Abstract
Perinatal complications in both term- and preterm-born infants are a leading cause of neonatal morbidities and mortality. Infants face different challenges in the neonatal intensive care unit with long-term morbidities such as perinatal brain injury and bronchopulmonary dysplasia being particularly devastating. While advances in perinatal medicine have improved our understanding of the pathogenesis, effective therapies to prevent and/or reduce the severity of these disorders are still lacking. The potential of mesenchymal stem/stromal cell (MSC) therapy has emerged during the last two decades, and an increasing effort is conducted to address brain- and lung-related morbidities in neonates at risk. Various studies support the notion that MSCs have protective effects. MSCs are an easy source and may be readily available after birth in a clinical setting. MSCs’ mechanisms of action are diverse, including migration and homing, release of growth factors and immunomodulation, and the potential to replace injured cells. Here, we review the pathophysiology of perinatally acquired brain and lung injuries and focus on MSCs as potential candidates for therapeutic strategies summarizing preclinical and clinical evidence.
Similar content being viewed by others
Introduction
Despite advances in obstetrics and neonatology, both preterm- and term-born infants continue to face serious risks during pregnancy, parturition, and adaptation after birth. Perinatal medicine is therefore a major public health issue around the world. The clinical presentation after perinatal complications or preterm birth in an individual child is complex. This complexity results from multiple potential causal pathways, signs, and symptoms of injury. Typical pathologies in these newborns include perinatal brain injury and bronchopulmonary dysplasia (BPD), among others. New avenues to treat these morbidities have emerged among which stem cells being particularly promising. The body of knowledge of stem cell biology and their function in tissue regeneration and protection has broadened exponentially in the last two decades. This review will discuss mesenchymal stem/stromal cells (MSCs) as a therapeutic approach for both brain and lung diseases in infants at risk. Furthermore, we will highlight the preclinical and clinical data that have emerged on the role of MSCs in perinatal medicine.
Perinatally acquired organ injury
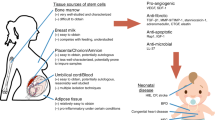
Perinatally acquired organ injury affects both term and preterm infants. Depending on the timing of injury and/or delivery, infants need to cope with different challenges. In the developing brain of a preterm infant, the spectrum of injury suggests that the underlying pathophysiology is not due to a single lesion but consists of white and gray matter disturbances [1]. Thus, a comprehensive multidimensional assessment of potential contributing factors such as maternal medical history, obstetric antecedents, intrapartum factors (including fetal heart rate monitoring results and issues related to the delivery itself), and placental pathology is recommended [2]. In the term-born infants, perinatal insults such as birth asphyxia or perinatal stroke affect 1 to 3 newborns out of 1000 [3, 4]. In contrast, in preterm-born infants morbidity and mortality strongly relate to the gestational age. While preterm birth before 37 weeks of gestation occurs in 5–8 % of all pregnancies, very low gestational age (VLGA) before 32 weeks of gestation occurs in about 1 % of singletons and 9 % of twin pregnancies [5]. Mortality of VLGA infants ranges between 7.3 and 21.4 % at 30 days and 9.0 and 22.7 % at 1 year [6]. Additionally, a large number of survivors suffer significant long-term disabilities including cerebral palsy (CP), epilepsy, increased hyperactivity, and developmental disorders [7]. For example, the risk to develop CP is 30 times higher in infants born before 33 weeks of gestation compared to term-born infants [8]. Moreover, injury in these infants is frequently exacerbated by fetal inflammation and preferentially affects cerebral white matter resulting in periventricular leukomalacia and germinal matrix hemorrhage [1]. Currently, the only intervention known to reduce the burden of perinatal brain injury in the term population is hypothermia. Several large clinical trials confirmed that hypothermia in infants with neonatal hypoxic-ischemic encephalopathy is associated with a significant reduction in death and disability [9]. However, 40–50 % of infants treated with hypothermia still die or develop significant neurological disability [10]. In the preterm population, therapeutic options are lacking as hypothermia is contra-productive. Antenatal magnesium sulfate prior to birth at less than 30 weeks of gestation reduces CP and combined CP and mortality rate at 2 years of age. However, randomized control trials do not demonstrate long-term neurological benefits [11, 12]. Approximately 25 % of VLGA infants will develop BPD with long-lasting consequences such as chronic respiratory impairment and neurodevelopmental delay [13]. Changes in clinical management reduced the incidence of BPD significantly with a shift from VLGA to extreme low gestational age newborns developing BPD. Not surprisingly, the current pathogenesis of BPD is based on immaturity with disordered alveolar and capillary development and represents a developmental disorder [14]. The plasticity of the developing lung after preterm birth is poorly understood. Long-term follow-up studies suggest an incomplete regeneration of the lung growth in survivors with BPD. Current treatment options for BPD include vitamin A and caffeine in addition to supportive therapies, but their results often remain unsatisfactory [15].
Together, therapeutic approaches to counteract the consequences of perinatally acquired injury are sparse, and new measures are desperately needed especially for preterm infants [1, 15–17].
Pathophysiology of brain and lung injury
Experimental studies identified different phases of perinatally induced neuronal death. Primary neuronal death is related to depletion of tissue energy reserves with primary energy failure. The secondary and tertiary phases are related to excitotoxicity, mitochondrial dysfunction, and free radical accumulation leading to cell necrosis or apoptosis with impaired myelination and/or axonal function [18]. The secondary and tertiary phases may cause persistent inflammation and gliosis, sensitization to further injury, and impaired oligodendrocyte maturation and myelination [19]. Interestingly, inflammation is increasingly recognized as being a critical contributor to both normal development and injury outcome especially in the immature brain [20]. Maternal infection/inflammation is not only a major risk for preterm birth but is linked to systemic fetal inflammatory response which, in turn, may elicit injury in the fetus. Perinatal inflammation modulates vulnerability to and development of brain injury [21] and influences critical phases of myelination and cortical plasticity [20]. Several studies suggest that inflammation may play a critical role in autism and schizophrenia [22]. Together, brain development, myelination, vascularization, and apoptosis are strongly influenced by inflammatory responses in both physiologic and pathophysiologic conditions [20, 23]. Pivotal regulators of inflammatory responses in the brain are glial cells which orchestrate the release of pro- and anti-inflammatory cytokines [24, 25]. Pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α and interferon gamma (IFN-γ) are cytotoxic to oligodendrocytes [26]. However, glial cells may produce anti-inflammatory cytokines (e.g., IL-10), which suppress expansion of IL-1β and TNF-α and contribute to resolving inflammation and repair processes [25]. Further, astrogliotic scar formation installs a barrier around tissue lesions to restrict the crossing of inflammatory cells into surrounding healthy areas [24].
