Abstract
This paper identifies the materials used to make a brocade belt belonging to the Qajar courtiers in Iran. This belt consists of two fabric types: red support and golden brocade. Accordingly, this paper examined the skin and tannin type, fiber types, dyeing agents, mordant, and metal wrapping of brocade yarns and metal buckle. Technical imaging, ESI–MS, FORS, FTIR and SEM–EDS were used to identify the materials. Multi-band imaging methods included Vis, UVL, IRR, and UVR, in which UVFC and IRFC images were obtained after processing. The results showed that cotton yarn was used in both fabrics. The red fabric yarns were dyed using cochineal and alum mordant. Also, the yellow brocade yarns were dyed using catechins-rich plants, and in this dyeing process, polyphenols and alum have been as mordant. Madder-dyed yarns are also found in parts of the belt. Examination of the metal wrapping of brocade yarns suggests using strips of copper-nickel alloy with a thin layer of gold-silver alloy. This secondary coating protects the copper-nickel strips and increases their golden luster. The leather analysis of the leather parts also indicated the using cattle/calfskin tanned with gallotannins. Belt buckle analysis also shows the use of brass alloy with a high percentage of zinc, leading to a golden sheen and the buckle desired strength. The results show the use of low-cost materials in the manufacture of clothing for the lower levels of the Qajar court.
Similar content being viewed by others
Introduction
Various analytical tools and methods are used to study historical fabrics for over three decades. These methods and multi-analytical approaches are applied to identify the parts of historical textiles, such as fibers and dyes, and also dyeing and fabrication techniques [1,2,3,4,5,6]. Studying historical textiles contributes to a better understanding of art history, trade connections, popular culture, beliefs, and economic conditions [1, 3]. Textiles are a significant part of the cultural heritage of every region of the world, and Iran is one of the richest one. The delicacy of the art-industry of textile weaving in Iran has been such that the fabrics of the Safavid period were considered the one of the most beautiful fabrics of their time in the world. This importance and position, along with texture and design delicacy, was due to the use of gold and silver metal fibers in the yarns used in textile weaving. These yarns were called golabatun, and the fabric woven with this yarn was called zaribaft (brocade). This weaving method began in the Achaemenid era (ca. 550–330 BCE) and peaked in the Sassanid era (ca. 224–641 CE). However, during the Qajar period (1789–1925 CE), Iranian textile weaving generally lost its position in technique and design and suffered from a decline in quality [7].
Numerous examples of Iranian historical brocade fabrics, especially from the Safavid period, exist in museums worldwide [8]. The role of this fabric type in Iranian culture needs studying its materials and manufacturing process. However, studies on Iranian historical textiles have focused on the texture, fibers type, design, and especially the dye of ordinary textiles and carpets [3, 9,10,11,12,13,14,15,16]. Despite the brocade fabrics values, there are few studies concerning them, including Hardin and Duffield [7] report, which examined metal yarns in several Iranian brocade specimens at the Auburn University, United States. Therefore, although some of the information on the process of making historical artifacts can be obtained from traditional treatises and instructions, in many cases, analytical studies are necessary to understand the nature of the constituent materials of the artifacts. Fibers, metal strips, dyeing materials and methods can be examined in the historical brocade fabrics. The history of dyeing is started with the history of costume production, and in fact, analysis of dyes and dying techniques in historical textiles is a requirement for conservation measures and history of clothing research [17]. Various chromatographic [17,18,19,20,21,22,23,24] or spectroscopic techniques [18, 20, 23,24,25,26,27,28,29,30] have been used to analyze the historical dyed textiles. In addition, some studies on dyes identification in fabrics are based on hyperspectral and multispectral imaging [31,32,33]. However, mass spectrometric-based methods such as liquid chromatography-mass spectroscopy (LC–MS) [34,35,36,37,38] provide reliable results on dye identification. Using these methods to analyze the dye agents in Iranian historical textiles has shown the application of cochineal, lac, madder, yellow larkspur, safflower, weld, tannins, turmeric, tamarisk and indigo [9, 12,13,14,15,16, 20].
In addition, metal strips and mordant constitute other main elements used in textiles (e.g. brocades). In general, X-ray-based methods have a good performance in identifying the constituents of these inorganic matters. Meanwhile, due to the possibility of simultaneous evaluation of morphology and chemical composition of the material's microstructure, scanning electron microscopy-energy dispersive X-ray spectroscopy (SEM–EDS) provides good results in identifying the mordant used in dyeing and metals in golabatun yarns [1, 7, 15, 39].
Accordingly, this paper intends to examine a historical belt attributed to the courtiers of Qajar in Iran to identify the fiber types, dyes, mordants, and metals used in its construction for future conservation measures and interpreting the historical significance and traditional making materials and processes. Therefore, electrospray ionization mass spectrometry (ESI–MS), fiber optics reflectance spectroscopy (FORS) and technical imaging methods have been used to determine the type of dye agents. Also, a SEM–EDS was used to identify the fiber types, mineral mordants, and metals composition in the belt. In addition, Fourier transform infrared spectroscopy (FTIR) was used to identify the type of polyphenols used in tanning the leather part of the belt. In fact, the aim of this research is to use a multianalytical approach to clarify technical aspects of the production methods of materials used to manufacture this artistic work.
