cult Herit. 2017;17:139–47. https://doi.org/10.6092/issn.1973-9494/7952.
Prati S, Sciutto G, Volpi F, Rehorn C, Vurro R, Blümich B, Mazzocchetti L, Giorgini L, Samorì C, Galletti P. Cleaning oil paintings: NMR relaxometry and SPME to evaluate the effects of green solvents and innovative green gels. New J Chem. 2019;43:8229–38. https://doi.org/10.1039/C9NJ00186G.
Pensabene Buemi L, Petruzzellis ML, Chelazzi D, Baglioni M, Mastrangelo R, Giorgi R, Baglioni P. Twin-chain polymer networks loaded with nanostructured fluids for the selective removal of a non-original varnish from Picasso’s L’Atelier at the Peggy Guggenheim Collection, Venice. Herit Sci. 2020;8:77. https://doi.org/10.1186/s40494-020-00420-0.
Segel K, Brajer I, Taube M, Martin de Fonjaudran C, Baglioni M, Chelazzi D, Giorgi R, Baglioni P. Removing ingrained soiling from medieval lime-based wall paintings using nanorestore Gel® peggy 6 in combination with aqueous cleaning liquids, studies in conservation 65 (2020) P284–91. https://doi.org/10.1080/00393630.2020.1790890.
Kevin J, D’Emilio E, Cranston ED, Geiger T, Nyström G. Dual physically and chemically crosslinked regenerated cellulose–gelatin composite hydrogels towards art restoration. Carbohydr Polym. 2020;234:115885. https://doi.org/10.1016/j.carbpol.2020.115885.
Baglioni M, Poggi G, Giorgi R, Rivella P, Ogura T, Baglioni P. Selective removal of over-paintings from street art using an environmentally friendly nanostructured fluid loaded in highly retentive hydrogels. J Colloid Interface Sci. 2021;595:187–201. https://doi.org/10.1016/j.jcis.2021.03.054.
Manfredda N, Buscaglia P, Gallo P, Borla M, Aicardi S, Poggi G, Baglioni P, Nervo M, Scalarone D, Borghi A. An ancient Egyptian multilayered polychrome wooden sculpture belonging to the museo egizio of torino: characterization of painting materials and design of cleaning processes by means of highly retentive hydrogels. Coatings. 2021;11:1335. https://doi.org/10.3390/coatings11111335.
Jia Y, Sciutto G, Botteon A, Conti C, Focarete ML, Gualandi C, Samorì C, Prati S, Mazzeo R. Deep eutectic solvent and agar: a new green gel to remove proteinaceous-based varnishes from paintings. J Cult Herit. 2021;51:138–44. https://doi.org/10.1016/j.culher.2021.08.001.
Rosciardi V, Chelazzi D, Baglioni P. Green biocomposite poly (vinyl alcohol)/starch cryogels as new advanced tools for the cleaning of artifacts. J Colloid Interface Sci. 2022;613:697–708. https://doi.org/10.1016/j.jcis.2021.12.145.
Kaniewska K, Pilecka-Pietrusińska Eb, Karbarz M. Nanocomposite organogel for art conservation a novel wax resin removal system. ACS Appl Mater Interfaces. 2023;15:24798–811. https://doi.org/10.1021/acsami.3c00321.
Chelazzi D, Baglioni P. From nanoparticles to gels: a breakthrough in art conservation science, Langmuir (2023). https://doi.org/10.1021/acs.langmuir.3c01324.
Yiming J, Sciutto G, Prati S, Catelli E, Galeotti M, Porcinai S, Mazzocchetti L, Samorì C, Galletti P, Giorgini L. A new bio-based organogel for the removal of wax coating from indoor bronze surfaces. Herit Sci. 2019;7:34. https://doi.org/10.1186/s40494-019-0276-8.
Cuvillier L, Passaretti A, Raimon A, Dupuy V, Guilminot E, Joseph E. in: Metal 2022, proceedings of the interim meeting of the ICOM-CC metals working group, (ICOM-CC; The National Museum of Finland, 2022).
Guaragnone T, Rossi M, Chelazzi D, Mastrangelo R, Severi M, Fratini E, Baglioni P. pH-responsive semi-interpenetrated polymer networks of pHEMA/PAA for the capture of copper ions and corrosion removal. ACS Appl Mater Interfaces. 2022;14:7471–85. https://doi.org/10.1021/acsami.1c22837.