The regulation of the inflammatory responses in the newborn appears to be a link that may explain some of the common features of organ injury in preterm infants. Many studies identified characteristic inflammatory changes and altered growth factor signaling in BPD such as initial influx of neutrophils into the lung followed by increased numbers of macrophages [27]. The release of cytokines and disturbance of growth factor signaling such as transforming growth factor (TGF)-β results in increased apoptotic process [28]. Furthermore, several studies showed that dysmorphic capillaries and subsequent development of pulmonary hypertension are related to an altered pattern of angiogenic growth factors such as vascular endothelial growth factor (VEGF) and its receptors [29]. Finally, lung injury leads to remodeling or early alveolar epithelial dysfunction which in turn promotes lung inflammation [30].
Taken together, perinatal inflammation is a strong modulator of both physiologic and pathophysiologic development of the neonatal organs. The fetal inflammatory response to certain cues such as lipopolysaccharides (LPS) [31] can be detected not only in the brain and the lung but also in remote tissues not directly exposed to LPS such as the spleen, liver, and mediastinal lymph nodes [32–34]. Protective strategies to counteract the cascades leading to injury should therefore not focus on one organ or system but rather treat perinatally acquired injury globally, in which the immune system plays a key role. MSCs possess a regenerative potential. They were shown to modulate innate and adaptive immune responses, to have antiapoptotic effects, to decrease inflammation, and to enhance tissue repair, mostly through the release of paracrine factors [35, 36].
Mesenchymal stem/stromal cells
Stem cells are broadly defined as cells with self-renewing and differentiation capacity. Although stem cells derived from embryonic tissue were identified first, the clinical use is limited due to ethical concerns and tumorigenic potential [37, 38]. Clinical and animal stem cell-based studies to prevent or repair perinatally acquired injury have emerged during the recent years with MSCs being particularly promising. These cells are considered somatic stem cells as they originate from stem cell niches such as bone marrow (BM), skin, adipose umbilical cord, and placental tissues [39]. MSCs can be isolated from placental membranes and tissues [40, 41], amniotic fluid [42–44], umbilical cord blood [45, 46], and the umbilical cord connective tissues (Wharton’s jelly; WJ) [47, 48]. Although all of these cells are MSCs, specific considerations with respect to clinical use, time of application, application route, availability, and ethical aspects need to be made.
MSC-based therapies are an attractive strategy since the pathophysiology of perinatally acquired injury is heterogeneous and MSCs have the capacity to adapt to the microenvironment of injured organs. The strategy may be either or both replacement/restoration of lost tissue and/or protection/salvage of injured cells. In term infants at risk for hypoxic-ischemic injury or neonatal ischemic stroke, MSCs could exert a neuroprotective effect starting at the acute phase of injury. The timing and presentation of the injury are usually well defined. MSCs could provide trophic support and/or amelioration of the inflammatory responses, leading to repair or reduced cell death. However, the different cell types, transplantation routes, and the timing need to be accounted for. Given the gold standard therapy of hypothermia for this kind of injury, MSCs have to proof additive/synergistic effects in order to be considered [49]. In contrast, in the preterm population, the timing of the injury is often unclear and the pathophysiology is more complex. The clinical diagnosis of infants at risk is challenging as symptoms such as CP or BPD are diagnosed in early childhood years. Thus, the injury may be considered more chronic as extensive atrophy and gliosis of the white matter tract or dysfunction of lung architecture are present. MSCs could modulate not only the inflammatory response after delivery but also the degree and magnitude of the injured white matter and epithelial cells as well. However, many questions such as altered pattern of growth factors and intercellular matrix proteins that could affect proliferation or differentiation of the desired cell types need to be addressed first.
MSCs: homing and migration
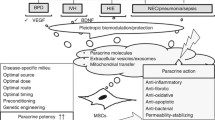
The approach of MSCs as a therapy for perinatal injury is based on several crucial properties of MSCs, including delivery of the cells “homing” to the site of injury. Migration and homing to the tissue of injury is influenced by multiple factors including age, passage, and number of cells; culture conditions; and delivery method [50]. The apparent migration and homing abilities of MSCs without tumorigenic potential were described by several groups and in different disease models [51–54]. In BPD animal models, peripherally injected cells were detected in the hyperoxia-induced injured lung [55, 56]. Experimental studies identified chemokines as major molecules responsible for cell homing with chemokine receptors CXCR3, CXCR4, and CXCR6 being particularly important [57–59]. Further secretion of factors such as stromal cell-derived factor-1α (SDF-1α), which is a CXCR4 ligand, promotes migration of MSC to the injury site [60]. Interestingly, the phenotype of MSCs is an important criterion as well. CD9 (high)-positive MSCs display improved engraftment compared to the CD9 (low)-positive population in a murine ischemic hind limb model [61]. This observation highlights the rather heterogeneous MSC population and the importance of proper MSC characterization and definition for future studies. Currently, MSC characterization is based on a set of minimal criteria [62], and they display a cell surface repertoire and gene expression pattern which differ among MSCs from various tissues of origin and culture conditions used [63–66]. For example, MSCs derived from amniotic fluid express many cell surface markers characteristic for BM-derived MSCs including CD73, CD90, CD105, and major histocompatibility complex (MHC) class I [44]. The lack of MHC class II, CD40, CD80, and CD86 molecules suggests a low immunogenic phenotype of MSCs when compared to other stem cell sources [67]. In contrast, WJ-MSCs express cell surface markers CD29, CD44, CD73, CD90, CD105, CD146, and CD166 [68, 69] and are considered more primitive cell population relative to BM-derived MSCs [70]. As a result, WJ-MSCs differentiate more efficiently into neural progenitors compared to BM-derived MSC [71]. In addition, the underlying clinical condition may also affect the phenotype of MSCs. WJ-MSCs derived from umbilical cords collected after preeclamptic pregnancies seem to be more committed to neuroglial differentiation compared to cords from uncomplicated pregnancies [66].
MSCs: secretome and immunomodulation
While MSCs have a proven restorative capacity in response to injury cues, the question of potential protective mechanisms remains unclear. Most of the available data comes from adult neurodegenerative and lung diseases or in vitro studies. Studies identified the induction of cytokines, interleukins, and trophic factors predominately involved in neurogenesis, angiogenesis, hematopoiesis, and cardiovascular regeneration being crucial for the mostly paracrine effects [58, 72]. For example, WJ-MSCs’ secretome triggers neuronal survival and differentiation in vitro and in vivo [73, 74]. Secreted factors such as VEGF-A, angiopoietin-1, fibroblast growth factor (FGF)-I, hepatocyte growth factor (HGF), FGF-II, brain-derived neurotrophic factor (BDNF), glial cell line-derived neurotrophic factor (GDNF), and platelet-derived growth factor (PDGF)-AB were identified [75–79]. Importantly, MSCs’ secretome alters both adaptive and innate immune responses [55, 80]. MSCs inhibit autoreactive T cell responses in animal models of multiple sclerosis and hypoxic-ischemic brain injury [81–83]. MSCs shift the alveolar macrophages from a M1 (pro-inflammatory) to a M2 (protective) phenotype ameliorating pulmonary injury in acute LPS-induced acute lung injury model [84]. Thus, the shifting from the M1 to the M2 states and promoting regulatory T cells are a function unique to MSCs [85]. Besides T cell modulation, MSCs inhibit B cell proliferation, neutrophil and monocyte function, and NK toxicity [86–89]. Although these modulatory effects are partially understood, direct cell-to-cell contact and soluble factors are relevant [90]. Additionally, MSC effects expand beyond constitutive immune modulatory properties with the release of cytokines and growth factors such as VEGF, transforming growth factor beta-1 (TGF-β1), TNF-α, interleukin-1 (IL-1), interleukin-6 (IL-6), and IFN-γ [91–94].
