Abstract
The serine-glycine-one-carbon (SGOC) metabolic pathway is critical for DNA methylation, histone methylation, and redox homeostasis, in addition to protein, lipid, and nucleotide biosynthesis. The SGOC pathway is a crucial metabolic network in tumorigenesis, wherein the outputs are required for cell survival and proliferation and are particularly likely to be co-opted by aggressive cancers. SGOC metabolism provides an integration point in cell metabolism and is of crucial clinical significance. The mechanism of how this network is regulated is the key to understanding tumor heterogeneity and overcoming the potential mechanism of tumor recurrence. Herein, we review the role of SGOC metabolism in cancer by focusing on key enzymes with tumor-promoting functions and important products with physiological significance in tumorigenesis. In addition, we introduce the ways in which cancer cells acquire and use one-carbon unit, and discuss the recently clarified role of SGOC metabolic enzymes in tumorigenesis and development, as well as their relationship with cancer immunotherapy and ferroptosis. The targeting of SGOC metabolism may be a potential therapeutic strategy to improve clinical outcomes in cancers.
Similar content being viewed by others
Introduction
Cell metabolic reprogramming is a common feature of human tumors and refers to the reconnection of cell metabolic flux to produce enough metabolites to support rapid cell proliferation under limited nutrition and stress conditions [1, 2]. Cell growth and proliferation require the construction of new cell components, including proteins, nucleic acids, and lipids, as well as the maintenance of redox, genetic and epigenetic states [3,4,5]. The metabolic unit known as SGOC metabolism, which provides serine, glycine, one-carbon units and other intermediates, can satisfy many of these requirements [6,7,8]. Furthermore, SGOC metabolism provides substrates for methylation reactions and affects cellular antioxidative capacity, thus promoting tumor homeostasis [9,10,11]. In 2014, Mehrmohamadi and his colleagues first proposed the concept of the SGOC metabolic network, and determined its extensive and heterogeneous functions in human cancer [11].
Recent studies have suggested a new role for SGOC metabolism in cancer pathogenesis. In neuroendocrine prostate cancer, SGOC metabolic networks are highly expressed and activated, thus suggesting a targetable vulnerability [12]. In MYCN-amplified neuroblastoma, SGOC metabolism is very active in supplying glucose-derived carbon for serine and glycine synthesis and presents a MYCN-dependent metabolic vulnerability [13,14,15]. In colorectal cancer (CRC) with ILF3 overexpression, SGOC metabolic enzymes are deregulated under tumorigenic conditions and may be potential targets for cancer therapy [16]. In breast cancer, the SGOC network is a metabolic hallmark inherent to CDK12-induced tumorigenesis, which indicates that an actionable vulnerability exists for breast cancer therapy [17]. Taken together, SGOC metabolism may represent a vulnerability in all highly SGOC-activated tumors in future scenarios. Herein, we summarize the roles of SGOC metabolism in tumorigenesis and development, and discuss their relationship with tumor immunotherapy and ferroptosis. SGOC metabolic enzymes may be potential therapeutic target genes for cancer treatment.
Serine, glycine and one-carbon metabolism
Serine and glycine metabolism
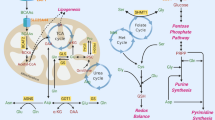
Serine is the main donor of one-carbon units, which can enter cells via many different transporter proteins or be synthesized de novo by the cell [18]. Extracellular serine supports the survival and proliferation of many types of cancer cells. A set of metabolite profiles of 60 different cancer cells showed that cancer cells voraciously consume extracellular serine, wherein this consumption ranks second only to that of glutamine among the amino acids [19]. Serine starvation can induce stress and metabolic remodeling, and inhibit cancer progression [4, 20, 21]. Moreover, Yang and colleagues found that tumor protein p53-mediated cell death was significantly enhanced in response to Nutlin-3 treatment during serine starvation [22]. The inhibition of the serine synthesis pathway and dietary serine depletion synergistically inhibit one-carbon metabolism and cancer cell growth [4]. In addition, cancer cells can also obtain serine via lysosomal degradation of proteins, as occurs during macrophage phagocytosis and autophagy [23,24,25]. The key metabolic enzymes in serine and glycine metabolism include phosphoglycerate dehydrogenase (PHGDH), phosphoserine aminotransferase 1 (PSAT1), phosphoserine phosphatase (PSPH), serine hydroxymethyltransferase 1/2 (SHMT1/2). Serine can be converted to glycine by SHMT1 in the cytoplasm or SHMT2 in the mitochondria [26,27,28]. During this process, a one-carbon unit separated from serine is transferred to tetrahydrofolate (THF) to produce 5-methyltetrahydrofolate (CH2-THF) [29]. CH2-THF is a precursor of folate and is reduced to 5-methyltetrahydrofolate (5-CH3-THF) by 5,10-methylenetetrahydrofolate reductase (MTHFR); finally, 5-CH3-THF is demethylated to yield folate to complete the folate cycle [30].
In addition, many cancer cells contain a glycine cleavage system through which glycine is cleaved in the presence of the glycine decarboxylase complex to produce ammonia, carbon dioxide, and methylenetetrahydrofolate to fuel the production of one-carbon units [31].
Folate-mediated one-carbon metabolism (FOCM)
Folic acid is a water-soluble B vitamin that can be converted to THF in vivo and is involved in many biochemical reactions in vivo (Fig. 1). Folate metabolism often occurs in both the cytoplasm and mitochondria and is compartmentalized in distinct regions in the cytoplasm, nucleus and mitochondria, depending on whether the one-carbon units are derived from serine or glycine catabolism [32,33,34]. The key metabolic enzymes in FOCM metabolism include methylenetetrahydrofolate dehydrogenase 1/1L (MTHFD1/1L), methylenetetrahydrofolate dehydrogenase 2/2L (MTHFD2/2L) and aldehyde dehydrogenase 1 family member L1/L2 (ALDH1L1/2) and so on. In most cultured cells, mitochondrial SHMT2 transfers the β-carbon atom from serine to THF to generate CH2-THF. This folic acid intermediate can also be produced by separating a one-carbon unit from glycine in a reaction catalyzed by the glycine cleavage system [35]. Subsequently, MTHFD2 or MTHFD2L uses NAD+ or NADP+ to oxidize CH2-THF to generate 10-formyltetrahydrofolate (10-CHO-THF) and produce a molecule of Nicotinamide adenine dinucleotide phosphate (NADPH) [36, 37]. Moreover, 10-CHO-THF can be used for the formylation of mitochondrial promoters [38, 39]. In addition, it can provide fuel for cytoplasmic and nuclear reactions or be excreted from the cell [40]. Mitochondrial 10-CHO-THF does not cross the mitochondrial membrane; thus, one of the one-carbon units in 10-CHO-THF is converted to formate in an MTHFD1L-mediated reaction, and formate can be exported to the cytoplasm [41]. During this process, adenosine diphosphate (ADP) can be phosphorylated to adenosine triphosphate (ATP) or used to generate THF and release CO2 via ALDH1L2, accompanied by NADPH production [42]. The formate transferred to the cytoplasm is dehydrogenated by MTHFD1 in a reaction that consumes ATP to regenerate cytosolic 10-CHO-THF for the de novo synthesis of purines [43]. This reaction can generate cytosolic CH2-THF for homocysteine remethylation and thymidylate synthesis via MTHFR or thymidylate synthase (TYMS) [35]. This CH2-THF can be reduced to THF via cytoplasmic SHMT1, which completes the folate cycle and the conversion of glycine to serine [44]. In addition, folic acid is reduced to 7,8-dihydrofolate (DHF) and then to THF by dihydrofolate reductase (DHFR) [30]. In conclusion, the folic acid cycle, as the common metabolic pathway between SGOC and one-carbon metabolism, has high plasticity.
Methionine cycle and transsulfuration pathway
The methionine cycle is commonly used to produce S-adenosyl-l-methionine (SAM), which is an ubiquitous methyl donor used by a large class of SAM-dependent methyltransferases for DNA, RNA, protein and lipid methylation [45]. The SGOC pathway is interconnected with the methionine cycle through the action of MTHFR, which catalyzes the irreversible conversion of CH2-THF to 5-CH3-THF. Afterwards, 5-CH3-THF is used by ubiquitously expressed methionine synthase (MS) to remethylate homocysteine in a vitamin B12-dependent reaction [46]. Furthermore, serine apparently plays an important role in the methionine cycle in vivo, and stable isotope tracing studies have shown that most methyl groups used for systemic homocysteine remethylation are derived from serine [47]. However, some reports have shown that serine-derived one-carbon units cannot be used to support remethylation when exogenous methionine levels are high [48, 49]. Glutathione (GSH) is synthesized from cysteine, glutamate and glycine in cytoplasmic lysates and can be transported to various cellular compartments [50]. Serine and homocysteine are also linked to the methionine cycle via the transsulfuration pathway, and both homocysteine and serine are precursors of cysteine synthesis. Due to the fact that both glycine and cysteine are products of serine metabolism, the depletion of serine results in lower GSH levels, whereas the activation of the SGOC pathway increases GSH synthesis [51]. In conclusion, the SGOC pathway is closely associated with the methionine cycle and transsulfuration pathway.
Serine-glycine-one-carbon metabolism in tumors
The SGOC network is a metabolic hallmark that is frequently upregulated in tumors and orchestrates two nearly identical, intertwined methylation cycles in the cytoplasm and mitochondria, thus having high clinical relevance [18, 52, 53]. The key metabolic enzymes in SGOC (folate cycle) metabolism include PHGDH, PSAT1, PSPH, SHMT1/2, MTHFD1/1L and MTHFD2/2L. Recently, an increasing number of studies have reported that SGOC metabolic enzymes are highly expressed in various cancers and indicate poor prognosis. PHGDH is the major rate-limiting enzyme in the first step of the SGOC pathway, which is abnormal in various diseases, especially in cancers [54, 55]. The expression of PHGDH in pancreatic cancer patients is related to tumor size, lymph node metastasis, and TNM stage of pancreatic cancer patients; in addition, it is an independent prognostic indicator [56]. In lung cancer, SHMT1 and SHMT2 are both highly associated with the infiltration of different types of immune cells, and are potential prognostic biomarkers [57, 58]. A study of 7,309 patients with non-Hodgkin’s lymphoma showed that the SHMT1 C1420T polymorphism may be associated with the risk of developing non-Hodgkin’s lymphoma [59]. These data mentioned above suggest that SGCO metabolic enzymes may be a marker of tumor prognosis.