Materials and methods
Object
This paper examines a golden brocade belt belonging to a private collection in Tabriz, Iran. This belt was part of the ceremonial and formal clothes of the Qajar courtiers, which has been inherited by its current owners. According to the history of the owners of the work, it dates back to around 1850 -1900 CE. The length of the belt is 91 cm. The top fabric is brocade with a yellow background, with a red fabric as the lining. The belt metal buckle is golden with a lion and sun inscription as the national symbol of Iran in the Qajar period. Leather is also used in parts of the belt (Fig. 1).
The belt consists of red fabric, golden brocade, a metal buckle with a lion and sun inscription, and a leather part. The studied samples were obtained from the marked sections; a yellow fibers and metal strips of brocade fabric, b red fiber of lining fabric, c the metal belt buckle (sampling from back of buckle), d leather part of belt, e the pink yarns used in sewing
SEM–EDS
Pieces of 0.5 cm length were selected from two red and brocade yarns, with a part containing metal wrapping and a part without. The SEM equipped with an energy dispersive X-ray spectrometer was used to examine the probable mordant used in dyeing and also the chemical composition of the metal wrapping. Scanning electron microscopy was performed using a TESCAN MIRA3 field-emission SEM with 15 keV accelerator voltage. The brocade yarns and buckle were examined without gold coating to investigate the possibility of gold presence.
ESI–MS
To extract the fibers dye, approximately 1 cm length of yarn was boiled in a test tube containing 0.5 cm3 of 3 M HCl aqueous solution. The acid was used to break the bond between the dye and the mordant. Then 0.5 cm3 of methanol was added to the test tube and reboiled for extraction. After concentration, 0.5 cm3 of methanol was added again and reboiled. This was repeated five times. The last step of this process was performed using 0.5 cm3 dimethylformamide. The extract was injected into an electrospray ionization mass spectrometry (ESI–MS) for analysis. ESI–MS was carried out on an Agilent 6410 Triple Quadrupole LC–MS device. Isocratic elution was performed with two solvents, water + 0.1% acetic acid and methanol, and an eluent flowrate of 0.3 mL/min.
Technical photography
Same as previous study [40], all images were captured by the modified camera Nikon D750 after removing the inbuilt UV-IR blocking filter to exploit the full sensitivity of the CMOS sensor (ca. 350–1100 nm). The camera was equipped with a Nikon AF Nikkor 50 mm f/1.8D lens. The camera was operated in fully manual mode. Two Youngenu NY660 xenon flashlights placed at 45 degrees angle to the subject were used as illuminating sources. An X-rite color checker was used as a spectral reference to correct images and compare them with reference samples.
Technical images, including Visible-Reflected (VIS), Infrared-Reflected (IRR), Ultraviolet-Reflected (UVR) and Ultraviolet-induced Visible Luminescence (UVL) were recorded in RAW format and highest resolution (24MP: 6016 × 4016 pixel) using the filters described in Table 1. Raw images obtained from the camera were converted into 16bit TIF format in Adobe Photoshop software. Post-processing and calibration procedures were performed according to Kushel method [41] and Cosentino recommendations [42]. False-color infrared (IRFC) and false-color ultraviolet (UVFC) images were obtained by combining VIS with IRR and UVR images, respectively, based on the method proposed by Dyer et al. [43].
Fiber optics reflectance spectroscopy (FORS)
Fiber optics reflectance spectroscopy (FORS) was applied to the analysis of red dye on belt lining fabric. The UV–Vis-NIR reflectance spectrum were obtained using an AvaSpec-2048 fiber optic spectrometer, an AvaLight-DHc compact deuterium-halogen light source, and a glass fiber reflection probe (Avantes Inc., Netherlands), operating in the 190–1100 nm. Spectrum was recorded with a 1 s integration time and 3 average. Finally, the range of 400–800 nm was compared with the spectra of reference dyes. All reference spectra are from Pigments Checker v.5, a free spectra database of pigments from cultural heritage science open source (CHSOS).
Polyphenols identification
The extraction procedure followed the method of Wouters [44] and Falcão and Araújo [45]. Polyphenols were extracted from 10 mg leather fibers (precision 0.0001 g), from the reticular layer, with 1 mL of aqueous-acetone solution (1:1) in capped vials, under continuous shaking, for 48 h at normal room temperature. Then, the extracts were filtered by whatman® filter paper No.42 and used for spot test and fourier transform infrared spectroscopy (FTIR), after evaporating the solvent. The FTIR analysis was carried out using a FT/IR-680Plus (Jasco, Japan). Spectrum was collected in the range of 400–4000 cm−1 at 2 cm−1 resolutions with 64 numbers of scans.
Results and discussion
The different parts and layers of the belt and their arrangement are presented in Fig. 2. In this belt, between the brocade fabric and the red lining, a cardboard layer is also placed to create strength and maintain its shape. The red lining fabric shows a plain weave. Also, in both fabrics, brocade and lining, the yarns are double-ply and have a Z-twist. The fibers identification is an essential part of studying historical textiles. The fibers identification provides valuable information about economic conditions, agricultural or livestock prosperity, trade between regions, the prevailing cultural conditions in the target period, and sometimes the authenticity of materials attributed to a region. Examining the fiber’s micromorphology using a microscope makes it possible to distinguish fibers of animal and plant origin from each other. On the other hand, microscopy provides a better understanding of the structural condition and degradation of the fibers and the penetration of dust and contamination into the fabrics. The longitudinal view of cotton fiber appears ribbon-like with a smooth and twisted surface. Wool fibers are seen on top of each other with scaly layers, as the main characteristics of these fibers [46]. The longitudinal view of linen fiber is also smooth and bamboo-like with cross marking nodes [47]. Under a microscope, silk fibers appear straight with a smooth surface [48, 49]. Examination of the fibers using the backscattered mode (SEM-BSE), shows the ribbon-like form in both brocade and red fabrics, indicating the use of cotton fibers (Fig. 3). Both fabric’s yarn is plied S (2Z) (two Z-spun yarns, which are then twisted together in an S). In addition, the brocade yarn fibers show more degradation than the cotton fibers of the red fabric, probably due to their contact with the metal strip (Fig. 3b, d).