MSCs: regeneration/replacement of injured cells
MSCs’ multipotency and self-renewal properties make them valid candidates for providing both lung and brain cell regeneration/replacement. Although, this strategy for repair carries risks such as tumorigenic potential [37, 38], MSCs were successfully differentiated into various types of cells including cardiomyocytes, myocytes, and epidermal and endothelial cells [54, 95–97]. Importantly, MSCs express neuroglial commitment and can be differentiated into lung cells as well [98–102]. Not surprisingly, MSCs are currently tested in various animal models and clinical trials for lung and brain regeneration [103–105]. Although this line of investigation is particularly intriguing, MSCs’ potential to replace injured cells is not proven and is a matter of constant debate [103, 104, 106]. For example, intravenously injected MSCs improve myocardial infarction without permanent replacement of injured cells [107]. In the lung, MSCs embolize causing endothelial damage and are cleared in a matter of hours [107]. Taken together, the MSCs’ low rate of in vivo engraftment and differentiation suggests that transplanted cells affect tissue injury and repair through paracrine factors. Whether the factors released by the MSCs or the cells themselves are more promising for the therapy of lung and brain injury in the newborn still remains an open question.
MSCs: extracellular vehicles
The translation from bench to bedside requires the most efficacious and safest approach. Thus, the question of cell-based versus cell-free therapy needs to be addressed. Given that the MSCs’ therapeutic potential has been shown to be largely triggered via paracrine effects and not differentiation, recent studies focus on extracellular vehicles (EV) [108]. These are all types of vehicles present in the extracellular space, including shedding vesicles, apoptotic bodies, and exosomes. Exosomes (40–100 nm in diameter) are secreted by cells in a regulated fashion, possess the ability to transfer proteins and functional genetic materials such as mRNA and microRNAs, and are involved in cell-to-cell signaling and regulation [80]. Not surprisingly, MSC-derived EV are contributing to tissue repair in brain injury including stroke and Alzheimer’s disease [109–111]. The cell-free approach is very promising; however, it is still in its infancy. In fact, stem cells do not just secrete growth factors and/or cytokines but encourage the growth and even supplement (host) cells [112–114]. Importantly, the stem cells’ potential of immunomodulation and protection after injury seems to depend on the bidirectional communication between the injured host cells and the graft via the exchange of specific information [115].
MSCs: clinical trials
As a result of the remarkable regenerative potential of MSCs, MSCs are ideal candidates for clinical cell therapy. MSCs are easily available, have a good safety profile and homing capacity, and importantly are relatively immunoprivileged, allowing allogeneic transplantation. Not surprisingly, MSCs have been tested in clinical trials in several neurodegenerative diseases such as stroke [116–118], amyotrophic lateral sclerosis [119], multiple sclerosis [120, 121], and spinal cord injury [122]. Several clinical trials indicated no serious side effects or dose-limiting toxicity in acute respiratory distress syndrome [123] and chronic obstructive pulmonary disease [124, 125]. Also, safety and feasibility trials for CP [126–128] and BPD [129, 130] were successful. A recent double-blind randomized control study used allogeneic umbilical cord blood, in combination with erythropoietin, in children diagnosed with CP showing motor and cognitive benefits [131]. In contrast to previous studies, a large safety study used autologous umbilical cord blood directly after birth for infants at risk for hypoxic-ischemic encephalopathy [132]. This approach differs significantly from previous studies as it aims mainly to prevent and not to replace affected cells. Cells were transplanted directly after birth and in combination with hypothermia. Authors concluded that the collection, preparation, and infusion of fresh autologous umbilical cord blood cells for use in infants with hypoxic-ischemic encephalopathy are feasible. The feasibility and safety of intra-tracheal infusion of umbilical cord-derived MSCs in BPD was reported as well [129]. MSCs reduced BPD severity and retinopathy of prematurity and improved inflammatory cytokine profile in tracheal aspirates in a phase 1 dose escalation study. Several other clinical trials for neonatal brain and lung injury are currently listed as in progress or completed, and more results should become available in the near future (ClinicalTrials.gov Identifiers: NCT01297205, NCT01828957, NCT02023788, NCT01832454, NCT01962233, NCT01988584, NCT01207869).
Conclusions
Given the array of potential regenerative mechanisms of MSCs to protect neonatal brain and lungs, therapeutic applications can be envisioned in the near future. The mechanisms span anti-apoptotic/pro-mitotic capacities leading to neovascularization by the stimulation of angiogenesis and anti-inflammatory responses. Further, stimulation of neuro- and gliogenesis, synaptogenesis, neurite outgrowth, and also immunomodulation are crucial. Available data clearly demonstrate that MSC and secreted factors are beneficial to treat a variety of neurodegenerative and lung disorders. Although the data are very promising, critical questions need to be answered:
-
First, what are the appropriate age, passage, and dosage of the transplanted MSCs? Freshly isolated MSCs may be beneficial compared to cultured cells, but is this practicable in a clinical setting especially in the context of preterm infants [133]?
-
What is the best source and application route of MSCs? Banking of UCB and placental tissue offer an easily accessible and ethically responsible source of MSCs, and minimally invasive routes are currently tested [47, 66, 134–136].
-
Finally, how does the transplantation of MSCs impact the standard care in the neonatal intensive care unit? The lack of effective interventions for many morbidities related to prematurity unlocks the potential of cell-based personalized treatments. Safe and effective clinical interventions are future perspectives bearing hope to improve the lifelong outcomes of the infants in our care. This has to be proven in long-term follow-up studies.