Redox effect of SGOC in tumors
Recent studies have demonstrated the effects of SGOC metabolism on dynamic redox balance and epigenetics [48]. The redox state is mainly determined by the dynamic balance between the generation of reactive oxygen species (ROS) and the activation of the antioxidant system [60]. SGOC-related metabolic enzymes can affect the NADPH/NADP+ ratio in tumors and regulate the redox state of cells. In fact, genomic analyses have shown that many cancers, especially breast cancer and non-small cell lung cancer (NSCLC), exhibit amplification and upregulated expression of SGOC metabolic enzymes, such as PHGDH and SHMT2 [61, 62]. Research has shown that the serine catabolic enzyme SHMT2, is induced when MYC-transformed cells are subjected to hypoxia; in the mitochondria, SHMT2 can initiate serine degradation to CO2 and NH4+, resulting in the net production of NADPH from NADP+ [63]. Knockdown of SHMT2 in MYC-dependent cells reduced the cellular NADPH:NADP+ ratio, increased cellular reactive oxygen species, and triggered hypoxia-induced cell death [63, 64]. In addition, one-carbon units for purine and thymidine synthesis can be generated from serine by cytosolic or mitochondrial folate metabolism [40]. Mitochondrial folate metabolic enzymes play a crucial role in this process. Folate metabolism can produce mitochondrial NADPH through ALDH1L2 and potential MTHFD2, and the knockdown of SHMT2 in some cancer cell lines increases their vulnerability to oxidative stressors [65]. The NADPH/NADP+ ratio in turn may play an important role in regulating the cytosolic flux of one-carbon units through the MTHFD1 dehydrogenase reaction [66].
Epigenetic roles of SGOC in tumors
Histone methyltransferase G9A promotes the transcription of key SGOC metabolic enzymes by maintaining an active chromatin state marked by histone H3 lysine 9 monomethylation (H3K9me1) in an ATF4-dependent manner [67]. SUMOylation is a reversible post-translational modification by conjugating with small ubiquitin-like modifiers (SUMOs) and a common protein modification in cancers [68]. NRF2 SUMOylation promotes the elimination of ROS in cells by increasing the transcription of glutathione peroxidase 2 (Gpx2), which leads to the upregulation of PHGDH in hepatocellular carcinoma (HCC) cells. These changes promote the production of one-carbon units in the de novo synthesis of serine and purine, thus promoting HCC [69]. Researchers have found that KDM4C epigenetically activates pathway genes under steady-state and serine deprivation conditions by removing the repressive histone modification histone H3 lysine 9 trimethylation (H3K9me3), in the serine-glycine synthesis pathway [70]. This finding links KDM4C-mediated H3K9 demethylation and ATF4-mediated transactivation in amino acid metabolism reprogramming for cancer cell proliferation. The deprivation of the SGOC metabolic pathway can lead to a significant drop in total ATP levels in rapidly proliferating cells, thus reducing the transfer of methyl to DNA and RNA, which can lead to changes in methyl transfer but will not induce the activation of AMP activated protein kinase (AMPK) [48]. One study showed that SHMT2 desuccinylation is a key signal for cancer cells to adapt to the serine metabolism process to achieve rapid growth, and the authors emphasized that SIRT5, as a candidate target to inhibit serine catabolism, is a strategy to block tumor growth [71].
Transcriptional regulation of SGOC in tumors
In addition to the effects of SGOC metabolism on dynamic redox balance and epigenetics, all of the SGOC metabolic enzymes are transcriptionally regulated by various transcription factors during the stress response or oncogene activation [72]. Ma et al. identified interacting proteins and detected their regulatory effects on translation initiation [73]. They found that PHGDH not only catalyzes serine synthesis and activates the AKT pathway but also interacts with the translation initiation factors eIF4A1 and eIF4E to promote the assembly of eIF4F on the 5’mRNA structure to increase the expression of related proteins, thus promoting the development of pancreatic cancer [73]. Studies have shown that in the absence of amino acids, cancer cells induce the expression of PHGDH, PSAT1, and PSPH in a GCN2-ATF4-dependent manner to produce sufficient amino acids [70, 74, 75]. Other transcription factors such as NRF2 and MYC, can also activate SGOC metabolism [69, 76, 77]. There is a MYC binding site E-box at the PHGDH, PSAT1, and SHMT gene sites, and knockout of MYC reduces their expression [77, 78]. In addition, HIF-1 and HIF-2 transcription factors can induce the expression of PHGDH, PSAT1, and SHMTs in breast cancer cell lines under hypoxia [79, 80]. The transcription regulators TAZ and YAP (TAZ/YAP) have become tumor-promoting factors that drive many carcinogenic characteristics, including improving cell growth, resisting cell death, and promoting cell migration and invasion. TAZ/YAP can induce the expression of glutamate oxaloacetate transaminase 1 (GOT1) and PSAT1 to produce more α-ketoglutarate and to promote the growth of breast cancer cells [81]. Recently, Liu et al. found that the lysine 64 residue (SHMT2K64) on SHMT2 and β-catenin and the transcription factor TCF4 interact to form SHMT2/β-catenin/TCF4 positive feedback loop, which inhibits ubiquitination-mediated degradation of β-catenin and promotes the proliferation and metastasis of CRC cells [29].
In conclusion, enhanced SGOC pathway activity may affect cancer cell processes, especially metabolism. Metabolites in the SGOC pathway (synthetic precursors of macromolecules, reducing substances, etc.) meet the metabolic requirements of rapid growth and proliferation of cancer cells. Moreover, the targeting of SGOC metabolic enzymes undoubtedly provides a new direction for exploring tumor therapy and brings hope for further research on tumor therapy.
Serine-glycine-one-carbon metabolism in cancer immunotherapy
The immune system plays an important role in controlling cancer progression. From the perspective of oncogenesis, tumor cells are transformed from normal cells, and this process from “self” to “non-self” is often closely monitored by the immune system and affected by an effective immune response [82]. The innate and adaptive immune systems interact to achieve effective anti-tumor immune monitoring [83]. Cancer immunotherapy has changed the cancer treatment paradigm, and these therapies aim to improve the anti-tumor immune response [84]. T lymphocytes are sentinels of the adaptive immune system, which are specifically used to identify and eliminate threats to the host [85]. The demand for specific nutrients that support the function of T cells increases the possibility that the metabolic microenvironment and availability of nutrients affect immunity by affecting the function of T cells [86]. Recently, some researchers have reported on the key role of non-essential amino acid serine in the effector T-cell response. Serine is essential for many biosynthetic and signal transduction pathways, including protein, nucleotide and glutathione synthesis, and is crucial for the growth and survival of proliferating cells [87]. After T-lymphocyte activation, T cells upregulate the enzymes of the SGOC metabolic network, and rapidly increase the process of serine conversion to one-carbon metabolism [88]. From the perspective of mechanism, serine provides glycine and one-carbon unit for de novo synthesis of proliferating T cells, and one-carbon unit in formate can rescue T cells from serine deficiency [88]. This suggests that the availability of serine in vivo may have important therapeutic significance for the immunotherapy and anti-tumor T-cell responses. Folic acid dependent one-carbon metabolism is a key metabolic process supporting cell proliferation, thus providing a carbon source for the synthesis of nucleotides in DNA and RNA [89]. Luteijn et al. determined that SLC19A1, as a folic acid organophosphorus reverse transporter, is the main transporter of cyclic dinucleotides (CDNs) by using genome wide CRISPR interference screening technology [90]. The inhibition of SLC19A1 can reduce the transport of folic acid, thereby reducing the uptake of CDNs by cancer cells [90, 91]. This discovery is of great significance for cancer immunotherapy and the host’s responsiveness to pathogenic microorganisms that produce CDNs. Researchers have also found that an immunosuppressive subset of tumor cells can be distinguished from the nonimmunosuppressive population by its upregulation of folate receptor beta (FRβ) and restriction to immunosuppressive tumor microenvironment [92]. Pemetrexed, which is a folate pathway inhibitor, can increase the activation of T cells in mouse tumors, and effectively induce immunogenic cell death in mouse tumor cells, as well as exert the inherent effects of T cells in vitro, such as enhancing mitochondrial function and T-cell activation [93]. Interestingly, some researchers have found that tryptophan (rather than serine) is the theoretical source of IDO1 (an enzyme in tumor immune escape) metabolism of one-carbon unit, and their research results showed that when cancer cells express IDO1, it will promote tryptophan to generate a carbon unit for the de novo synthesis of purine nucleotides [94]. Under the condition of low serine, tryptophan can be used as an alternative carbon source to support proliferation [95, 96]. Cancer cells release tryptophan derived formate, which can be used by pancreatic stellate cells to support the synthesis of purine nucleotides, thus avoiding the use of immunotherapy [95].
Recently, it has been reported that PSAT1 hypermethylation is related to T-cell dysfunction, shortened survival time and immune cell infiltration in breast cancer [97]. In addition, the expression of PSAT1 in lung cancer was significantly positively correlated with tumor mutational burden, and negatively correlated with tumor immune dysfunction and rejection [97]. It has been suggested that PSAT1 may be a new biomarker for the survival of lung cancer patients and for predicting the efficacy of immunotherapy. The infiltrating immune cells in ferroptosis-related genes (FRGs) in gastric cancer samples of the TCGA-STAD dataset were estimated by using the CIBERSORT and XCELL algorithms [98]. It was found that the overexpression of Hub FRGs (MYB, PSAT1, TP53 and LONP1) were positively correlated with the infiltration of activated CD4+ T cells, especially Th cells [98]. It is suggested that effective intervention on Hub FRGs is helpful to promote the activation of CD4+ T cells in patients with GC and to improve the efficacy of immunotherapy. In addition, researchers have found that the high expression of another key enzyme of SGOC metabolism (PSPH) was negatively correlated with CD8+ T cells, macrophages, and neutrophils, thus affecting the survival of patients with neuroblastoma [99]. Similarly, SHMT2 was significantly associated with CD8+ T cell infiltrates and highly expressed in breast, liver and lung cancer, and kidney renal papillary cell carcinoma [100]. These data suggested that PSPH and SHMT2 may be a promising indicator of the prognosis and cancer immunotherapy by affecting the infiltration level of immune cells. In bladder cancer, high expression of MTHFD2 was associated with PD‑L1 activation via the PI3K/AKT signaling pathway, suggesting that it could be a promising marker of cancer immunotherapy [101].