Figure 4 shows the Vis, UVL, UVR, IRR, UVFC and IRFC images of the belt to identify the red dye agents. Although the red dyes are varied in Iranian fabrics, madder and cochineal are generally the main agents used in red dyeing. Madder is obtained from the root of Rubiaceae family, including Rubia and Gallium species, [50] and has been repeatedly identified in Iranian historical textiles. In Iran, the most common species used in dyeing is Rubia tinctorum, which is also known as Persian Madder. As another of the most important dyes in red, cochineal is obtained from the body of the female insect of the same name. In addition, lac, which is mainly extracted from the body of the Kerria lacca insect, has been one of the leading dyes used in Iranian textiles. Of course, this dye is more common in India and was exported to other regions from India.
According to Cosentino report [51], insect-based red dyes, especially cochineal, appear dark green in the UVFC. The UVFC image is a good indicator of the separation of cochineal from red plant dyes, especially madder. In addition, the red fibers in the IRFC image also appear in orange. Based on the literature, cochineal, madder and lac appear in orange, yellow and red in IRFC images [51]. This issue reinforces the hypothesis of the use of cochineal in dyeing the belt lining red fabric. In addition, this dyed fabric can be seen as red, dark and bright in the UVL, UVR and IRR images, respectively, which according to the report of Walthew et al. [52], are the characteristics of cochineal dye, especially with alum mordant.
Moreover, the pink yarns have also been used in sewing some parts. These yarns, added after the belt was made and during recent subsequent repairs, can be seen in the IRFC and UVFC images in light yellow and green, respectively. This is one of the characteristics of madder dye in technical imaging [51, 53], indicating the madder used in dyeing these yarns. In the UVL image, this yarn emit a weak pinkish luminescence, perhaps suggesting the presence of madder dye [33, 54]. Due to the fact that these yarns are newer than the belt and do not provide historical information, no further investigation was done on them.
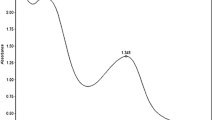
FORS spectra of red dyed fabric and the reference dyes are shown in Fig. 5. Fourier self-deconvolution (FSD) was used to better observe the features of the spectra. The main absorption bands of madder lake, lac and carmine lake from 500‐560, 450–560 and 475–570 nm were visible, respectively. The reflectance spectrum of the red dyed fabric also shows a great similarity to the carmine lake, which is obtained from cochineal. In this dyed fabric, the absorption band is also observed around 475–570 nm, which confirms the possibility of using cochineal dye. Also, the lac dye shows more absorbance than red fabric and carmine lake in the range of 600–750 nm.
a ESI-mass spectrum of dye extracted from red cloth; b Some of the significant fragments of carminic acid that can be identified in the mass spectrum of extracted dye [2]
Figure 6 shows the QqQ mass spectrum of the dye extracted from the red lining fabric. In this spectrum, ions 327, 339, and m/z 358 can be seen, which are the main fragments of carminic acid, according to Lech et al. [2, 55]. The fact that only carminic acid fragments were detected was probably due to the extraction method with hydrochloric acid and the destruction of the structure. Carminic acid is the main component of cochineal and its identification indicator. There are different types of cochineal, including Armenian (Porphyrophora polonica Linnaeus), Mexican (Dactylopius coccus Costa), or Polish (Porphyrophora hamelii Brandt), named depending on the geographical location [20, 56]. One of the most important sources of red dye extraction for dyeing is Armenian cochineal. Armenian cochineal is currently found near Mount Ararat. However, Armenian cochineal appears to have been harvested in other parts of the Caucasus, Turkey, and Iran [20, 57]. It has been reported in textiles found in Syria, Turkey, Khotan, Egypt, and Iran [20, 56,57,58]. As a consequence, although the results of this study do not support the identifying cochineal type, but it is more probable to use Armenian cochineal in this object, according to its period and geographical situation.
This dye must be part of the fibers to achieve proper stability. In this regard, fiber pre-treatment is performed using mordant, which has a long history. Mordanting is an important part of the dyeing process to achieve color stability and diversity and proper shade depth [9]. Mordanting involves adding fibers to hot water along with the mordant material, which can be done before, after, or during dyeing. The chemical bond between the natural dye molecule and the mordant leads to the formation of different colors in the fabrics. The final color depends on the type and chemical properties of the dye, the mordant, and the dye bath conditions (pH) [59]. Many synthetic and natural organic and inorganic materials have been used as mordant. The metal salts of chromium, aluminum, iron, tin, copper, and organic compounds such as polyphenols, citric acid, tartaric acid and oxalic acid, are some materials used in dyeing as the mordanting or dyeing assistants [9, 59, 60].
To identify the mordanting agent, the elements of the fiber surface were examined using SEM–EDS. The SEM–EDS results are presented in Table 2. The indicator element specified in the mineral particle on the fiber surface is aluminum. This indicates the possibility of using alum mordant in the dyeing process of this fabric. Alum mordant, one of the most common materials used in dyeing [61], provides good shade depth and excellent stability against light and moisture in dyeing with cochineal [62].