References
Volpe JJ (2009) Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol 8(1):110–124. doi:10.1016/S1474-4422(08)70294-1
Executive summary: Neonatal encephalopathy and neurologic outcome, second edition. Report of the American College of Obstetricians and Gynecologists' Task Force on Neonatal Encephalopathy (2014). Obstet Gynecol 123 (4):896-901. doi:10.1097/01.AOG.0000445580.65983.d2
Graham EM, Ruis KA, Hartman AL, Northington FJ, Fox HE (2008) A systematic review of the role of intrapartum hypoxia-ischemia in the causation of neonatal encephalopathy. Am J Obstet Gynecol 199(6):587–595. doi:10.1016/j.ajog.2008.06.094
Cowan FM, Mercuri E, Rutherford MA (2005) Perinatal stroke in term infants with neonatal encephalopathy. Neurology 64(3):579, Author reply 579
Schaaf JM, Mol BW, Abu-Hanna A, Ravelli AC (2011) Trends in preterm birth: singleton and multiple pregnancies in the Netherlands, 2000-2007. BJOG 118(10):1196–1204. doi:10.1111/j.1471-0528.2011.03010.x
Numerato D, Fattore G, Tediosi F, Zanini R, Peltola M, Banks H, Mihalicza P, Lehtonen L, Svereus S, Heijink R, Klitkou ST, Fletcher E, Heijden A, Lundberg F, Over E, Hakkinen U, Seppala TT (2015) Mortality and length of stay of very low birth weight and very preterm infants: a EuroHOPE study. PLoS ONE 10(6):e0131685, 10.1371/journal.pone.0131685
Robertson CM, Watt MJ, Yasui Y (2007) Changes in the prevalence of cerebral palsy for children born very prematurely within a population-based program over 30 years. JAMA 297(24):2733–2740. doi:10.1001/jama.297.24.2733
MacLennan AH, Thompson SC, Gecz J (2015) Cerebral palsy: causes, pathways, and the role of genetic variants. Am J Obstet Gynecol. doi:10.1016/j.ajog.2015.05.034.
Higgins RD, Raju T, Edwards AD, Azzopardi DV, Bose CL, Clark RH, Ferriero DM, Guillet R, Gunn AJ, Hagberg H, Hirtz D, Inder TE, Jacobs SE, Jenkins D, Juul S, Laptook AR, Lucey JF, Maze M, Palmer C, Papile L, Pfister RH, Robertson NJ, Rutherford M, Shankaran S, Silverstein FS, Soll RF, Thoresen M, Walsh WF (2011) Hypothermia and other treatment options for neonatal encephalopathy: an executive summary of the Eunice Kennedy Shriver NICHD workshop. J Pediatr 159(5):851–858. doi:10.1016/j.jpeds.2011.08.004, e851
Edwards AD, Brocklehurst P, Gunn AJ, Halliday H, Juszczak E, Levene M, Strohm B, Thoresen M, Whitelaw A, Azzopardi D (2010) Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ 340:c363. doi:10.1136/bmj.c363
Cahill AG, Stout MJ, Caughey AB (2010) Intrapartum magnesium for prevention of cerebral palsy: continuing controversy? Curr Opin Obstet Gynecol 22(2):122–127. doi:10.1097/GCO.0b013e3283372312
Doyle LW, Anderson PJ, Haslam R, Lee KJ, Crowther C, Australasian Collaborative Trial of Magnesium Sulphate Study G (2014) School-age outcomes of very preterm infants after antenatal treatment with magnesium sulfate vs placebo. JAMA 312(11):1105–1113. doi:10.1001/jama.2014.11189
Jensen EA, Schmidt B (2014) Epidemiology of bronchopulmonary dysplasia. Birth Defects Res A Clin Mol Teratol 100(3):145–157. doi:10.1002/bdra.23235
Van Marter LJ (2009) Epidemiology of bronchopulmonary dysplasia. Semin Fetal Neonatal Med 14(6):358–366. doi:10.1016/j.siny.2009.08.007
Donohue PK, Gilmore MM, Cristofalo E, Wilson RF, Weiner JZ, Lau BD, Robinson KA, Allen MC (2011) Inhaled nitric oxide in preterm infants: a systematic review. Pediatrics 127(2):e414–e422. doi:10.1542/peds.2010-3428
Hassell KJ, Ezzati M, Alonso-Alconada D, Hausenloy DJ, Robertson NJ (2015) New horizons for newborn brain protection: enhancing endogenous neuroprotection. Arch Dis Child Fetal Neonatal Ed. doi:10.1136/archdischild-2014-306284.
Buonocore G, Turrisi G, Kramer BW, Balduini W, Perrone S (2012) New pharmacological approaches in infants with hypoxic-ischemic encephalopathy., Curr Pharm Des
Pleasure D, Soulika A, Singh SK, Gallo V, Bannerman P (2006) Inflammation in white matter: clinical and pathophysiological aspects. Ment Retard Dev Disabil Res Rev 12(2):141–146. doi:10.1002/mrdd.20100
Favrais G, van de Looij Y, Fleiss B, Ramanantsoa N, Bonnin P, Stoltenburg-Didinger G, Lacaud A, Saliba E, Dammann O, Gallego J, Sizonenko S, Hagberg H, Lelievre V, Gressens P (2011) Systemic inflammation disrupts the developmental program of white matter. Ann Neurol 70(4):550–565. doi:10.1002/ana.22489
Hagberg H, Mallard C, Ferriero DM, Vannucci SJ, Levison SW, Vexler ZS, Gressens P (2015) The role of inflammation in perinatal brain injury. Nat Rev Neurol 11(4):192–208. doi:10.1038/nrneurol.2015.13
Eklind S, Mallard C, Leverin AL, Gilland E, Blomgren K, Mattsby-Baltzer I, Hagberg H (2001) Bacterial endotoxin sensitizes the immature brain to hypoxic-ischaemic injury. Eur J Neurosci 13(6):1101–1106
Meyer U, Feldon J, Dammann O (2011) Schizophrenia and autism: both shared and disorder-specific pathogenesis via perinatal inflammation? Pediatr Res 69(5 Pt 2):26R–33R. doi:10.1203/PDR.0b013e318212c196
Shrivastava K, Chertoff M, Llovera G, Recasens M, Acarin L (2012) Short and long-term analysis and comparison of neurodegeneration and inflammatory cell response in the ipsilateral and contralateral hemisphere of the neonatal mouse brain after hypoxia/ischemia. Neurol Res Int 2012:781512. doi:10.1155/2012/781512
Sofroniew MV (2015) Astrocyte barriers to neurotoxic inflammation. Nat Rev Neurosci 16(5):249–263. doi:10.1038/nrn3898
Brown GC, Neher JJ (2010) Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol Neurobiol 41(2-3):242–247. doi:10.1007/s12035-010-8105-9
Buntinx M, Moreels M, Vandenabeele F, Lambrichts I, Raus J, Steels P, Stinissen P, Ameloot M (2004) Cytokine-induced cell death in human oligodendroglial cell lines: I. Synergistic effects of IFN-gamma and TNF-alpha on apoptosis. J Neurosci Res 76(6):834–845. doi:10.1002/jnr.20118