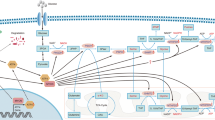
Serine-glycine-one-carbon metabolism in ferroptosis
Ferroptosis is a newly discovered form of programmed cell death, that is the result of excessive oxidation of iron-dependent polyunsaturated fatty acids [102, 103]. The three key characteristics of ferroptosis include membrane lipid peroxidation, availability of intracellular iron, and loss of antioxidant defense [104]. PHGDH, as the first rate-limiting enzyme of the SGOC metabolic pathway, plays an important role in ferroptosis-related pathways. Researchers have found that PHGDH can bind to the RNA-binding protein PCBP2 and inhibit its ubiquitination degradation; subsequently, PCBP2 stabilized SLC7A11 mRNA and increased its expression, thus inhibiting ferroptosis (Fig. 2) [105]. In gastric cancer, PSAT1 was identified as a ferroptosis-related gene and a new potential biomarker, papillary renal cell carcinoma and amyotrophic lateral sclerosis [98, 106, 107]. In liver cancer, the overexpression of c-Jun can activate the transcription of PSAT1 by directly binding with its promoter region, thereby antagonizing the ferroptosis induced by erastin [108]. In triple-negative breast cancer, MTHFD2 was identified as a ferroptosis regulator and prognostic biomarker [109].
Small molecular substances or metabolic pathways are also recognized as important influencing factors of ferroptosis. The amino acid glutamine was identified as the inducers of ferroptosis; and the glutamine-fueled intracellular metabolic pathway, glutaminolysis, was identified as the essential component of ferroptosis [110]. Homocysteine is an amino acid involved in gene methylation and can be generated by the SGOC metabolic pathway [111]. It was found that homocysteine promotes a degenerative cell phenotype (involving increased oxidative stress and cell death by ferroptosis) mediated by upregulated methylation of GPX4 [112].
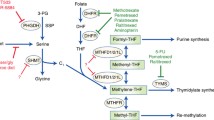
Serine-glycine-one-carbon metabolism and noncoding RNAs
PSAT1 and noncoding RNAs
Long noncoding RNAs (lncRNAs) can epigenetically regulate gene expression and cellular signaling pathways in different types of cancers [113, 114]. Accumulating evidence shows that lncRNAs are interlinked with PSAT1 and play a major role in cancer cell proliferation, angiogenesis and invasion [114,115,116]. The lncRNA RP4-694A7.2 levels in HCC tissues are higher than those in normal liver tissues and RP4-694A7.2 is also highly expressed in HCC cell lines [114]. RP4-694A7.2 regulates the glycolytic function of PSAT1 during HCC cell growth and invasion via the GSK3β/β-catenin pathway [114]. Interestingly, lncRNA MEG3 exerts tumor-suppressive effects and inhibits Epithelial-mesenchymal transition (EMT) by suppressing the PSAT1-dependent GSK3β/Snail signaling pathway in esophageal squamous cell carcinoma (ESCC) [115]. In NSCLC, the lncRNA MEG8 is expressed at higher levels in tumor tissues than in normal adjacent tissues and promotes tumor progression by regulating the miR-15a/b-5p/PSAT1 axis [117]. In addition, the lncRNA BC200 promotes the migration and invasion of cancer cells via the regulation of ATF4 expression, which in turn regulates the expression of PSAT1 in ESCC [116] (Fig. 3).
Abnormal expression of miRNAs plays an important role in the development of various cancers. In ESCC, the expression of miR-340 is negatively correlated with that of PSAT1 and significantly lower in tumor tissues than in paraneoplastic tissues [118]. Moreover, the high expression of miR-365 inhibits cell proliferation, invasion, colony formation and EMT and these inhibitions are reversed by the overexpression of PSAT1 [119]. In CRC, miR-424 directly inhibits PSAT1 expression at the transcriptional level, thereby supressing cell proliferation and inducing apoptosis [120]. In ovarian cancer, the overexpression of miR-195-5p reduces cisplatin resistance and angiogenesis by inhibiting PSAT1-dependent GSK3β/β-catenin [121]. Interestingly, miR-15a/b-5p is also regulated by the lncRNA MEG8 and affects the proliferation, migration and invasion of NSCLC cells through the downregulation of PSAT1 expression [117]. In summary, PSAT1 may provide a more effective therapeutic strategy for cancer treatment.
SHMT and noncoding RNAs
The overexpression of the lncRNA Gm15290 exerts tumor-stimulating effects through the inhibition of miR-615-5p, which targets the genes insulin-like growth factor 2 (IGF2), AKT2, and SHMT2 [122, 123]. In colon cancer, LINC01234 is highly expressed and acts as a competing endogenous RNA for miR-642a-5p, which targets SHMT2 [124]. Furthermore, miR-6778-5p positively regulates SHMT1, thus mediating compensatory activation of cytoplasmic one-carbon metabolism, which plays an essential role in the maintenance of gastric cancer stem cells (GCSCs) [125]. The deletion of miR-6778-5p or SHMT1 significantly reduces GCSC sphere formation and increases 5-FU sensitivity in Drosha-knockdown cells [125]. In lung cancer, miR-198 inhibits cell proliferation in vitro and in vivo by directly targeting SHMT1 [126]. In addition, miR-218-5p suppresses the cytotoxic effect of natural killer cells by targeting SHMT1 in lung cancer [127]. In addition, the circRNA circ_0072995 was demonstrated to promote a malignant phenotype and anaerobic glycolysis by competitively binding miR-149-5p to upregulate its downstream gene SHMT2 in breast cancer [128].
MTHFD2 and noncoding RNAs
MTHFD2 has been confirmed to be a target gene of miR-33a-5p that suppresses CRC cell growth by inhibiting MTHFD2 [129]. In acute myeloid leukemia (AML), miR-92a inhibits cell proliferation and induces apoptosis by directly suppressing MTHFD2 expression [130]. The high-expression of miR-504-3p is associated with good prognosis in AML patients and may serve as a tumor suppressor by targeting MTHFD2 [131]. In breast cancer, miR-9 exerts anti-proliferative, anti-invasive and proapoptotic effects by targeting MTHFD2 [132]. Interestingly, the lncRNA taurine upregulated gene 1 (TUG1) was found to negatively regulate miR-9 expression but positively regulate the expression of MTHFD2 in breast cancer cells [133]. In glioma, miR-940 suppresses tumor progression by inhibiting mitochondrial folate metabolism, which directly targets MTHFD2 [134]. In gastric cancer, miR-22 inhibits cell proliferation by inducing a deficiency in endogenous SAM by reducing MTHFD2 and MTHFR expression [135]. In head and neck squamous cell carcinoma (HNSCC), low expression of miR-99a-3p, which targets MTHFD2, significantly predicts poor prognosis [136]. These results indicate that miRNAs targeting MTHFD2 regulate tumor progression and may be new biomarkers.
Red font, Promoting the expression of targeted SGOC metabolic enzymes; Green font, Inhibiting the expression of targeted SGOC-metabolic enzymes.
Therapeutic targeting of serine-glycine-one-carbon metabolic enzymes
Inhibitors of enzymes in serine-glycine biosynthesis
PHGDH inhibitors can be divided into two main types: synthetic and natural chemicals (Table 1). The synthetic chemicals include BI-4924, CBR-5884, NCT-503, PKUMDL-WQ-2201 and so on [165, 138]. The natural chemicals include azacoccone E and ixocarpalactone A [141, 142] Researchers have found that the most effective PSPH inhibitor using L-phosphoserine as the substrate is p-chloromercuriphenylsulfonic acid (CMPSA), followed by Glyceryl phosphocholine [144]. Moreover, clofazimine is a specific inhibitor of PSPH [143]; D-AP3 is selective and is the most effective competitive inhibitor of PSPH [166]. As an SHMT inhibitor, the compound SHIN1 with the pyrazolopyran scaffold exerts potent and specific on target activity against SHMTs [36]. In addition to having selective activity against SHMT1, compound 2.12 also displayed anticancer activity in the mid-micromolar range [147]. AGF347, a folate mimetic, exerts significant in vivo anti-tumor effect, providing the candidates for therapeutic targeting of SHMTs [146].
Inhibitors of enzymes in one-carbon metabolism
One-carbon metabolism supports vital events for the growth and survival of proliferating cells whose enzymes are associated with cancer progression [72, 100, 167]. Aminopterin is an anti-folate drug that has been found to relieve childhood acute lymphoblastic leukemia (ALL) [168]. Based on this discovery, a series of one-carbon-metabolism-targeted drugs have been developed, including methotrexate, pemetrexed, and 5-FU, which are of great significance in cancer treatment, especially immunotherapy [93, 169,170,171,172,173,174,175,176,177]. Ly345889 is the first synthetic inhibitor of MTHFD1/2 [148]. Subsequently, researchers found that carolacton, which is a macrolide ketone carbonic acid, inhibits folic acid-dependent one-carbon metabolism by targeting MTHFD1/2, and its inhibitory activity is higher than that of Ly345889 [149]. DHFR and TYMS were the early enzymes of one-carbon metabolism to be clinically validated as targets for cancer therapy and remain the most successful in this context to date, and almost all Food and Drug Administration (FDA)-approved DHFR and TYMS inhibitors are classical or non-classical folate derivatives [178]. There are also several compounds targeting these two enzymes in the clinical trial pipeline: Trimetrexate [156], Raltitrexed [157], Piritrexim [158], ZD-9331 [159], GS7904L [160], and ONX-0801 [161]. Folate transporters play important roles in the efficacy of anti-folate chemotherapies [179]. Farletuzumab is a monoclonal antibody specifically targeting folate receptor beta (FOLR1) [180, 181]. Despite encouraging preclinical data, farletuzumab has not successfully completed the Phase III trial [180]. The biosynthesis of purines is carried out by purinosome and requires phosphoribosylglycinamide formyltransferase (GART) [182]. Lometrexol has the strongest inhibitory effect on GART, which is in clinical trials [163, 183]. Moreover, the methionine cycle is crucial for one-carbon metabolism because the one-carbon unit is transferred to S-adenosyl-l-homocysteine (SAH) to form SAM in this process, which is necessary for many life activities [184]. AG-270, targeting methionine adenosyltransferase 2 A (MAT2A) in the methionine cycle, is currently in a cinical trial (NCT03435250) [162]. Cystathionine beta-synthase (CBS) is the first rate-limiting enzyme in the transsulfuration pathway and play vital roles in the occurrence, development, and treatment of cancer [185]. As a classical inhibitor of CBS, aminooxyacetic acid is the most common, but it also inhibits cystathionine gamma-lyase (CSE), which is another enzyme in the transsulfuration pathway [164].