Figure 7 shows the mass spectra of yellow dye extracted from the brocade yarns. The m/z 443 ion has been identified as the main compound in positive ion mode. This ion indicates the presence of epi/catechin gallate (442 Da) [63] in the yellow dye. The negative ion mode also shows an ion at m/z 290, corresponding to epi/catechin [63]. The presence of epi/catechin gallate and epi/catechin indicate the use of flavonoid-rich plant dyes, especially catechin derivatives [64]. Although this type of dye can be used in protein fibers without mordant, polyphenols are commonly used as mordant in cotton dyeing. According to Fig. 7, the ion at m/z 786 with various fragments, especially at m/z 168.9 (corresponding to gallic acid), indicates the presence of hydrolyzable tannins and gallic acid derivatives. In addition, gallic acid, as one of the main components extracted from this dye, was detected with a specific negative ion at m/z 169 [65]. Tannins generally do not produce yellow tones in cotton fibers dyeing, alone. However, the identification of gallic acid and its derivatives suggests their use as mordant agents in the dyeing of yellow brocade yarns. In other words, gallic acid have affinity for dyeing substrates due to the presence of auxochrome groups –OH [66]. This not only introduces gallic acid as a biomordant but also brings the possibility of using it as a color modifier.
The ESI-mass spectrum (positive ions) of the extracted dye from yellow yarns and the detection of an ion at m/z 443, related to epi/catechin gallate (a); The negative ESI-mass spectra shows ions at m/z 290 related to epi/catechin (b), m/z 786 related to gallotannins and gallic acid derivatives (c) and m/z 169 for gallic acid (d)
However, it should be noted that in the glycosidic dyes, flavonoid dyes particularly, some of the dye components are decomposed by HCl during the heat extraction [67]. Therefore, despite the identification of key components such as epi/catechin, epi/catechin gallate, and gallic acid and its derivatives, some components have also been decomposed. This makes it a challenge to identify the main source of color. Therefore, it is not possible to identify the type of plant used in the dyeing of this fabric.
Fiber surface elements were investigated using SEM–EDS, and the results are summarized in Table 2. Among the detected elements, aluminum was identified as the main element. The presence of 39% of this element indicates that the alum mordant was used in the dyeing of the fibers, as mordant, in addition to polyphenols. The simultaneous use of alum and polyphenols, especially tannins, as mordant has been common in the dyeing of cotton fibers using flavonoids and plants rich in catechins, including acacia [68, 69].
The metal wrapping surface of the brocade yarn was studied using SEM–EDS analysis. The SEM-BSE micrograph of the metal wrapping surface clearly shows the presence of a very thin layer on a metal plate (Fig. 8). According to the EDS results in Table 2, the composition of the metal plate of brocade yarns consists of an alloy of copper and nickel with about 87 wt% copper and 10 wt% nickel (Analysis A). However, a very thin silver-gold alloy coating is seen on this metal plate (Analysis B). The presence of significant amounts of copper and nickel in the thin metal coating composition can be due to the penetration of electrons below the coating surface during EDS analysis and measurement of these elements.
SEM-BSE images of surface (a) and cross-section (b) of yellow yarn with metal wrapping; EDS analysis was performed at the marked points, which can be seen in Table 2
Also, to observe the thickness of the metal plate and the thin silver-gold coating, a cross-section of yarn with metal wrapping was prepared and analyzed using the SEM–EDS. However, the thin silver-gold layer is not visible at all at low magnification, but it is possible to analyze it at very high magnification. EDS analysis of metal plate cross-section (Analyses C and D) also indicates copper-nickel alloy. The EDS results of the very thin layer (Analysis E) also show the using silver-gold alloy. Based on this, it can be concluded that the metal wrapping used on the yarn is made of copper-nickel alloy, which is coated using a very thin layer of silver-gold alloy.
Figure 9 shows the SEM-BSE image of the cross-section of the metal buckle of the belt. The SEM image shows a single-phase structure with a scattered fine light phase on a metal background. The EDS analysis of the main phase indicates a combination of copper and zinc (brass alloy) with a zinc content of about 27 wt% (Analysis F in Table 2). This can be classified as alpha brass, a Cu–Zn alloy with less than 35 wt% of zinc that forms a single-phase microstructure with high ductility and tensile strength [70,71,72]. On the other hand, EDS analysis of one of the very fine phases in the microstructure indicates the presence of lead along with copper and zinc (Analysis G in Table 2). These results show that these fine and bright phases are, in fact, lead globules that have been segregated as very fine phases due to the immiscibility of lead in copper [72].
SEM-BSE image of a cross-section of the metal belt buckle, based on the EDS results in Table 2, is made of brass. The G points indicate lead globules
There is ample evidence of using copper-nickel alloys, or cupronickel, in manufacturing artifacts throughout history [73,74,75]. However, this alloy has been more widely used in recent centuries. Cupronickel has been considered for its relatively silver luster and high corrosion resistance [76, 77]. Interestingly, this alloy is used to manufacture metal wrapping of yarns and a very thin layer of gold-silver alloy to cover it. It can be said that the metal wrapping of the studied brocade yarns was made using copper-nickel alloy, and to create glitter and a protective coating, a very thin layer of the gold-silver alloy was made used on it. Also, the belt buckle is made of alpha-brass alloy with a high amount of zinc, which also has a golden shine and, at the same time, provides the necessary hardness for shaping and using the buckle.