Speer CP (2006) Pulmonary inflammation and bronchopulmonary dysplasia. J Perinatol 26(Suppl 1):S57–S62. doi:10.1038/sj.jp.7211476, Discussion S63-54
Kunzmann S, Speer CP, Jobe AH, Kramer BW (2007) Antenatal inflammation induced TGF-beta1 but suppressed CTGF in preterm lungs. Am J Physiol Lung Cell Mol Physiol 292(1):L223–L231. doi:10.1152/ajplung.00159.2006
Thebaud B (2007) Angiogenesis in lung development, injury and repair: implications for chronic lung disease of prematurity. Neonatology 91(4):291–297. doi:10.1159/000101344
Atochina-Vasserman EN, Bates SR, Zhang P, Abramova H, Zhang Z, Gonzales L, Tao JQ, Gochuico BR, Gahl W, Guo CJ, Gow AJ, Beers MF, Guttentag S (2011) Early alveolar epithelial dysfunction promotes lung inflammation in a mouse model of Hermansky-Pudlak syndrome. Am J Respir Crit Care Med 184(4):449–458. doi:10.1164/rccm.201011-1882OC
Martinez-Lopez DG, Funderburg NT, Cerissi A, Rifaie R, Aviles-Medina L, Llorens-Bonilla BJ, Sleasman J, Luciano AA (2014) Lipopolysaccharide and soluble CD14 in cord blood plasma are associated with prematurity and chorioamnionitis. Pediatr Res 75(1-1):67–74. doi:10.1038/pr.2013.182
Maneenil G, Kemp MW, Kannan PS, Kramer BW, Saito M, Newnham JP, Jobe AH, Kallapur SG (2015) Oral, nasal and pharyngeal exposure to lipopolysaccharide causes a fetal inflammatory response in sheep. PLoS ONE 10(3):e0119281. doi:10.1371/journal.pone.0119281
Strackx E, Sparnaaij MA, Vlassaks E, Jellema R, Kuypers E, Vles JS, Kramer BW, Gavilanes AW (2015) Lipopolysaccharide-induced chorioamnionitis causes acute inflammatory changes in the ovine central nervous system. CNS Neurol Disord Drug Targets 14(1):77–84
Kuypers E, Willems MG, Jellema RK, Kemp MW, Newnham JP, Delhaas T, Kallapur SG, Jobe AH, Wolfs TG, Kramer BW (2015) Responses of the spleen to intraamniotic lipopolysaccharide exposure in fetal sheep. Pediatr Res 77(1-1):29–35. doi:10.1038/pr.2014.152
Lee JW, Fang X, Krasnodembskaya A, Howard JP, Matthay MA (2011) Concise review: mesenchymal stem cells for acute lung injury: role of paracrine soluble factors. Stem Cells 29(6):913–919. doi:10.1002/stem.643
Phillips AW, Johnston MV, Fatemi A (2013) The potential for cell-based therapy in perinatal brain injuries. Transl Stroke Res 4(2):137–148. doi:10.1007/s12975-013-0254-5
Ramalho-Santos M, Willenbring H (2007) On the origin of the term "stem cell". Cell Stem Cell 1(1):35–38. doi:10.1016/j.stem.2007.05.013
Caplan AI (1991) Mesenchymal stem cells. J Orthop Res 9(5):641–650. doi:10.1002/jor.1100090504
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH (2001) Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7(2):211–228. doi:10.1089/107632701300062859
In 't Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE, Kanhai HH (2004) Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells 22(7):1338–1345. doi:10.1634/stemcells.2004-0058
Igura K, Zhang X, Takahashi K, Mitsuru A, Yamaguchi S, Takashi TA (2004) Isolation and characterization of mesenchymal progenitor cells from chorionic villi of human placenta. Cytotherapy 6(6):543–553
Stefanidis K, Loutradis D, Anastasiadou V, Bletsa R, Kiapekou E, Drakakis P, Beretsos P, Elenis E, Mesogitis S, Antsaklis A (2008) Oxytocin receptor- and Oct-4-expressing cells in human amniotic fluid. Gynecol Endocrinol 24(5):280–284. doi:10.1080/09513590801977167
Prusa AR, Marton E, Rosner M, Bernaschek G, Hengstschlager M (2003) Oct-4-expressing cells in human amniotic fluid: a new source for stem cell research? Hum Reprod 18(7):1489–1493
In 't Anker PS, Scherjon SA, Kleijburg-van der Keur C, Noort WA, Claas FH, Willemze R, Fibbe WE, Kanhai HH (2003) Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood 102(4):1548–1549. doi:10.1182/blood-2003-04-1291
Wang JF, Wang LJ, Wu YF, Xiang Y, Xie CG, Jia BB, Harrington J, McNiece IK (2004) Mesenchymal stem/progenitor cells in human umbilical cord blood as support for ex vivo expansion of CD34(+) hematopoietic stem cells and for chondrogenic differentiation. Haematologica 89(7):837–844
Erices A, Conget P, Minguell JJ (2000) Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol 109(1):235–242
Ma L, Feng XY, Cui BL, Law F, Jiang XW, Yang LY, Xie QD, Huang TH (2005) Human umbilical cord Wharton's Jelly-derived mesenchymal stem cells differentiation into nerve-like cells. Chin Med J (Engl) 118(23):1987–1993
Wang HS, Hung SC, Peng ST, Huang CC, Wei HM, Guo YJ, Fu YS, Lai MC, Chen CC (2004) Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem Cells 22(7):1330–1337. doi:10.1634/stemcells.2004-0013
Park WS, Sung SI, Ahn SY, Yoo HS, Sung DK, Im GH, Choi SJ, Chang YS (2015) Hypothermia augments neuroprotective activity of mesenchymal stem cells for neonatal hypoxic-ischemic encephalopathy. PLoS ONE 10(3):e0120893. doi:10.1371/journal.pone.0120893
Sohni A, Verfaillie CM (2013) Mesenchymal stem cells migration homing and tracking. Stem Cells Int 2013:130763. doi:10.1155/2013/130763
Prasad VK, Kurtzberg J (2009) Umbilical cord blood transplantation for non-malignant diseases. Bone Marrow Transplant 44(10):643–651. doi:10.1038/bmt.2009.290
Yang WZ, Zhang Y, Wu F, Min WP, Minev B, Zhang M, Luo XL, Ramos F, Ichim TE, Riordan NH, Hu X (2010) Safety evaluation of allogeneic umbilical cord blood mononuclear cell therapy for degenerative conditions. J Transl Med 8:75. doi:10.1186/1479-5876-8-75
Schoeberlein A, Mueller M, Reinhart U, Sager R, Messerli M, Surbek DV (2011) Homing of placenta-derived mesenchymal stem cells after perinatal intracerebral transplantation in a rat model. Am J Obstet Gynecol 205(3):277. doi:10.1016/j.ajog.2011.06.044, e271-276
De Coppi P, Bartsch G Jr, Siddiqui MM, Xu T, Santos CC, Perin L, Mostoslavsky G, Serre AC, Snyder EY, Yoo JJ, Furth ME, Soker S, Atala A (2007) Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol 25(1):100–106. doi:10.1038/nbt1274