In summary, the investigation of inhibitors of SGOC metabolism will help to clarify the role of one-carbon metabolism in different stages of cancer progression and to verify whether one-carbon metabolism is the right pathway to drugs for cancer treatment. The newer generation of drugs selectively targeting key metabolic enzymes in SGOC metabolism, such as PHGDH, MTHFDs, DHFR, TYMS, GART and CBS, will provide a new strategy for cancer treatment in the future.
Conclusion and prospects
More than 70 years ago, Faber and his colleagues found that folic acid can stimulate the proliferation of acute lymphoblastic leukemia cells, and used aminopterin to induce clinical remission in patients [168]. At later time periods, more drugs targeting the one-carbon metabolic pathway were found, such as the folic acid metabolism and thymidine acid synthesis inhibitors: methotrexate and 5-Fu, which are among the first successful cancer treatments [186]. These drugs are still used to treat various cancers. When the PHGDH-specific inhibitor NCT-503 or shRNA was used to inhibit PHGDH expression, the antitumor effect of doxorubicin in TNBC was significantly improved in vivo and in vitro [187]. In addition, pemetrexed could increase the activation of T cells in mouse tumors in vivo and exerted the inherent effect of T cells in vitro; moreover, combined with PD-1 pathway blockade, pemetrexed enhanced the anti-tumor effect [93]. These evidences indicate that the inhibition of specific enzymes in SGOC metabolism can be a more effective mechanism for synergistic treatment of drug resistance and enhancing tumor immunotherapy.
To generate enough one-carbon unit to meet their own proliferation needs, tumor cells usually increase their intake of extracellular serine, glycine and other raw materials [48, 52]. Therefore, limitations of serine and glycine in the diet may be a good treatment strategy [21]. Tumors with amplified SGOC metabolic enzymes are unlikely to be affected by exogenous serine consumption, and p53 deletion may aggravate this dependence [20]. This scenario may be due to the impaired transformation between glycolysis and oxidative phosphorylation when p53 is deficient, thus resulting in insufficient ATP production [22]. It has been found that the growth rate of tumors and the final volume of tumors in mice treated with a serine- or glycine-deficient diet combined with metformin significantly decreased [188]. The mechanism may be that serine deficiency can inhibit the compensatory increase in the metformin induced glycolysis pathway. Therefore, the identification of metabolic dependencies related to the environment may help to identify tumor types that may benefit from existing approved therapies and can be more easily reused for new applications [8, 189].
SGOC metabolism not only serves as precursors to protein synthesis, but also provides one-carbon precursors for nucleotide synthesis, as well as head groups for sphingolipid and phospholipid synthesis [18, 44]. It seems that many cancer cells depend on the availability of one-carbon unit to some extent, which indicates that the limitation of one-carbon unit supply can have more therapeutic benefits. This review comprehensively analyzes the expression pattern and metabolic flux of the SGOC pathway in multiple cancer backgrounds at the system level and to describe the possible role of the SGOC metabolic network in tumor immunotherapy and ferroptosis. It is expected that new research and more effective and specific compounds may provide much-needed breakthroughs in targeting this pathway against cancer.
Availability of data and materials
Not applicable.
Abbreviations
- α-KG:
-
α-ketoglutarate
- ADP:
-
Adenosine diphosphate
- ALDH1L1/2:
-
Aldehyde dehydrogenase 1 family member L1/L2
- AML:
-
Acute myeloid leukemia
- AMPK:
-
AMP activated protein kinase
- ATP:
-
Adenosine triphosphate
- BHMT:
-
betaine-homocysteine S-methyltransferase
- B12:
-
Vitamin B12
- B6:
-
Vitamin B6
- CBS:
-
Cystathionine beta-synthase
- CDNs:
-
Cyclic dinucleotides
- CH+-THF:
-
5,10-methenyl-THF
- CH2-THF:
-
5,10-methylenetetrahydrofolate
- CMPSA:
-
p-chloromercuriphenylsulfonic acid
- CRC:
-
Colorectal cancer
- CSE:
-
Cystathionine gamma-lyase
- DHF:
-
Dihydrofuran
- DHFR:
-
Dihydrofolate reductase
- DMG:
-
Dimethylglycine
- EMT:
-
Epithelial-mesenchymal transition
- ESCC:
-
Esophageal squamous cell carcinoma
- FDA:
-
Food and Drug Administration
- FOCM:
-
Folate-mediated one-carbon metabolism
- FOLR1:
-
Folate Receptor Alpha
- FRGs:
-
Ferroptosis-related genes
- FRβ:
-
Folate receptor beta
- GCSCs:
-
Gastric cancer stem cells
- GART:
-
Phosphoribosylglycinamide formyltransferase
- GCLC:
-
Gamma-glutamyl cysteine synthetase
- GGCT:
-
Gamma-Glutamylcyclotransferase
- GLDC:
-
Glycine dehydrogenase
- GLS:
-
Glutaminase
- Glu:
-
Glutamate
- GOT1:
-
Glutamate oxaloacetate transaminase
- Gpx2:
-
Glutathione peroxidase 2
- GSH:
-
Glutathione
- GSS:
-
Glutathione synthetase
- H3K9me1:
-
Histone H3 lysine 9 monomethylation
- H3K9me3:
-
Histone H3 lysine 9 trimethylation
- HCC:
-
Hepatocellular carcinoma
- HNSCC:
-
Head and neck squamous cell carcinoma
- IGF2:
-
Insulin-like growth factor 2
- ILF3:
-
Interleukin enhancer binding factor 3
- LncRNAs:
-
Long noncoding RNAs
- MAT:
-
Methionine adenosyltransferase
- MAT2A:
-
Methionine adenosyltransferase 2 A
- MS:
-
Methionine synthase
- MTHF:
-
Methylenetetrahydrofolate
- MTHFD1/1L:
-
Methylenetetrahydrofolate dehydrogenase 1/1L
- MTHFD2/2L:
-
Methylenetetrahydrofolate dehydrogenase 2/2L
- MTHFR:
-
Methylenetetrahydrofolate reductase
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- NSC127755:
-
Trichloroethylene anti folic acid
- NSCLC:
-
Non-small cell lung cancer
- PCNSL:
-
Primary central nervous system lymphoma
- PEP:
-
Phosphoenolpyruvate
- PHGDH:
-
Phosphoglycerate dehydrogenase
- PSAT1:
-
Phosphoserine aminotransferase 1
- PSPH:
-
Phosphoserine phosphatase
- ROS:
-
Reactive oxygen species
- SAH:
-
S-adenosyl-l-homocysteine
- SAM:
-
S-adenosyl-l-methionine
- SGOC:
-
Serine-glycine-one-carbon
- SHMT1/2:
-
Serine hydroxymethyltransferase 1/2
- SUMOs:
-
Small ubiquitin-like modifiers
- THF:
-
Tetrahydrofolate
- TYMS:
-
Thymidylate synthase
- 2PG:
-
2-phosphoglycerate
- 3PG:
-
3-phosphoglycerate
- 3PH:
-
3-phosphohydroxypyruvate
- 3PS:
-
3-phosphoserine
- 10-CHO-THF:
-
10-formyltetrahydrofolate
- 5-Fu:
-
5-fluorouracil
- 5-CH3-THF:
-
5-methyltetrahydrofolate
References
Ben-Sahra I, Hoxhaj G, Ricoult SJH, Asara JM, Manning BD. mTORC1 induces purine synthesis through control of the mitochondrial tetrahydrofolate cycle. Science. 2016;351:728–33.
Chen L, Zhang Z, Hoshino A, Zheng HD, Morley M, Arany Z, et al. NADPH production by the oxidative pentose-phosphate pathway supports folate metabolism. Nat Metab. 2019;1:404–15.
de Koning TJ, Snell K, Duran M, Berger R, Poll-The BT, Surtees R. L-serine in disease and development. Biochem J. 2003;371:653–61.
Labuschagne CF, van den Broek NJ, Mackay GM, Vousden KH, Maddocks OD. Serine, but not glycine, supports one-carbon metabolism and proliferation of cancer cells. Cell Rep. 2014;7:1248–58.
Locasale JW. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat Rev Cancer. 2013;13:572–83.
Kalhan SC, Hanson RW. Resurgence of serine: an often neglected but indispensable amino acid. J Biol Chem. 2012;287:19786–91.
DeBerardinis RJ, Cheng T. Q’s next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene. 2010;29:313–24.
Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33.
Amelio I, Cutruzzola F, Antonov A, Agostini M, Melino G. Serine and glycine metabolism in cancer. Trends Biochem Sci. 2014;39:191–8.
Reina-Campos M, Diaz-Meco MT, Moscat J. The complexity of the serine glycine one-carbon pathway in cancer. J Cell Biol. 2020;219:e201907022.
Mehrmohamadi M, Liu X, Shestov AA, Locasale JW. Characterization of the usage of the serine metabolic network in human cancer. Cell Rep. 2014;9:1507–19.
Gao X, Locasale JW, Reid MA. Serine and methionine metabolism: vulnerabilities in lethal prostate cancer. Cancer Cell. 2019;35:339–41.
Xia Y, Ye B, Ding J, Yu Y, Alptekin A, Thangaraju M, et al. Metabolic reprogramming by MYCN confers dependence on the serine-glycine-one-carbon biosynthetic pathway. Cancer Res. 2019;79:3837–50.
Zhao E, Hou J, Cui H. Serine-glycine-one-carbon metabolism: vulnerabilities in MYCN-amplified neuroblastoma. Oncogenesis. 2020;9:14.
Garcia AR, Arsenian-Henriksson M. Serine-glycine-one-carbon metabolism: the hidden Achilles heel of MYCN-amplified neuroblastoma? Cancer Res. 2019;79:3818–9.