The follicles pattern on the leather surface was examined to identify the skin type. The stereomicroscopic photograph of this pattern is presented in Fig. 10a. The follicles in the leather are mostly equal in size and are arranged in regular rows. This arrangement of follicles indicates the use of cattle/calfskin to make leather [78]. In addition to the skin type, the type of tannins used in leather tanning was also investigated by FTIR spectroscopy. According to the studies of Falcão and Araújo [45], each group of tannins presents characteristic absorption bands in the FTIR spectrum. These indicator absorption bands are summarized and presented in Fig. 10b [79]. Accordingly, the type of tannin used in leather tanning was investigated by FTIR spectroscopy (Fig. 10c). Examination of spectral data has confirmed the presence of tannins in leather, by absorption bands at 1605 and 1445 cm−1 (aromatic ring stretching vibration), 1205 and 1035 cm−1 (C–O bond stretching vibration) and 1506 cm−1 (skeletal vibration of aromatic rings). The vibration of C–O–C stretching and OH deformation bands and the vibration of C=O stretching bonds of free gallic acid at 1330 cm−1 and 1710 cm−1, respectively, showed the existence of hydrolysable tannins. In addition, the absorption bands in 1073, 870 and 760 cm−1 related to aryl phenolic ester C–O–C symmetric stretching, C–C stretching, and sugar ring-breathing vibration, respectively, confirmed that the type of tannins in the leather was gallotannins [45, 79,80,81].
Conclusion
This paper investigated a brocade court ceremonial belt related to the Qajar period. Although the use of silk fibers has been common in Iranian brocade fabrics, the study of this belt indicated the use of S(2z) cotton yarns in both support and brocade fabric. The red-dyed support appeared in UVFC and IRFC images in green and orange, respectively, characteristic of cochineal dye. Identifying carminic acid fragments' ions in this dye's mass spectrum and FORS also confirmed the use of cochineal. This dye is commonly used in cotton fibers with a mordant. EDS analysis of particles on the fiber surface indicates aluminum accumulation, which has been used as a mordant. This shows the dyeing of cotton yarns of support fabric using cochineal dye and an alum mordant. Moreover, according to the technical imaging, evidence of yarns dyed with madder was evident in sewing parts of the belt. Cochineal and madder dyes can be considered the most common historical red dyes used in Iranian textiles.
The ESI-mass spectra of yellow dye extracted from brocade yarns suggest the presence of epi/catechin and epi/catechin gallate. This is evidence for flavonoid-rich plants, especially catechins, in the dyeing process, originating from an unidentified natural yellow dyestuff or use as polyphenolic biomordants. In addition, the negative ions at m/z 169 and 786 related to gallic acid and gallotannins were detected in this specimen. Identifying these compounds and catechin indicates the use of polyphenols as organic mordanting agents in fiber dyeing. However, polyphenols are not the only mordanting agents. The accumulation of aluminum on the surface of these fibers indicates the combined use of an aluminium metallic salt mordant like alum. SEM–EDS analysis of the brocade yarn's metal wrapping suggests using a hammered wire of copper-nickel alloy, or cupronickel, with a very fine coating layer of gold-silver alloy. In fact, a coating of gold-silver alloy has been placed on the copper-nickel plate to increase its luster and golden appearance. SEM–EDS analysis of the belt buckle also showed the using a standard copper-zinc alloy (brass). This alloy has a significant hardness and a golden shining due to its high zinc content. The analysis of the leather part of the belt also showed the use of tanned cattle/calfskin with gallotannins.
Although exquisite specimens of Iranian brocades are made of silk yarns with a wrapping of gold; but, cotton fibers with a wrapping of copper-nickel and only a thin layer of gold-silver alloy are used in this brocade belt. Therefore, it seems that high-quality and expensive materials were not used to produce the clothes of the people in the lower levels of the Qajar court.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Al-Sharairi N, Sandu ICA, Vasilache V, Sandu I. Recognition of natural silk fibers, dyes and metal threads of historical Romanian textile fragments using the multi-analytical techniques approach. Text Res J. 2020;90(15–16):1671–88.
Lech K, Witkoś K, Wileńska B, Jarosz M. Identification of unknown colorants in pre-Columbian textiles dyed with American cochineal (Dactylopius coccus Costa) using high-performance liquid chromatography and tandem mass spectrometry. Anal Bioanal Chem. 2015;407(3):855–67.
Koochakzaei A, Mohammadi Achachluei M, Saidi MM. Identification of fibers and weaving technology in the remains of fabrics discovered from Kuh-e Khwaja, a Parthian archaeological site in Sistan, Iran. J Anc Hist Archaeol. 2021;8(3):148–55.
Pozzi F, Poldi G, Bruni S, De Luca E, Guglielmi V. Multi-technique characterization of dyes in ancient Kaitag textiles from Caucasus. Archaeol Anthropol Sci. 2012;4(3):185–97.
Balakina GG, Vasiliev VG, Karpova EV, Mamatyuk VI. HPLC and molecular spectroscopic investigations of the red dye obtained from an ancient Pazyryk textile. Dyes Pigm. 2006;71(1):54–60.
Al-Gaoudi HA, Aly NM. The characterization of some ancient Egyptian funerary linens from the twenty-first dynasty discovered in the Bab El-Gasus excavation. J Egypt Archaeol. 2021;107(1–2):115–28.