Aslam M, Baveja R, Liang OD, Fernandez-Gonzalez A, Lee C, Mitsialis SA, Kourembanas S (2009) Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am J Respir Crit Care Med 180(11):1122–1130. doi:10.1164/rccm.200902-0242OC
Zhang X, Wang H, Shi Y, Peng W, Zhang S, Zhang W, Xu J, Mei Y, Feng Z (2012) Role of bone marrow-derived mesenchymal stem cells in the prevention of hyperoxia-induced lung injury in newborn mice. Cell Biol Int 36(6):589–594. doi:10.1042/CBI20110447
Jaerve A, Muller HW (2012) Chemokines in CNS injury and repair. Cell Tissue Res 349(1):229–248. doi:10.1007/s00441-012-1427-3
Gao LR, Zhang NK, Ding QA, Chen HY, Hu X, Jiang S, Li TC, Chen Y, Wang ZG, Ye Y, Zhu ZM (2013) Common expression of stemness molecular markers and early cardiac transcription factors in human Wharton's jelly-derived mesenchymal stem cells and embryonic stem cells. Cell Transplant 22(10):1883–1900. doi:10.3727/096368912X662444
Yang JX, Zhang N, Wang HW, Gao P, Yang QP, Wen QP (2015) CXCR4 receptor overexpression in mesenchymal stem cells facilitates treatment of acute lung injury in rats. J Biol Chem 290(4):1994–2006. doi:10.1074/jbc.M114.605063
Yang DY, Sheu ML, Su HL, Cheng FC, Chen YJ, Chen CJ, Chiu WT, Yiin JJ, Sheehan J, Pan HC (2012) Dual regeneration of muscle and nerve by intravenous administration of human amniotic fluid-derived mesenchymal stem cells regulated by stromal cell-derived factor-1alpha in a sciatic nerve injury model. J Neurosurg 116(6):1357–1367. doi:10.3171/2012.2.JNS111360
Kim YJ, Yu JM, Joo HJ, Kim HK, Cho HH, Bae YC, Jung JS (2007) Role of CD9 in proliferation and proangiogenic action of human adipose-derived mesenchymal stem cells. Pflugers Arch 455(2):283–296. doi:10.1007/s00424-007-0285-4
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8(4):315–317. doi:10.1080/14653240600855905
Roobrouck VD, Vanuytsel K, Verfaillie CM (2011) Concise review: culture mediated changes in fate and/or potency of stem cells. Stem Cells 29(4):583–589. doi:10.1002/stem.603
Brooke G, Tong H, Levesque JP, Atkinson K (2008) Molecular trafficking mechanisms of multipotent mesenchymal stem cells derived from human bone marrow and placenta. Stem Cells Dev 17(5):929–940. doi:10.1089/scd.2007.0156
Mariotti E, Mirabelli P, Abate G, Schiattarella M, Martinelli P, Fortunato G, Di Noto R, Del Vecchio L (2008) Comparative characteristics of mesenchymal stem cells from human bone marrow and placenta: CD10, CD49d, and CD56 make a difference. Stem Cells Dev 17(6):1039–1041. doi:10.1089/scd.2008.0212
Joerger-Messerli M, Bruhlmann E, Bessire A, Wagner A, Mueller M, Surbek DV, Schoeberlein A (2015) Preeclampsia enhances neuroglial marker expression in umbilical cord Wharton's jelly-derived mesenchymal stem cells. J Matern Fetal Neonatal Med 28(4):464–469. doi:10.3109/14767058.2014.921671
Moorefield EC, McKee EE, Solchaga L, Orlando G, Yoo JJ, Walker S, Furth ME, Bishop CE (2011) Cloned, CD117 selected human amniotic fluid stem cells are capable of modulating the immune response. PLoS ONE 6(10):e26535. doi:10.1371/journal.pone.0026535
Fong CY, Chak LL, Biswas A, Tan JH, Gauthaman K, Chan WK, Bongso A (2011) Human Wharton's jelly stem cells have unique transcriptome profiles compared to human embryonic stem cells and other mesenchymal stem cells. Stem Cell Rev 7(1):1–16. doi:10.1007/s12015-010-9166-x
Subramanian A, Fong CY, Biswas A, Bongso A (2015) Comparative characterization of cells from the various compartments of the human umbilical cord shows that the Wharton's jelly compartment provides the best source of clinically utilizable mesenchymal stem cells. PLoS ONE 10(6):e0127992. doi:10.1371/journal.pone.0127992
Troyer DL, Weiss ML (2008) Wharton's jelly-derived cells are a primitive stromal cell population. Stem Cells 26(3):591–599. doi:10.1634/stemcells.2007-0439
Balasubramanian S, Thej C, Venugopal P, Priya N, Zakaria Z, Sundarraj S, Majumdar AS (2013) Higher propensity of Wharton's jelly derived mesenchymal stromal cells towards neuronal lineage in comparison to those derived from adipose and bone marrow. Cell Biol Int 37(5):507–515. doi:10.1002/cbin.10056
Hsieh JY, Wang HW, Chang SJ, Liao KH, Lee IH, Lin WS, Wu CH, Lin WY, Cheng SM (2013) Mesenchymal stem cells from human umbilical cord express preferentially secreted factors related to neuroprotection, neurogenesis, and angiogenesis. PLoS ONE 8(8):e72604. doi:10.1371/journal.pone.0072604
Pires AO, Neves-Carvalho A, Sousa N, Salgado AJ (2014) The secretome of bone marrow and Wharton jelly derived mesenchymal stem cells induces differentiation and neurite outgrowth in SH-SY5Y cells. Stem Cells Int 2014:438352. doi:10.1155/2014/438352
Teixeira FG, Carvalho MM, Neves-Carvalho A, Panchalingam KM, Behie LA, Pinto L, Sousa N, Salgado AJ (2015) Secretome of mesenchymal progenitors from the umbilical cord acts as modulator of neural/glial proliferation and differentiation. Stem Cell Rev 11(2):288–297. doi:10.1007/s12015-014-9576-2
Constantin G, Marconi S, Rossi B, Angiari S, Calderan L, Anghileri E, Gini B, Bach SD, Martinello M, Bifari F, Galie M, Turano E, Budui S, Sbarbati A, Krampera M, Bonetti B (2009) Adipose-derived mesenchymal stem cells ameliorate chronic experimental autoimmune encephalomyelitis. Stem Cells 27(10):2624–2635. doi:10.1002/stem.194
Bai L, Lennon DP, Caplan AI, DeChant A, Hecker J, Kranso J, Zaremba A, Miller RH (2012) Hepatocyte growth factor mediates mesenchymal stem cell-induced recovery in multiple sclerosis models. Nat Neurosci 15(6):862–870. doi:10.1038/nn.3109
Voulgari-Kokota A, Fairless R, Karamita M, Kyrargyri V, Tseveleki V, Evangelidou M, Delorme B, Charbord P, Diem R, Probert L (2012) Mesenchymal stem cells protect CNS neurons against glutamate excitotoxicity by inhibiting glutamate receptor expression and function. Exp Neurol 236(1):161–170. doi:10.1016/j.expneurol.2012.04.011