Cuvillier L, Passaretti A, Guilminot E, Joseph E. Testing of the siderophore deferoxamine amended in hydrogels for the cleaning of iron corrosion. Eur Phys J Plus. 2023;138:569. https://doi.org/10.1140/epjp/s13360-023-04159-y.
Çakmak Y, Çakmakçi E, Apohan NK, Karadag R. Isosorbide, pyrogallol, and limonene-containing thiol-ene photocured bio-based organogels for the cleaning of artworks. J Cult Herit. 2022;55:391–8. https://doi.org/10.1016/j.culher.2022.04.013.
Toreno G, Isola D, Meloni P, Carcangiu G, Selbmann L, Onofri S, Caneva G, Zucconi L. Biological colonization on stone monuments: a new low impact cleaning method. J Cult Herit. 2018;30:100–9. https://doi.org/10.1016/j.culher.2017.09.004.
Bertasa M, Canevali C, Sansonetti A, Lazzari M, Malandrino M, Simonutti R, Scalarone D. An in-depth study on the agar gel effectiveness for built heritage cleaning. J Cult Herit. 2021;47:12–20. https://doi.org/10.1016/j.culher.2020.10.007.
Campos B, Marco A, Cadeco G, Freire-Lista DM, Silvestre-Albero J, Algarra M, Vieira E, Pintado M, Moreira P. Green Chitosan: thiourea dioxide cleaning gel for manganese stains on granite and glass substrates. Herit Sci. 2021;9:160. https://doi.org/10.21203/rs.3.rs-779247/v1.
Weththimuni ML, Fiocco G, Girella A, Vigani B, Sacchi D, Rossi S, Licchelli M. Removing aged Polymer Coatings from Porous Stone surfaces using the gel cleaning method. Coatings. 2024;14:482. https://doi.org/10.3390/coatings14040482.
Puoti F, Jervis AV, Ciabattoni R, Cossa E, Di Giovanni A, Giuliani MR, Iodele M. Evaluation of Leather cleaning with a rigid hidrogel of gellan gum on two composite amharic shields from the museo nazionale preistorico etnografico luigi pigorini. Rome: Archetype; 2017.
Bartoletti A, Maor T, Chelazzi D, Bonelli N, Baglioni P, Angelova LV, Ormsby BA. Facilitating the conservation treatment of Eva Hesse’s Addendum through practice-based research, including a comparative evaluation of novel cleaning systems. Herit Sci. 2020;8:35. https://doi.org/10.1186/s40494-020-00378-z.
Rapti S, Boyatzis S, Rivers S, Velios A, Pournou A, Desferrioxamine B. Investigating the efficacy of hydrogels and ethanol gels for removing Akaganeite and Maghemite from Dry Wooden substrates. Forests. 2023;14:247. https://doi.org/10.3390/f14020247.
Mabrouk N. Experimental and applied study on the removal of polyurethane adhesive from the archaeological textiles. J Archit Arts Humanistic Sci. 2021;6:926–42. https://doi.org/10.21608/MJAF.2020.33731.1675.
Jedrusik A, Krasnan V, Rehakova M, Rebros M. Future prospects of biocleaning application in textile conservation. Eur Phys J Plus. 2023;138:864. https://doi.org/10.1140/epjp/s13360-023-04447-7.
Smets A, De Vis K, Ortega-Saez N. A challenging treatment of an 18th century embroidered textile using gel cleaning in combination with decamethylcyclopentasiloxane (D5) silicone solvent barriers. Conservar Património. 2019;31:41–52. https://doi.org/10.14568/cp2018023.
Acknowledgements
Our sincere gratitude goes out to Tabriz Islamic Art University for their priceless financial and moral support during the entire course of our research. This article is a segment of the outcomes obtained from the thesis of the first author, who was supervised by two co-authors at Tabriz Islamic Art University.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
NK: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Visualization, Writing - original draft. AK: Conceptualization, Formal analysis, Methodology, Project administration, Resources, Supervision, Validation, Writing - original draft, Writing - review & editing. YH: Supervision, Writing - review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Khaksar-Baghan, N., Koochakzaei, A. & Hamzavi, Y. An overview of gel-based cleaning approaches for art conservation. Herit Sci 12, 248 (2024). https://doi.org/10.1186/s40494-024-01369-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40494-024-01369-0