Hardin IR, Duffield FJ. Characterization of metallic yarns in historic persian textiles by microanalysis. historic textile and paper materials. Advances in Chemistry. 212: American Chemical Society; 1986. p. 231–52.
Vryzidis N. Persian textiles in the Ottoman Empire: evidence from Greek sacristies. Iran. 2018;56(2):228–36.
Smith GD, Esson JM, Chen VJ, Hanson RM. Forensic dye analysis in cultural heritage: unraveling the authenticity of the earliest Persian knotted-pile silk carpet. Forensic Sci Int Synerg. 2021;3: 100130.
Kateb f, Mafitabar A. Restudying the historical textiles in Iran (based on methods of classifying designs and motifs). Glory of Art (Jelve-y Honar) Alzahra Sci Q. 2019;11(2):67–80.
Moravej E. Comparative study of graphic aspects of textiles in Indian Gurakani and Iranian Safavid eras. Bull Soc Roy Sci Liège. 2016;85:793–806.
Mabrouk N. Identification of natural dyes in some archaeological Iranian carpets by non-destructive MALDI TOF MS. J Archit Arts Hum Sci. 2021;6(29):766–80.
Mouri C, Aali A, Zhang X, Laursen R. Analysis of dyes in textiles from the Chehrabad salt mine in Iran. Herit Sci. 2014;2(1):20.
Potts DT. On the history of madder (Rubia peregrina L., and Rubia tinctorum L.) in pre-modern Iran and the Caucasus. Asiatische Studien Études Asiatiques. 2022. https://doi.org/10.1515/asia-2021-0039.
Chahardoli Z, Vanden Berghe I, Mazzeo R. Twentieth century Iranian carpets: investigation of red dye molecules and study of traditional madder dyeing techniques. Herit Sci. 2019;7(1):57.
Sharif S. Natural yellow dyes in persian carpets: a holistic approach. Universidade NOVA de Lisboa; 2021.
Baek Y-M, Kwon Y-S, Goto-Doshida S, Saito M. Analysis of dyes and mordants of 16–17th century textiles excavated from Daejeon. J Conserv Sci. 2012;28:119–29.
Karadağ R, Dölen E. Examination of historical textiles with dyestuff analyses by TLC and derivative spectrophotometry. Turk J Chem. 1997;21(2):126–33.
Schweppe H. Identification of red madder and insect dyes by thin-layer chromatography. Historic textile and paper materials II. ACS symposium series, vol 410. American Chemical Society; 1989. p. 188–219.
Shibayama N, Wypyski M, Gagliardi-Mangilli E. Analysis of natural dyes and metal threads used in 16th -18th century Persian/Safavid and Indian/Mughal velvets by HPLC-PDA and SEM-EDS to investigate the system to differentiate velvets of these two cultures. Herit Sci. 2015;3(1):12.
Valianou L, Karapanagiotis I, Chryssoulakis Y. Comparison of extraction methods for the analysis of natural dyes in historical textiles by high-performance liquid chromatography. Anal Bioanal Chem. 2009;395(7):2175–89.
Karapanagiotis I, Lakka A, Valianou L, Chryssoulakis Y. High-performance liquid chromatographic determination of colouring matters in historical garments from the Holy Mountain of Athos. Microchim Acta. 2008;160(4):477–83.
Wertz JH, Quye A, France D, Tang PL, Richmond L, editors. Authenticating Turkey red textiles through material investigations by FTIR and UHPLC. ICOM-CC 18th triennial meeting; 2017 4–8 Sept; Copenhagen, Denmark.
Zaffino C, Bedini GD, Mazzola G, Guglielmi V, Bruni S. Online coupling of high-performance liquid chromatography with surface-enhanced Raman spectroscopy for the identification of historical dyes. J Raman Spectrosc. 2016;47(5):607–15.
Yusa Marco D, Domenech Carbo M, Vaccarella I, Batista Dos Santos A, Vicente Palomino S, Fuster López L. Characterization of colouring compounds in annatto (Bixa Orellana L.) Used in historic textiles by means of UV-vis spectrophotometry and FT-IR spectroscopy. Arché. 2008;3:153–8.
Gillard RD, Hardman SM, Thomas RG, Watkinson DE. The detection of dyes by FTIR microscopy. Stud Conserv. 1994;39(3):187–92.
Brosseau CL, Gambardella A, Casadio F, Grzywacz CM, Wouters J, Van Duyne RP. Ad-hoc surface-enhanced raman spectroscopy methodologies for the detection of artist dyestuffs: thin layer chromatography-surface enhanced Raman spectroscopy and in situ on the fiber analysis. Anal Chem. 2009;81(8):3056–62.
Lee J, Kim MJ, Elslande EV, Walter P, Lee Y. Identification of natural dyes in ancient textiles by time-of-flight secondary ion mass spectrometry and surface-enhanced Raman spectroscopy. J Nanosci Nanotechnol. 2015;15(11):8701–5.
Pozzi F, Porcinai S, Lombardi JR, Leona M. Statistical methods and library search approaches for fast and reliable identification of dyes using surface-enhanced Raman spectroscopy (SERS). Anal Methods. 2013;5(16):4205–12.
Bruni S, Guglielmi V, Pozzi F, Mercuri AM. Surface-enhanced Raman spectroscopy (SERS) on silver colloids for the identification of ancient textile dyes. Part II: pomegranate and sumac. J Raman Spectrosc. 2011;42(3):465–73.