Gu W, Zhang F, Xue Q, Ma Z, Lu P, Yu B (2010) Transplantation of bone marrow mesenchymal stem cells reduces lesion volume and induces axonal regrowth of injured spinal cord. Neuropathology 30(3):205–217. doi:10.1111/j.1440-1789.2009.01063.x
Meng F, Meliton A, Moldobaeva N, Mutlu G, Kawasaki Y, Akiyama T, Birukova AA (2015) Asef mediates HGF protective effects against LPS-induced lung injury and endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 308(5):L452–L463. doi:10.1152/ajplung.00170.2014
Bruno S, Deregibus MC, Camussi G (2015) The secretome of mesenchymal stromal cells: role of extracellular vesicles in immunomodulation. Immunol Lett. doi:10.1016/j.imlet.2015.06.007
Gerdoni E, Gallo B, Casazza S, Musio S, Bonanni I, Pedemonte E, Mantegazza R, Frassoni F, Mancardi G, Pedotti R, Uccelli A (2007) Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Ann Neurol 61(3):219–227. doi:10.1002/ana.21076
Jellema RK, Wolfs TG, Lima Passos V, Zwanenburg A, Ophelders DR, Kuypers E, Hopman AH, Dudink J, Steinbusch HW, Andriessen P, Germeraad WT, Vanderlocht J, Kramer BW (2013) Mesenchymal stem cells induce T-cell tolerance and protect the preterm brain after global hypoxia-ischemia. PLoS ONE 8(8):e73031. doi:10.1371/journal.pone.0073031
Duffy MM, Ritter T, Ceredig R, Griffin MD (2011) Mesenchymal stem cell effects on T-cell effector pathways. Stem Cell Res Ther 2(4):34. doi:10.1186/scrt75
Maron-Gutierrez T, Silva JD, Asensi KD, Bakker-Abreu I, Shan Y, Diaz BL, Goldenberg RC, Mei SH, Stewart DJ, Morales MM, Rocco PR, Dos Santos CC (2013) Effects of mesenchymal stem cell therapy on the time course of pulmonary remodeling depend on the etiology of lung injury in mice. Crit Care Med 41(11):e319–e333. doi:10.1097/CCM.0b013e31828a663e
Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM (2002) Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99(10):3838–3843
Franquesa M, Hoogduijn MJ, Bestard O, Grinyo JM (2012) Immunomodulatory effect of mesenchymal stem cells on B cells. Front Immunol 3:212. doi:10.3389/fimmu.2012.00212
Raffaghello L, Bianchi G, Bertolotto M, Montecucco F, Busca A, Dallegri F, Ottonello L, Pistoia V (2008) Human mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow niche. Stem Cells 26(1):151–162. doi:10.1634/stemcells.2007-0416
Ivanova-Todorova E, Bochev I, Mourdjeva M, Dimitrov R, Bukarev D, Kyurkchiev S, Tivchev P, Altunkova I, Kyurkchiev DS (2009) Adipose tissue-derived mesenchymal stem cells are more potent suppressors of dendritic cells differentiation compared to bone marrow-derived mesenchymal stem cells. Immunol Lett 126(1-2):37–42. doi:10.1016/j.imlet.2009.07.010
Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L (2008) Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood 111(3):1327–1333. doi:10.1182/blood-2007-02-074997
Rahmat Z, Jose S, Ramasamy R, Vidyadaran S (2013) Reciprocal interactions of mouse bone marrow-derived mesenchymal stem cells and BV2 microglia after lipopolysaccharide stimulation. Stem Cell Res Ther 4(1):12. doi:10.1186/scrt160
Prasanna SJ, Gopalakrishnan D, Shankar SR, Vasandan AB (2010) Pro-inflammatory cytokines, IFNgamma and TNFalpha, influence immune properties of human bone marrow and Wharton jelly mesenchymal stem cells differentially. PLoS ONE 5(2):e9016. doi:10.1371/journal.pone.0009016
Chang YS, Ahn SY, Jeon HB, Sung DK, Kim ES, Sung SI, Yoo HS, Choi SJ, Oh WI, Park WS (2014) Critical role of vascular endothelial growth factor secreted by mesenchymal stem cells in hyperoxic lung injury. Am J Respir Cell Mol Biol 51(3):391–399. doi:10.1165/rcmb.2013-0385OC
Guan XJ, Song L, Han FF, Cui ZL, Chen X, Guo XJ, Xu WG (2013) Mesenchymal stem cells protect cigarette smoke-damaged lung and pulmonary function partly via VEGF-VEGF receptors. J Cell Biochem 114(2):323–335. doi:10.1002/jcb.24377
Aggarwal S, Pittenger MF (2005) Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105(4):1815–1822. doi:10.1182/blood-2004-04-1559
Nartprayut K, U-Pratya Y, Kheolamai P, Manochantr S, Chayosumrit M, Issaragrisil S, Supokawej A (2013) Cardiomyocyte differentiation of perinatally derived mesenchymal stem cells. Mol Med Rep 7(5):1465–1469. doi:10.3892/mmr.2013.1356
Li D, Chai J, Shen C, Han Y, Sun T (2014) Human umbilical cord-derived mesenchymal stem cells differentiate into epidermal-like cells using a novel co-culture technique. Cytotechnology 66(4):699–708. doi:10.1007/s10616-013-9569-z
Wu KH, Zhou B, Lu SH, Feng B, Yang SG, Du WT, Gu DS, Han ZC, Liu YL (2007) In vitro and in vivo differentiation of human umbilical cord derived stem cells into endothelial cells. J Cell Biochem 100(3):608–616. doi:10.1002/jcb.21078
Leite C, Silva NT, Mendes S, Ribeiro A, de Faria JP, Lourenco T, dos Santos F, Andrade PZ, Cardoso CM, Vieira M, Paiva A, da Silva CL, Cabral JM, Relvas JB, Graos M (2014) Differentiation of human umbilical cord matrix mesenchymal stem cells into neural-like progenitor cells and maturation into an oligodendroglial-like lineage. PLoS ONE 9(10):e111059. doi:10.1371/journal.pone.0111059
Yan ZJ, Hu YQ, Zhang HT, Zhang P, Xiao ZY, Sun XL, Cai YQ, Hu CC, Xu RX (2013) Comparison of the neural differentiation potential of human mesenchymal stem cells from amniotic fluid and adult bone marrow. Cell Mol Neurobiol 33(4):465–475. doi:10.1007/s10571-013-9922-y
Mendez JJ, Ghaedi M, Steinbacher D, Niklason LE (2014) Epithelial cell differentiation of human mesenchymal stromal cells in decellularized lung scaffolds. Tissue Eng Part A 20(11-12):1735–1746. doi:10.1089/ten.TEA.2013.0647
Huang K, Kang X, Wang X, Wu S, Xiao J, Li Z, Wu X, Zhang W (2015) Conversion of bone marrow mesenchymal stem cells into type II alveolar epithelial cells reduces pulmonary fibrosis by decreasing oxidative stress in rats. Mol Med Rep 11(3):1685–1692. doi:10.3892/mmr.2014.2981