Li K, Wu JL, Qin B, Fan Z, Tang Q, Lu W, et al. ILF3 is a substrate of SPOP for regulating serine biosynthesis in colorectal cancer. Cell Res. 2020;30:163–78.
Filippone MG, Gaglio D, Bonfanti R, Tucci FA, Ceccacci E, Pennisi R, et al. CDK12 promotes tumorigenesis but induces vulnerability to therapies inhibiting folate one-carbon metabolism in breast cancer. Nat Commun. 2022;13:2642.
Li AM, Ye J. Reprogramming of serine, glycine and one-carbon metabolism in cancer. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165841.
Jain M, Nilsson R, Sharma S, Madhusudhan N, Kitami T, Souza AL, et al. Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation. Science. 2012;336:1040–4.
Maddocks ODK, Berkers CR, Mason SM, Zheng L, Blyth K, Gottlieb E, et al. Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. Nature. 2013;493:542–6.
Maddocks ODK, Athineos D, Cheung EC, Lee P, Zhang T, van den Broek NJF, et al. Modulating the therapeutic response of tumours to dietary serine and glycine starvation. Nature. 2017;544:372–6.
Ou Y, Wang SJ, Jiang L, Zheng B, Gu W. p53 protein-mediated regulation of phosphoglycerate dehydrogenase (PHGDH) is crucial for the apoptotic response upon serine starvation. J Biol Chem. 2015;290:457–66.
Galluzzi L, Pietrocola F, Bravo-San Pedro JM, Amaravadi RK, Baehrecke EH, Cecconi F, et al. Autophagy in malignant transformation and cancer progression. EMBO J. 2015;34:856–80.
Commisso C, Davidson SM, Soydaner-Azeloglu RG, Parker SJ, Kamphorst JJ, Hackett S, et al. Macropinocytosis of protein is an amino acid supply route in ras-transformed cells. Nature. 2013;497:633–7.
Kamphorst JJ, Nofal M, Commisso C, Hackett SR, Lu W, Grabocka E, et al. Human pancreatic cancer tumors are nutrient poor and tumor cells actively scavenge extracellular protein. Cancer Res. 2015;75:544–53.
Choi YJ, Lee G, Yun SH, Lee W, Yu J, Kim SK, et al. The role of SHMT2 in modulating lipid metabolism in hepatocytes via glycine-mediated mTOR activation. Amino Acids. 2022;54:823–34.
Hebbring SJ, Chai YB, Ji Y, Abo RP, Jenkins GD, Fridley B, et al. Serine hydroxymethyltransferase 1 and 2: gene sequence variation and functional genomic charctaerization. J Neurochem. 2012;120:881–90.
Zhao EH, Hou JB, Ke XX, Abbas MN, Kausar S, Zhang L, et al. The roles of sirtuin family proteins in cancer progression. Cancers. 2019;11:1949.
Liu C, Wang L, Liu X, Tan Y, Tao L, Xiao Y, et al. Cytoplasmic SHMT2 drives the progression and metastasis of colorectal cancer by inhibiting beta-catenin degradation. Theranostics. 2021;11:2966–86.
Tibbetts AS, Appling DR. Compartmentalization of mammalian folate-mediated one-carbon metabolism. Annu Rev Nutr. 2010;30:57–81.
Zhang WC, Shyh-Chang N, Yang H, Rai A, Umashankar S, Ma S, et al. Glycine decarboxylase activity drives non-small cell lung cancer tumor-initiating cells and tumorigenesis. Cell. 2012;148:259–72.
Pasternack LB, Laude DA Jr, Appling DR. 13 C NMR detection of folate-mediated serine and glycine synthesis in vivo in Saccharomyces cerevisiae. Biochemistry. 1992;31:8713–9.
Herbig K, Chiang EP, Lee LR, Hills J, Shane B, Stover PJ. Cytoplasmic serine hydroxymethyltransferase mediates competition between folate-dependent deoxyribonucleotide and S-adenosylmethionine biosyntheses. J Biol Chem. 2002;277:38381–9.
Gregory JF 3rd, Cuskelly GJ, Shane B, Toth JP, Baumgartner TG, Stacpoole PW. Primed, constant infusion with [2H3]serine allows in vivo kinetic measurement of serine turnover, homocysteine remethylation, and transsulfuration processes in human one-carbon metabolism. Am J Clin Nutr. 2000;72:1535–41.
Zhao LN, Bjorklund M, Caldez MJ, Zheng J, Kaldis P. Therapeutic targeting of the mitochondrial one-carbon pathway: perspectives, pitfalls, and potential. Oncogene. 2021;40:2339–54.
Yang C, Zhang J, Liao M, Yang Y, Wang Y, Yuan Y, et al. Folate-mediated one-carbon metabolism: a targeting strategy in cancer therapy. Drug Discov Today. 2021;26:817–25.
Lan X, Field MS, Stover PJ. Cell cycle regulation of folate-mediated one-carbon metabolism. Wiley Interdiscip Rev Syst Biol Med. 2018;10:e1426.
Tucker EJ, Hershman SG, Kohrer C, Belcher-Timme CA, Patel J, Goldberger OA, et al. Mutations in MTFMT underlie a human disorder of formylation causing impaired mitochondrial translation. Cell Metab. 2011;14:428–34.
Nilsson R, Jain M, Madhusudhan N, Sheppard NG, Strittmatter L, Kampf C, et al. Metabolic enzyme expression highlights a key role for MTHFD2 and the mitochondrial folate pathway in cancer. Nat Commun. 2014;5:3128.
Ducker GS, Chen L, Morscher RJ, Ghergurovich JM, Esposito M, Teng X, et al. Reversal of cytosolic one-carbon flux compensates for loss of the mitochondrial folate pathway. Cell Metab. 2016;23:1140–53.
Chen ZW, Hu JF, Wang ZW, Liao CY, Kang FP, Lin CF, et al. Circular RNA circ-MTHFD1L induces HR repair to promote gemcitabine resistance via the miR-615-3p/RPN6 axis in pancreatic ductal adenocarcinoma. J Exp Clin Cancer Res. 2022;41:153.
Krupenko SA, Krupenko NI. ALDH1L1 and ALDH1L2 folate regulatory enzymes in cancer. Adv Exp Med Biol. 2018;1032:127–43.
Burda P, Kuster A, Hjalmarson O, Suormala T, Burer C, Lutz S, et al. Characterization and review of MTHFD1 deficiency: four new patients, cellular delineation and response to folic and folinic acid treatment. J Inherit Metab Dis. 2015;38:863–72.
Yang M, Vousden KH. Serine and one-carbon metabolism in cancer. Nat Rev Cancer. 2016;16:650–62.
Struck AW, Thompson ML, Wong LS, Micklefield J. S-adenosyl-methionine-dependent methyltransferases: highly versatile enzymes in biocatalysis, biosynthesis and other biotechnological applications. ChemBioChem. 2012;13:2642–55.
Raghubeer S, Matsha TE. Methylenetetrahydrofolate (MTHFR), the one-carbon cycle, and cardiovascular risks. Nutrients. 2021;13:4562.
Davis SR, Stacpoole PW, Williamson J, Kick LS, Quinlivan EP, Coats BS, et al. Tracer-derived total and folate-dependent homocysteine remethylation and synthesis rates in humans indicate that serine is the main one-carbon donor. Am J Physiol Endocrinol Metab. 2004;286:E272–9.
Maddocks OD, Labuschagne CF, Adams PD, Vousden KH. Serine metabolism supports the methionine cycle and DNA/RNA methylation through de novo ATP synthesis in cancer cells. Mol Cell. 2016;61:210–21.
Mentch SJ, Mehrmohamadi M, Huang L, Liu X, Gupta D, Mattocks D, et al. Histone methylation dynamics and gene regulation occur through the sensing of one-carbon metabolism. Cell Metab. 2015;22:861–73.
Hultberg B, Hultberg M. High glutathione turnover in human cell lines revealed by acivicin inhibition of gamma-glutamyltranspeptidase and the effects of thiol-reactive metals during acivicin inhibition. Clin Chim Acta. 2004;349:45–52.
Yoshida K, Furuya S, Osuka S, Mitoma J, Shinoda Y, Watanabe M, et al. Targeted disruption of the mouse 3-phosphoglycerate dehydrogenase gene causes severe neurodevelopmental defects and results in embryonic lethality. J Biol Chem. 2004;279:3573–7.
Zheng Y, Lin TY, Lee G, Paddock MN, Momb J, Cheng Z, et al. Mitochondrial one-carbon pathway supports cytosolic folate integrity in cancer cells. Cell. 2018;175:1546–60e17.
Liu ML, Xia YF, Ding JN, Ye BW, Zhao EH, Choi JH, et al. Transcriptional profiling reveals a common metabolic program in high-risk human neuroblastoma and mouse neuroblastoma sphere-forming cells. Cell Rep. 2016;17:609–23.
Zhao JY, Feng KR, Wang F, Zhang JW, Cheng JF, Lin GQ, et al. A retrospective overview of PHGDH and its inhibitors for regulating cancer metabolism. Eur J Med Chem. 2021;217:113379.
Annibali D, Fendt SM. Nuclear PHGDH protects cancer cells from nutrient stress. Nat Metab. 2021;3:1284–5.
Ross KC, Andrews AJ, Marion CD, Yen TJ, Bhattacharjee V. Identification of the serine biosynthesis pathway as a critical component of BRAF inhibitor resistance of melanoma, pancreatic, and non-small cell lung cancer cells. Mol Cancer Ther. 2017;16:1596–609.
Luo L, Zheng Y, Lin Z, Li X, Li X, Li M, et al. Identification of SHMT2 as a potential prognostic biomarker and correlating with immune infiltrates in lung adenocarcinoma. J Immunol Res. 2021;2021:6647122.
Bouzidi A, Magnifico MC, Paiardini A, Macone A, Boumis G, Giardina G, et al. Cytosolic serine hydroxymethyltransferase controls lung adenocarcinoma cells migratory ability by modulating AMP kinase activity. Cell Death Dis. 2020;11:1012.
Wang YW, Zhang SD, Xue WJ, Zhu ML, Zheng LZ. SHMT1 C1420T polymorphism contributes to the risk of non-hodgkin lymphoma: evidence from 7309 patients. Chin J Cancer. 2015;34:573–82.