De La Codre H, Daniel F, Chapoulie R, Servant L, Mounier A. Investigating the materials used in eighteenth-century tapestries from the three French Royal Manufactories: inputs of hyperspectral approaches. Eur Phys J Plus. 2021. https://doi.org/10.1140/epjp/s13360-021-02184-3.
Peruzzi G, Cucci C, Picollo M, Quercioli F, Stefani L. Non-invasive identification of dyed textiles by using VIS-NIR FORS and hyperspectral imaging techniques. Cultura e Scienza del Colore Color Cult Sci. 2021;13(01):61–9.
Dyer J, Tamburini D, O’Connell ER, Harrison A. A multispectral imaging approach integrated into the study of Late Antique textiles from Egypt. PLoS ONE. 2018;13(10): e0204699.
Szostek B, Orska-Gawrys J, Surowiec I, Trojanowicz M. Investigation of natural dyes occurring in historical Coptic textiles by high-performance liquid chromatography with UV–Vis and mass spectrometric detection. J Chromatogr A. 2003;1012(2):179–92.
Petroviciu I, Albu F, Medvedovici A. LC/MS and LC/MS/MS based protocol for identification of dyes in historic textiles. Microchem J. 2010;95(2):247–54.
Tamburini D. Investigating Asian colourants in Chinese textiles from Dunhuang (7th-10th century AD) by high performance liquid chromatography tandem mass spectrometry—towards the creation of a mass spectra database. Dyes Pigm. 2019;163:454–74.
Zhang X, Laursen R. Application of LC–MS to the analysis of dyes in objects of historical interest. Int J Mass Spectrom. 2009;284(1):108–14.
Petroviciu I, Vanden Berghe I, Cretu I, Albu F, Medvedovici A. Identification of natural dyes in historical textiles from Romanian collections by LC–DAD and LC–MS (single stage and tandem MS). J Cult Herit. 2012;13(1):89–97.
Indictor N, Koestler RJ, Sheryll R. The detection of mordants by energy dispersive X-ray spectrometry: part I. Dyed woolen textile fibers. J Am Inst Conserv. 1985;24(2):104–9.
Koochakzaei A, Alizadeh Gharetapeh S, Jelodarian BB. Identification of pigments used in a Qajar manuscript from Iran by using atomic and molecular spectroscopy and technical photography methods. Herit Sci. 2022;10(1):30.
Kushel D. Photographic techniques for conservation. The AIC guide to digital photography and conservation documentation. 3rd ed. Washington, DC: American Institute for Conservation; 2017. p. 109–71.
Cosentino A. Practical notes on ultraviolet technical photography for art examination. Notas práticas sobre a fotografia de ultravioleta para o exame de obras de arte. 2015;21:53–62.
Dyer J, Verri G, Cupitt J. Multispectral imaging in reflectance and photo-induced luminescence modes: a user manual. British Museum; 2013.
Wouters J, editor High-performance liquid chromatography of vegetable tannins extracted from new and old leathers. In: The 10th Triennal meeting ICOM committee for conservation. Washington, DC, USA: James & James for ICOM-CC; 1993 22–27 August 1993.
Falcão L, Araújo MEM. Tannins characterization in historic leathers by complementary analytical techniques ATR-FTIR, UV-Vis and chemical tests. J Cult Herit. 2013;14(6):499–508.
Suomela JA, Vajanto K, Räisänen R. Seeking nettle textiles—utilizing a combination of microscopic methods for fibre identification. Stud Conserv. 2018;63(7):412–22.
Haugan E, Holst B. Flax look-alikes: pitfalls of ancient plant fibre identification. Archaeometry. 2014;56(6):951–60.
Gleba M, VandenBerghe I, Aldenderfer M. Textile technology in Nepal in the 5th–7th centuries CE: the case of Samdzong. STAR Sci Technol Archaeol Res. 2016;2(1):25–35.
Goodway M. Fiber identification in practice. J Am Inst Conserv. 1987;26(1):27–44.
Mantzouris D, Karapanagiotis I. Armenian cochineal (Porphyrophora hamelii) and purpurin-rich madder in ancient polychromy. Color Technol. 2015;131(5):370–3.
Cosentino A. Identification of pigments by multispectral imaging; a flowchart method. Herit Sci. 2014;2(1):8.
Walthew J, Eng-Wilmot K, Nguyen P. Spotlight on current research: multiband imaging for dyed textiles: Cooper Hewitt, Smithsonian Design Museum; 2019. https://www.cooperhewitt.org/2019/11/22/spotlight-on-current-research-multiband-imaging-for-dyed-textiles/.
Al-Gaoudi HA, Iannaccone R. Multiband imaging techniques incorporated into the study of dyed ancient Egyptian textile fragments. Int J Conserv Sci. 2021;12(3):893–906.
Tamburini D, Dyer J. Fibre optic reflectance spectroscopy and multispectral imaging for the non-invasive investigation of Asian colourants in Chinese textiles from Dunhuang (7th–10th century AD). Dyes Pigm. 2019;162:494–511.
Lech K, Jarosz M. Identification of Polish cochineal (Porphyrophora polonica L.) in historical textiles by high-performance liquid chromatography coupled with spectrophotometric and tandem mass spectrometric detection. Anal Bioanal Chem. 2016;408(12):3349–58.
Serrano A, Sousa MM, Hallett J, Lopes JA, Oliveira MC. Analysis of natural red dyes (cochineal) in textiles of historical importance using HPLC and multivariate data analysis. Anal Bioanal Chem. 2011;401(2):735–43.