Bradshaw PC. Cytoplasmic and mitochondrial NADPH-coupled redox systems in the regulation of aging. Nutrients. 2019;11:504.
Bernhardt S, Bayerlova M, Vetter M, Wachter A, Mitra D, Hanf V, et al. Proteomic profiling of breast cancer metabolism identifies SHMT2 and ASCT2 as prognostic factors. Breast Cancer Res. 2017;19:112.
Ju HQ, Lu YX, Chen DL, Zuo ZX, Liu ZX, Wu QN, et al. Modulation of redox homeostasis by inhibition of MTHFD2 in colorectal cancer: mechanisms and therapeutic implications. J Natl Cancer Inst. 2019;111:584–96.
Ye J, Fan J, Venneti S, Wan YW, Pawel BR, Zhang J, et al. Serine catabolism regulates mitochondrial redox control during hypoxia. Cancer Discov. 2014;4:1406–17.
Clark RA, Qiao J, Jacobson JC, Chung DH. Induction of serine hydroxymethyltransferase 2 promotes tumorigenesis and metastasis in neuroblastoma. Oncotarget. 2022;13:32–45.
Konno M, Asai A, Kawamoto K, Nishida N, Satoh T, Doki Y, et al. The one-carbon metabolism pathway highlights therapeutic targets for gastrointestinal cancer (review). Int J Oncol. 2017;50:1057–63.
Meiser J, Tumanov S, Maddocks O, Labuschagne CF, Athineos D, Van Den Broek N, et al. Serine one-carbon catabolism with formate overflow. Sci Adv. 2016;2:e1601273.
Ding J, Li T, Wang X, Zhao E, Choi JH, Yang L, et al. The histone H3 methyltransferase G9A epigenetically activates the serine-glycine synthesis pathway to sustain cancer cell survival and proliferation. Cell Metab. 2013;18:896–907.
Yau TY, Sander W, Eidson C, Courey AJ. SUMO interacting motifs: structure and function. Cells. 2021;10:2825.
Guo H, Xu J, Zheng Q, He J, Zhou W, Wang K, et al. NRF2 SUMOylation promotes de novo serine synthesis and maintains HCC tumorigenesis. Cancer Lett. 2019;466:39–48.
Zhao E, Ding J, Xia Y, Liu M, Ye B, Choi JH, et al. KDM4C and ATF4 cooperate in transcriptional control of amino acid metabolism. Cell Rep. 2016;14:506–19.
Yang X, Wang Z, Li X, Liu B, Liu M, Liu L, et al. SHMT2 desuccinylation by SIRT5 drives cancer cell proliferation. Cancer Res. 2018;78:372–86.
Sun W, Zhao E, Cui H. Target enzymes in serine-glycine-one-carbon metabolic pathway for cancer therapy. Int J Cancer. 2022;2022:1–18.
Ma X, Li B, Liu J, Fu Y, Luo Y. Phosphoglycerate dehydrogenase promotes pancreatic cancer development by interacting with eIF4A1 and eIF4E. J Exp Clin Cancer Res. 2019;38:66.
Gao S, Ge A, Xu S, You Z, Ning S, Zhao Y, et al. PSAT1 is regulated by ATF4 and enhances cell proliferation via the GSK3beta/beta-catenin/cyclin D1 signaling pathway in ER-negative breast cancer. J Exp Clin Cancer Res. 2017;36:179.
Adams CM. Role of the transcription factor ATF4 in the anabolic actions of insulin and the anti-anabolic actions of glucocorticoids. J Biol Chem. 2007;282:16744–53.
DeNicola GM, Chen PH, Mullarky E, Sudderth JA, Hu Z, Wu D, et al. NRF2 regulates serine biosynthesis in non-small cell lung cancer. Nat Genet. 2015;47:1475–81.
Nilsson LM, Forshell TZ, Rimpi S, Kreutzer C, Pretsch W, Bornkamm GW, et al. Mouse genetics suggests cell-context dependency for myc-regulated metabolic enzymes during tumorigenesis. PLoS Genet. 2012;8:e1002573.
Sun L, Song L, Wan Q, Wu G, Li X, Wang Y, et al. Cmyc-mediated activation of serine biosynthesis pathway is critical for cancer progression under nutrient deprivation conditions. Cell Res. 2015;25:429–44.
Yoshino H, Nohata N, Miyamoto K, Yonemori M, Sakaguchi T, Sugita S, et al. PHGDH as a key enzyme for serine biosynthesis in HIF2α-targeting therapy for renal cell carcinoma. Cancer Res. 2017;77:6321–9.
Nagao A, Kobayashi M, Koyasu S, Chow CCT, Harada H. HIF-1-dependent reprogramming of glucose metabolic pathway of cancer cells and its therapeutic significance. Int J Mol Sci. 2019;20:238.
Yang CS, Stampouloglou E, Kingston NM, Zhang L, Monti S, Varelas X. Glutamine-utilizing transaminases are a metabolic vulnerability of TAZ/YAP-activated cancer cells. EMBO Rep. 2018;19:e43577.
Sangro B, Sarobe P, Hervas-Stubbs S, Melero I. Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18:525–43.
Wargo JA, Reddy SM, Reuben A, Sharma P. Monitoring immune responses in the tumor microenvironment. Curr Opin Immunol. 2016;41:23–31.
Riley RS, June CH, Langer R, Mitchell MJ. Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov. 2019;18:175–96.
Buck MD, O’Sullivan D, Pearce EL. T cell metabolism drives immunity. J Exp Med. 2015;212:1345–60.
Chang CH, Curtis JD, Maggi LB Jr, Faubert B, Villarino AV, O’Sullivan D, et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153:1239–51.
Pan SJ, Fan M, Liu ZN, Li X, Wang HJ. Serine, glycine and one-carbon metabolism in cancer (review). Int J Oncol. 2021;58:158–70.
Ma EH, Bantug G, Griss T, Condotta S, Johnson RM, Samborska B, et al. Serine is an essential metabolite for effector T cell expansion. Cell Metab. 2017;25:482.
Tedeschi PM, Markert EK, Gounder M, Lin H, Dvorzhinski D, Dolfi SC, et al. Contribution of serine, folate and glycine metabolism to the ATP, NADPH and purine requirements of cancer cells. Cell Death Dis. 2013;4:e877.
Luteijn RD, Zaver SA, Gowen BG, Wyman SK, Garelis NE, Onia L, et al. SLC19A1 transports immunoreactive cyclic dinucleotides. Nature. 2019;573:434–8.
Ritchie C, Cordova AF, Hess GT, Bassik MC, Li L. SLC19A1 is an importer of the immunotransmitter cGAMP. Mol Cell. 2019;75:372-81.e5.
Cresswell GM, Wang B, Kischuk EM, Broman MM, Alfar RA, Vickman RE, et al. Folate receptor beta designates immunosuppressive tumor-associated myeloid cells that can be reprogrammed with folate-targeted drugs. Cancer Res. 2021;81:671–84.
Schaer DA, Geeganage S, Amaladas N, Lu ZH, Rasmussen ER, Sonyi A, et al. The folate pathway inhibitor pemetrexed pleiotropically enhances effects of cancer immunotherapy. Clin Cancer Res. 2019;25:7175–88.
Shi D, Wu X, Jian Y, Wang J, Huang C, Mo S, et al. USP14 promotes tryptophan metabolism and immune suppression by stabilizing IDO1 in colorectal cancer. Nat Commun. 2022;13:5644.
Newman AC, Falcone M, Huerta Uribe A, Zhang T, Athineos D, Pietzke M, et al. Immune-regulated IDO1-dependent tryptophan metabolism is source of one-carbon units for pancreatic cancer and stellate cells. Mol Cell. 2021;81:2290-302.e7.
Wang Y, Chen J, Tian J, Wang G, Luo W, Huang Z, et al. Tryptophan-sorbitol based carbon quantum dots for theranostics against hepatocellular carcinoma. J Nanobiotechnol. 2022;20:78.
Feng M, Cui H, Tu W, Li L, Gao Y, Chen L, et al. An integrated pan-cancer analysis of PSAT1: a potential biomarker for survival and immunotherapy. Front Genet. 2022;13:975381.
Yao F, Zhan Y, Pu Z, Lu Y, Chen J, Deng J, et al. LncRNAs target ferroptosis-related genes and impair activation of CD4(+) T cell in gastric cancer. Front Cell Dev Biol. 2021;9:797339.
Zeng L, Liu XY, Chen K, Qin LJ, Wang FH, Miao L, et al. Phosphoserine phosphatase as an indicator for survival through potentially influencing the infiltration levels of immune cells in neuroblastoma. Front Cell Dev Biol. 2022;10:873710.
Usman M, Hameed Y, Ahmad M, Iqbal MJ, Maryam A, Mazhar A, et al. SHMT2 is associated with tumor purity, CD8 + T immune cells infiltration, and a novel therapeutic target in four different human cancers. Curr Mol Med. 2023;23:161–76.
Deng XX, Liu XQ, Hu B, Liu JY, Fu B, Zhang WS. Upregulation of MTHFD2 is associated with PD-L1 activation in bladder cancer via the PI3K/AKT pathway. Int J Mol Med. 2023;51:14.
Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021;31:107–25.
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72.
Chen X, Li J, Kang R, Klionsky DJ, Tang D. Ferroptosis: machinery and regulation. Autophagy. 2021;17:2054–81.
Shen L, Zhang J, Zheng Z, Yang F, Liu S, Wu Y, et al. PHGDH inhibits ferroptosis and promotes malignant progression by upregulating SLC7A11 in bladder cancer. Int J Biol Sci. 2022;18:5459–74.
Zhang Q, Zhao H, Luo M, Cheng X, Li Y, Li Q, et al. The classification and prediction of ferroptosis-related genes in ALS: a pilot study. Front Genet. 2022;13:919188.
Da Q, Ren M, Huang L, Qu J, Yang Q, Xu J, et al. Identification and validation of a ferroptosis-related signature for predicting prognosis andimmune microenvironment in papillary renal cell carcinoma. Int J Gen Med. 2022;15:2963–77.
Chen Y, Zhu G, Liu Y, Wu Q, Zhang X, Bian Z, et al. O-GlcNAcylated c-Jun antagonizes ferroptosis via inhibiting GSH synthesis in liver cancer. Cell Signal. 2019;63:109384.
Zhang H, Zhu SL, Zhou HT, Li R, Xia XH, Xiong HH. Identification of MTHFD2 as a prognostic biomarker and ferroptosis regulator in triple-negative breast cancer. Front Oncol. 2023;13:1098357.
Gao M, Monian P, Quadri N, Ramasamy R, Jiang X. Glutaminolysis and transferrin regulate ferroptosis. Mol Cell. 2015;59:298–308.
Watanabe M, Chiba Y, Hirai MY. Metabolism and regulatory functions of O-acetylserine, S-adenosylmethionine, homocysteine, and serine in plant development and environmental responses. Front Plant Sci. 2021;12:643403.
Zhang X, Huang Z, Xie Z, Chen Y, Zheng Z, Wei X, et al. Homocysteine induces oxidative stress and ferroptosis of nucleus pulposus via enhancing methylation of GPX4. Free Radic Biol Med. 2020;160:552–65.
Kong S, Tao M, Shen X, Ju S. Translatable circRNAs and lncRNAs: driving mechanisms and functions of their translation products. Cancer Lett. 2020;483:59–65.
Fan Y, Wang L, Ding Y, Sheng Q, Zhang C, Li Y, et al. Long non-coding RNA RP4-694A7.2 promotes hepatocellular carcinoma cell proliferation and metastasis through the regulation of PSAT1. J Cancer. 2021;12:5633–43.
Li MK, Liu LX, Zhang WY, Zhan HL, Chen RP, Feng JL, et al. Long noncoding RNA MEG3 suppresses epithelialtomesenchymal transition by inhibiting the PSAT1dependent GSK3beta/Snail signaling pathway in esophageal squamous cell carcinoma. Oncol Rep. 2020;44:2130–42.
Zhao R, Cao X, Jin S, Li R, Zhong Q, Jiang M, et al. LncRNA BC200 promotes esophageal squamous cell cancer migration and invasion and can regulate ATF4 expression. Front Oncol. 2020;10:1392.
Guo K, Qi D, Huang B. LncRNA MEG8 promotes NSCLC progression by modulating the miR-15a-5p-miR-15b-5p/PSAT1 axis. Cancer Cell Int. 2021;21:84.
Yan S, Jiang H, Fang S, Yin F, Wang Z, Jia Y, et al. MicroRNA-340 inhibits esophageal cancer cell growth and invasion by targeting phosphoserine aminotransferase 1. Cell Physiol Biochem. 2015;37:375–86.
Sun C, Zhang X, Chen Y, Jia Q, Yang J, Shu Y. MicroRNA-365 suppresses cell growth and invasion in esophageal squamous cell carcinoma by modulating phosphoserine aminotransferase 1. Cancer Manag Res. 2018;10:4581–90.
Fang Y, Liang X, Xu J, Cai X. miR-424 targets AKT3 and PSAT1 and has a tumor-suppressive role in human colorectal cancer. Cancer Manag Res. 2018;10:6537–47.
Dai J, Wei R, Zhang P, Kong B. Overexpression of microRNA-195-5p reduces cisplatin resistance and angiogenesis in ovarian cancer by inhibiting the PSAT1-dependent GSK3beta/beta-catenin signaling pathway. J Transl Med. 2019;17:190.
Dong Y, Huo X, Sun R, Liu Z, Huang M, Yang S. LncRNA Gm15290 promotes cell proliferation and invasion in lung cancer through directly interacting with and suppressing the tumor suppressor miR-615-5p. Biosci Rep. 2018;38:BSR20181150.
Wu X, Deng L, Tang D, Ying G, Yao X, Liu F, et al. Mir-615-5p prevents proliferation and migration through negatively regulating serine hydromethyltransferase 2 (SHMT2) in hepatocellular carcinoma. Tumour Biol. 2016;37:6813–21.
Lin C, Zhang Y, Chen Y, Bai Y, Zhang Y. Long noncoding RNA LINC01234 promotes serine hydroxymethyltransferase 2 expression and proliferation by competitively binding miR-642a-5p in colon cancer. Cell Death Dis. 2019;10:137.
Zhao M, Hou Y, Du YE, Yang L, Qin Y, Peng M, et al. Drosha-independent mir-6778-5p strengthens gastric cancer stem cell stemness via regulation of cytosolic one-carbon folate metabolism. Cancer Lett. 2020;478:8–21.
Wu S, Zhang G, Li P, Chen S, Zhang F, Li J, et al. miR-198 targets SHMT1 to inhibit cell proliferation and enhance cell apoptosis in lung adenocarcinoma. Tumour Biol. 2016;37:5193–202.
Yang Q, Li J, Hu Y, Tang X, Yu L, Dong L, et al. MiR-218-5p suppresses the killing effect of natural killer cell to lung adenocarcinoma by targeting SHMT1. Yonsei Med J. 2019;60:500–8.
Qi C, Qin X, Zhou Z, Wang Y, Yang Q, Liao T. Circ_0072995 promotes cell carcinogenesis via up-regulating miR-149-5p-mediated SHMT2 in breast cancer. Cancer Manag Res. 2020;12:11169–81.
Yan Y, Zhang D, Lei T, Zhao C, Han J, Cui J, et al. MicroRNA-33a-5p suppresses colorectal cancer cell growth by inhibiting MTHFD2. Clin Exp Pharmacol Physiol. 2019;46:928–36.
Gu Y, Si J, Xiao X, Tian Y, Yang S. miR-92a inhibits proliferation and induces apoptosis by regulating methylenetetrahydrofolate dehydrogenase 2 (MTHFD2) expression in acute myeloid leukemia. Oncol Res. 2017;25:1069–79.
Li SM, Zhao YQ, Hao YL, Liang YY. Upregulation of mir-504-3p is associated with favorable prognosis of acute myeloid leukemia and may serve as a tumor suppressor by targeting MTHFD2. Eur Rev Med Pharmacol Sci. 2019;23:1203–13.
Selcuklu SD, Donoghue MT, Rehmet K, de Souza Gomes M, Fort A, Kovvuru P, et al. MicroRNA-9 inhibition of cell proliferation and identification of novel miR-9 targets by transcriptome profiling in breast cancer cells. J Biol Chem. 2012;287:29516–28.
Zhao XB, Ren GS. LncRNA taurine-upregulated gene 1 promotes cell proliferation by inhibiting MicroRNA-9 in MCF-7 cells. J Breast Cancer. 2016;19:349–57.
Xu T, Zhang K, Shi J, Huang B, Wang X, Qian K, et al. MicroRNA-940 inhibits glioma progression by blocking mitochondrial folate metabolism through targeting of MTHFD2. Am J Cancer Res. 2019;9:250–69.
Tong D, Zhang J, Wang X, Li Q, Liu L, Lu A, et al. MiR-22, regulated by MeCP2, suppresses gastric cancer cell proliferation by inducing a deficiency in endogenous S-adenosylmethionine. Oncogenesis. 2020;9:99.
Okada R, Koshizuka K, Yamada Y, Moriya S, Kikkawa N, Kinoshita T, et al. Regulation of oncogenic targets by miR-99a-3p (passenger strand of miR-99a-duplex) in head and neck squamous cell carcinoma. Cells. 2019;8:1535.
Weinstabl H, Treu M, Rinnenthal J, Zahn SK, Ettmayer P, Bader G, et al. Intracellular trapping of the selective phosphoglycerate dehydrogenase (PHGDH) inhibitor BI-4924 disrupts serine biosynthesis. J Med Chem. 2019;62:7976–97.
Mullarky E, Lucki NC, Zavareh RB, Anglin JL, Gomes AP, Nicolay BN, et al. Identification of a small molecule inhibitor of 3-phosphoglycerate dehydrogenase to target serine biosynthesis in cancers. Proc Natl Acad Sci U S A. 2016;113:1778–83.
Pacold ME, Brimacombe KR, Chan SH, Rohde JM, Lewis CA, Swier LJ, et al. A PHGDH inhibitor reveals coordination of serine synthesis and one-carbon unit fate. Nat Chem Biol. 2016;12:452–8.
Wang Q, Liberti MV, Liu P, Deng XB, Liu Y, Locasale JW, et al. Rational design of selective allosteric inhibitors of PHGDH and serine synthesis with anti-tumor activity. Cell Chem Biol. 2017;24:55–65.
Guo J, Gu XX, Zheng MZ, Zhang YH, Chen LX, Li H. Azacoccone E inhibits cancer cell growth by targeting 3-phosphoglycerate dehydrogenase. Bioorg Chem. 2019;87:16–22.
Zheng MZ, Guo J, Xu JM, Yang KY, Tang RT, Gu XX, et al. Ixocarpalactone a from dietary tomatillo inhibits pancreatic cancer growth by targeting PHGDH. Food Funct. 2019;10:3386–95.
Haufroid M, Wouters J. Targeting the serine pathway: a promising approach against tuberculosis? Pharmaceuticals (Basel). 2019;12:66.
Hawkinson JE, Acosta-Burruel M, Ta ND, Wood PL. Novel phosphoserine phosphatase inhibitors. Eur J Pharmacol. 1997;337:315–24.
Ducker GS, Ghergurovich JM, Mainolfi N, Suri V, Jeong SK, Hsin-Jung Li S, et al. Human SHMT inhibitors reveal defective glycine import as a targetable metabolic vulnerability of diffuse large B-cell lymphoma. Proc Natl Acad Sci U S A. 2017;114:11404–9.
Dekhne AS, Shah K, Ducker GS, Katinas JM, Wong-Roushar J, Nayeen MJ, et al. Novel pyrrolo[3,2-d]pyrimidine compounds target mitochondrial and cytosolic one-carbon metabolism with broad-spectrum antitumor efficacy. Mol Cancer Ther. 2019;18:1787–99.
Marani M, Paone A, Fiascarelli A, Macone A, Gargano M, Rinaldo S, et al. A pyrazolopyran derivative preferentially inhibits the activity of human cytosolic serine hydroxymethyltransferase and induces cell death in lung cancer cells. Oncotarget. 2016;7:4570–83.
Gustafsson R, Jemth AS, Gustafsson NM, Farnegardh K, Loseva O, Wiita E, et al. Crystal structure of the emerging cancer target MTHFD2 in complex with a substrate-based inhibitor. Cancer Res. 2017;77:937–48.