Cardon D. Natural dyes: sources, tradition, technology and science. Archetype; 2007.
de Graaff JHH, Roelofs WGT, van Bommel MR. The colourful past: origins, chemistry and identification of natural dyestuffs. Abegg-Stiftung; 2004.
Ammayappan L, Shakyawar DBB. Dyeing of carpet woolen yarn using natural dye from cochineal. J Nat Fibers. 2016;13(1):42–53.
Shahmoradi Ghaheh F, Moghaddam MK, Tehrani M. Comparison of the effect of metal mordants and bio-mordants on the colorimetric and antibacterial properties of natural dyes on cotton fabric. Color Technol. 2021;137(6):689–98.
Imani H, Gharanjig K, Ahmadi Z. A novel efficient method for eco-friendly deep dyeing of wool yarns by extracted madder dyes in the presence of additives. Ind Crops Prod. 2022;183: 114970.
Arroyo-Figueroa G, Ruiz-Aguilar GML, Cuevas-Rodriguez G, Sanchez GG. Cotton fabric dyeing with cochineal extract: influence of mordant concentration. Color Technol. 2011;127(1):39–46.
Susanti E, Ratnawati R, Rudijanto A. Qualitative analysis of catechins from green tea GMB-4 clone using HPLC and LC-MS/MS. Asian Pac J Trop Biomed. 2015;5(12):1046–50.
Sequin-Frey M. The chemistry of plant and animal dyes. J Chem Educ. 1981;58(4):301.
Sandhu AK, Gu L. Antioxidant capacity, phenolic content, and profiling of phenolic compounds in the seeds, skin, and pulp of Vitis rotundifolia (Muscadine Grapes) as determined by HPLC-DAD-ESI-MSn. J Agric Food Chem. 2010;58(8):4681–92.
Shahid ul I, Rather LJ, Shabbir M, Sheikh J, Bukhari MN, Khan MA, et al. Exploiting the potential of polyphenolic biomordants in environmentally friendly coloration of wool with natural dye from Butea monosperma flower extract. J Nat Fibers. 2019;16(4):512–23.
Zhang X, Laursen RA. Development of mild extraction methods for the analysis of natural dyes in textiles of historical interest using LC-diode array detector-MS. Anal Chem. 2005;77(7):2022–5.
Brahma S, Islam MR, Shimo SS, Dina RB, editors. Influence of natural and artificial mordants on the dyeing performance of cotton knit fabric with natural dyes 2019.
Chakraborty JN. 17—Dyeing with basic dye. In: Chakraborty JN, editor. Fundamentals and practices in colouration of textiles. Woodhead Publishing India; 2014. p. 200–8.
Rizzuto BC. Chapter 13 quantitative analysis of archaeological and historical brasses using handheld X-ray fluorescence spectrometry. Advances in portable X-ray fluorescence spectrometry: instrumentation, application and interpretation. The Royal Society of Chemistry; 2023. p. 364–99.
Laws KJ, Crosby C, Sridhar A, Conway P, Koloadin LS, Zhao M, et al. High entropy brasses and bronzes—microstructure, phase evolution and properties. J Alloys Compd. 2015;650(8):949–61.
Scott DA. Metallogrpahy and microstrcuture of ancient and hitoric metals. Los Angeles: Getty Conservation Institute; 1991.
Cheng CF, Schwitter CM. Nickel in ancient bronzes. Am J Archaeol. 1957;61(4):351–65.
Schwitter CM, Cheng CF. Bactrian Nickel and Chinese bamboo. Am J Archaeol. 1962;66(1):87–92.
Chen K, Rehren T, Mei J, Zhao C. Special alloys from remote frontiers of the Shang Kingdom: scientific study of the Hanzhong bronzes from southwest Shaanxi, China. J Archaeol Sci. 2009;36(10):2108–18.
Mathiyarasu J, Palaniswamy N, Muralidharan VS. Corrosion resistance of cupronickels—an overview. Corros Rev. 2000;18(1):65–103.
Chandra K, Mahanti A, Singh AP, Kain V, Gujar HG. Microbiologically influenced corrosion of 70/30 cupronickel tubes of a heat-exchanger. Eng Fail Anal. 2019;105:1328–39.
Haines BM. The fibre structure of leather. In: Kite M, Thomson R, editors. Conservation of leather and related materials. London: ButterworthHeinemann; 2006. p. 11–21.
Koochakzaei A, Mallakpour S. Identification of surface dyeing agents of two bookbinding leathers from 19th-century Qajar, Iran, using LC–MS, μXRF and FTIR spectroscopy. Archaeometry. 2022. https://doi.org/10.1111/arcm.12832.
Fernández K, Agosin E. Quantitative analysis of red wine tannins using fourier-transform mid-infrared spectrometry. J Agric Food Chem. 2007;55(18):7294–300.
Grasel FDS, Ferrão MF, Wolf CR. Development of methodology for identification the nature of the polyphenolic extracts by FTIR associated with multivariate analysis. Spectrochimica Acta Part A Mol Biomol Spectrosc. 2016;153:94–101.
Acknowledgements
The authors would like to thank B. Jelodarian, from Tabriz Islamic art university, for his advice and help in technical imaging.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
AK designed the work process and analyzed and interpreted the data. OO participated in experiments. Both authors discussed the results and contributed to the final manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Koochakzaei, A., Oudbashi, O. A courtly brocade belt belonging to Qajar period (1789–1925), Iran. Herit Sci 11, 29 (2023). https://doi.org/10.1186/s40494-023-00875-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40494-023-00875-x