Fu C, Sikandar A, Donner J, Zaburannyi N, Herrmann J, Reck M, et al. The natural product carolacton inhibits folate-dependent C1 metabolism by targeting FolD/MTHFD. Nat Commun. 2017;8:1529.
Chattopadhyay S, Moran RG, Goldman ID. Pemetrexed: biochemical and cellular pharmacology, mechanisms, and clinical applications. Mol Cancer Ther. 2007;6:404–17.
Kremer JM. Toward a better understanding of methotrexate. Arthritis Rheum. 2004;50:1370–82.
Danenberg PV. Thymidylate synthetase - a target enzyme in cancer chemotherapy. Biochim Biophys Acta. 1977;473:73–92.
Konner JA, Bell-McGuinn KM, Sabbatini P, Hensley ML, Tew WP, Pandit-Taskar N, et al. Farletuzumab, a humanized monoclonal antibody against folate receptor alpha, in epithelial ovarian cancer: a phase I study. Clin Cancer Res. 2010;16:5288–95.
Armstrong DK, White AJ, Weil SC, Phillips M, Coleman RL. Farletuzumab (a monoclonal antibody against folate receptor alpha) in relapsed platinum-sensitive ovarian cancer. Gynecol Oncol. 2013;129:452–8.
Vergote I, Armstrong D, Scambia G, Teneriello M, Sehouli J, Schweizer C, et al. A randomized, double-blind, placebo-controlled, phase III study to assess efficacy and safety of weekly farletuzumab in combination with carboplatin and taxane in patients with ovarian cancer in first platinum-sensitive relapse. J Clin Oncol. 2016;34:2271–8.
Senkovich O, Bhatia V, Garg N, Chattopadhyay D. Lipophilic antifolate trimetrexate is a potent inhibitor of Trypanosoma cruzi: prospect for chemotherapy of Chagas’ disease. Antimicrob Agents Chemother. 2005;49:3234–8.
Jackman AL, Taylor GA, Gibson W, Kimbell R, Brown M, Calvert AH, et al. ICI D1694, a quinazoline antifolate thymidylate synthase inhibitor that is a potent inhibitor of L1210 tumor cell growth in vitro and in vivo: a new agent for clinical study. Cancer Res. 1991;51:5579–86.
Fry DW, Jackson RC. Biological and biochemical properties of new anticancer folate antagonists. Cancer Metastasis Rev. 1987;5:251–70.
Jackman AL, Calvert AH. Folate-based thymidylate synthase inhibitors as anticancer drugs. Ann Oncol. 1995;6:871–81.
Beutel G, Glen H, Schoffski P, Chick J, Gill S, Cassidy J, et al. Phase I study of OSI-7904L, a novel liposomal thymidylate synthase inhibitor in patients with refractory solid tumors. Clin Cancer Res. 2005;11:5487–95.
Banerji U, Garces AHI, Michalarea V, Ruddle R, Raynaud FI, Riisnaes R, et al. An investigator-initiated phase I study of ONX-0801 a first-in-class alpha folate receptor targeted, small molecule thymidylate synthase inhibitor in solid tumors. J Clin Oncol. 2017;35:2503.
Guo J, Yang Y, Buettner R, Rosen ST. Targeting the methionine-methionine adenosyl transferase 2A- S -adenosyl methionine axis for cancer therapy. Curr Opin Oncol. 2022;34:546–51.
Brooks HB, Meier TI, Geeganage S, Fales KR, Thrasher KJ, Konicek SA, et al. Characterization of a novel AICARFT inhibitor which potently elevates ZMP and has anti-tumor activity in murine models. Sci Rep. 2018;8:15458.
Zuhra K, Augsburger F, Majtan T, Szabo C. Cystathionine-beta-synthase: molecular regulation and pharmacological inhibition. Biomolecules. 2020;10:697.
Cuthbertson CR, Arabzada Z, Bankhead A 3rd, Kyani A, Neamati N. A review of small-molecule inhibitors of one-carbon enzymes: SHMT2 and MTHFD2 in the spotlight. ACS Pharmacol Transl Sci. 2021;4:624–46.
Hawkinson JE, Acosta-Burruel M, Wood PL. The metabotropic glutamate receptor antagonist L-2-amino-3-phosphonopropionic acid inhibits phosphoserine phosphatase. Eur J Pharmacol. 1996;307:219–25.
Nilsson R, Nicolaidou V, Koufaris C. Mitochondrial MTHFD isozymes display distinct expression, regulation, and association with cancer. Gene. 2019;716:144032.
Farber S, Diamond LK. Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4-aminopteroyl-glutamic acid. N Engl J Med. 1948;238:787–93.
King EM, Mazor R, Cuburu N, Pastan I. Low-dose methotrexate prevents primary and secondary humoral immune responses and induces immune tolerance to a recombinant immunotoxin. J Immunol. 2018;200:2038–45.
Al-Toubah T, Morse B, Pelle E, Strosberg J. Efficacy of FOLFOX in patients with aggressive pancreatic neuroendocrine tumors after prior capecitabine/temozolomide. Oncologist. 2021;26:115–9.
Guo J, Yu Z, Sun D, Zou Y, Liu Y, Huang L. Two nanoformulations induce reactive oxygen species and immunogenetic cell death for synergistic chemo-immunotherapy eradicating colorectal cancer and hepatocellular carcinoma. Mol Cancer. 2021;20:10.
Baraniskin A, Schroers R. Liquid biopsy and other non-invasive diagnostic measures in PCNSL. Cancers (Basel). 2021;13:2665.
Norrington M, Rathi N, Jenkinson MD, Mills SJ. Neuroinflammation preceding primary central nervous system lymphoma (PCNSL) - case reports and literature review. J Clin Neurosci. 2021;89:381–8.
Grommes C, DeAngelis LM. Primary CNS lymphoma. J Clin Oncol. 2017;35:2410–8.
Doolittle ND, Jahnke K, Belanger R, Ryan DA, Nance RW Jr, Lacy CA, et al. Potential of chemo-immunotherapy and radioimmunotherapy in relapsed primary central nervous system (CNS) lymphoma. Leuk Lymphoma. 2007;48:1712–20.
Chukwueke U, Grommes C, Nayak L. Primary central nervous system lymphomas. Hematol Oncol Clin North Am. 2022;36:147–59.
Rosenberg AR, Tabacchi M, Ngo KH, Wallendorf M, Rosman IS, Cornelius LA, et al. Skin cancer precursor immunotherapy for squamous cell carcinoma prevention. JCI Insight. 2019;4:e125476.
Zhao M, Tan B, Dai X, Shao Y, He Q, Yang B, et al. DHFR/TYMS are positive regulators of glioma cell growth and modulate chemo-sensitivity to temozolomide. Eur J Pharmacol. 2019;863:172665.
Matherly LH, Hou Z, Gangjee A. The promise and challenges of exploiting the proton-coupled folate transporter for selective therapeutic targeting of cancer. Cancer Chemother Pharmacol. 2018;81:1–15.
Scaranti M, Cojocaru E, Banerjee S, Banerji U. Exploiting the folate receptor alpha in oncology. Nat Rev Clin Oncol. 2020;17:349–59.
Shimizu T, Fujiwara Y, Yonemori K, Koyama T, Sato J, Tamura K, et al. First-in-human phase 1 study of MORAb-202, an antibody-drug conjugate comprising farletuzumab linked to eribulin mesylate, in patients with folate receptor-alpha-positive advanced solid tumors. Clin Cancer Res. 2021;27:3905–15.
Pedley AM, Benkovic SJ. A new view into the regulation of purine metabolism: the purinosome. Trends Biochem Sci. 2017;42:141–54.
Zhang Y, Desharnais J, Marsilje TH, Li C, Hedrick MP, Gooljarsingh LT, et al. Rational design, synthesis, evaluation, and crystal structure of a potent inhibitor of human GAR Tfase: 10-(trifluoroacetyl)-5,10-dideazaacyclic-5,6,7,8-tetrahydrofolic acid. Biochemistry. 2003;42:6043–56.
Froese DS, Fowler B, Baumgartner MR. Vitamin B(12), folate, and the methionine remethylation cycle-biochemistry, pathways, and regulation. J Inherit Metab Dis. 2019;42:673–85.
Menezo Y, Clement P, Dale B, Elder K. Modulating oxidative stress and epigenetic homeostasis in preimplantation IVF embryos. Zygote. 2022;30:149–58.
Murakami M, Ota K. [Current studies of sequential methotrexate and 5-fluorouracil treatment in cancer chemotherapy]. Gan To Kagaku Ryoho. 1988;15:409–17.
Arlt B, Mastrobuoni G, Wuenschel J, Astrahantseff K, Eggert A, Kempa S, et al. Inhibiting PHGDH with NCT-503 reroutes glucose-derived carbons into the TCA cycle, independently of its on-target effect. J Enzyme Inhib Med Chem. 2021;36:1282–9.
Nguyen MQ, Teh JLF, Purwin TJ, Chervoneva I, Davies MA, Nathanson KL, et al. Targeting PHGDH upregulation reduces glutathione levels and resensitizes resistant NRAS-mutant melanoma to MAPK kinase inhibition. J Invest Dermatol. 2020;140:2242–52.
DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20.
Acknowledgements
The figures in the manuscript were partly generated from BioRender (https://biorender.com/). We acknowledge all members of our lab for their help and assistance.
Funding
This work was supported by the Natural Science Foundation of Chongqing (CSTB2022NSCQ-MSX0631, cstc2022ycjh-bgzxm0113) and the Graduate Scientific Research Innovation Project of Chongqing (CYS22256).
Author information
Authors and Affiliations
Contributions
The work reported in the article has been performed by the authors unless clearly specified in the text. Wei Sun and Erhu Zhao conceived the project, provided direction and guidance on the whole project. Wei Sun and Ruochen Liu drafted the manuscript. Xinyue Gao, Zini Lin and Hongao Tang helped to draw the figures. Erhu Zhao and Hongjuan Cui reviewed and modified the manuscript. The final manuscript has been approved by all authors.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have read and approved the final manuscript.
Competing interests
The authors declare that they have no conflict of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sun, W., Liu, R., Gao, X. et al. Targeting serine-glycine-one-carbon metabolism as a vulnerability in cancers. Biomark Res 11, 48 (2023). https://doi.org/10.1186/s40364-023-00487-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40364-023-00487-4