Cerrada A, de la Torre P, Grande J, Haller T, Flores AI, Perez-Gil J (2014) Human decidua-derived mesenchymal stem cells differentiate into functional alveolar type II-like cells that synthesize and secrete pulmonary surfactant complexes. PLoS ONE 9(10):e110195. doi:10.1371/journal.pone.0110195
Kotton DN, Morrisey EE (2014) Lung regeneration: mechanisms, applications and emerging stem cell populations. Nat Med 20(8):822–832. doi:10.1038/nm.3642
Uccelli A, Benvenuto F, Laroni A, Giunti D (2011) Neuroprotective features of mesenchymal stem cells. Best Pract Res Clin Haematol 24(1):59–64. doi:10.1016/j.beha.2011.01.004
Stabler CT, Lecht S, Lazarovici P, Lelkes PI (2015) Mesenchymal stem cells for therapeutic applications in pulmonary medicine. Br Med Bull. doi:10.1093/bmb/ldv026
Bianco P, Cao X, Frenette PS, Mao JJ, Robey PG, Simmons PJ, Wang CY (2013) The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med 19(1):35–42. doi:10.1038/nm.3028
Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ (2009) Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 5(1):54–63. doi:10.1016/j.stem.2009.05.003
Katsuda T, Kosaka N, Takeshita F, Ochiya T (2013) The therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Proteomics 13(10-11):1637–1653. doi:10.1002/pmic.201200373
Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang X, Shang X, Zhang ZG, Chopp M (2012) Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells 30(7):1556–1564. doi:10.1002/stem.1129
Dreyer JL (2010) New insights into the roles of microRNAs in drug addiction and neuroplasticity. Genome Med 2(12):92. doi:10.1186/gm213
Katsuda T, Tsuchiya R, Kosaka N, Yoshioka Y, Takagaki K, Oki K, Takeshita F, Sakai Y, Kuroda M, Ochiya T (2013) Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Sci Rep 3:1197. doi:10.1038/srep01197
Ribeiro CA, Fraga JS, Graos M, Neves NM, Reis RL, Gimble JM, Sousa N, Salgado AJ (2012) The secretome of stem cells isolated from the adipose tissue and Wharton jelly acts differently on central nervous system derived cell populations. Stem Cell Res Ther 3(3):18. doi:10.1186/scrt109
Ribeiro CA, Salgado AJ, Fraga JS, Silva NA, Reis RL, Sousa N (2011) The secretome of bone marrow mesenchymal stem cells-conditioned media varies with time and drives a distinct effect on mature neurons and glial cells (primary cultures). J Tissue Eng Regen Med 5(8):668–672. doi:10.1002/term.365
Glenn JD, Whartenby KA (2014) Mesenchymal stem cells: emerging mechanisms of immunomodulation and therapy. World J Stem Cells 6(5):526–539. doi:10.4252/wjsc.v6.i5.526
Pluchino S, Cossetti C (2013) How stem cells speak with host immune cells in inflammatory brain diseases. Glia 61(9):1379–1401. doi:10.1002/glia.22500
Honmou O, Houkin K, Matsunaga T, Niitsu Y, Ishiai S, Onodera R, Waxman SG, Kocsis JD (2011) Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain 134(Pt 6):1790–1807. doi:10.1093/brain/awr063
Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY, collaborators S (2010) A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells 28(6):1099–1106. doi:10.1002/stem.430
Diez-Tejedor E, Gutierrez-Fernandez M, Martinez-Sanchez P, Rodriguez-Frutos B, Ruiz-Ares G, Lara ML, Gimeno BF (2014) Reparative therapy for acute ischemic stroke with allogeneic mesenchymal stem cells from adipose tissue: a safety assessment: a phase II randomized, double-blind, placebo-controlled, single-center, pilot clinical trial. J Stroke Cerebrovasc Dis 23(10):2694–2700. doi:10.1016/j.jstrokecerebrovasdis.2014.06.011
Mazzini L, Ferrero I, Luparello V, Rustichelli D, Gunetti M, Mareschi K, Testa L, Stecco A, Tarletti R, Miglioretti M, Fava E, Nasuelli N, Cisari C, Massara M, Vercelli R, Oggioni GD, Carriero A, Cantello R, Monaco F, Fagioli F (2010) Mesenchymal stem cell transplantation in amyotrophic lateral sclerosis: a phase I clinical trial. Exp Neurol 223(1):229–237. doi:10.1016/j.expneurol.2009.08.007
Connick P, Kolappan M, Crawley C, Webber DJ, Patani R, Michell AW, Du MQ, Luan SL, Altmann DR, Thompson AJ, Compston A, Scott MA, Miller DH, Chandran S (2012) Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open-label phase 2a proof-of-concept study. Lancet Neurol 11(2):150–156. doi:10.1016/S1474-4422(11)70305-2
Llufriu S, Sepulveda M, Blanco Y, Marin P, Moreno B, Berenguer J, Gabilondo I, Martinez-Heras E, Sola-Valls N, Arnaiz JA, Andreu EJ, Fernandez B, Bullich S, Sanchez-Dalmau B, Graus F, Villoslada P, Saiz A (2014) Randomized placebo-controlled phase II trial of autologous mesenchymal stem cells in multiple sclerosis. PLoS ONE 9(12):e113936. doi:10.1371/journal.pone.0113936
Pal R, Venkataramana NK, Bansal A, Balaraju S, Jan M, Chandra R, Dixit A, Rauthan A, Murgod U, Totey S (2009) Ex vivo-expanded autologous bone marrow-derived mesenchymal stromal cells in human spinal cord injury/paraplegia: a pilot clinical study. Cytotherapy 11(7):897–911. doi:10.3109/14653240903253857
Zheng G, Huang L, Tong H, Shu Q, Hu Y, Ge M, Deng K, Zhang L, Zou B, Cheng B, Xu J (2014) Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respir Res 15:39. doi:10.1186/1465-9921-15-39
Weiss DJ, Casaburi R, Flannery R, LeRoux-Williams M, Tashkin DP (2013) A placebo-controlled, randomized trial of mesenchymal stem cells in COPD. Chest 143(6):1590–1598. doi:10.1378/chest.12-2094
Ribeiro-Paes JT, Bilaqui A, Greco OT, Ruiz MA, Marcelino MY, Stessuk T, de Faria CA, Lago MR (2011) Unicentric study of cell therapy in chronic obstructive pulmonary disease/pulmonary emphysema. Int J Chron Obstruct Pulmon Dis 6:63–71. doi:10.2147/COPD.S15292
Lee YH, Choi KV, Moon JH, Jun HJ, Kang HR, Oh SI, Kim HS, Um JS, Kim MJ, Choi YY, Lee YJ, Kim HJ, Lee JH, Son SM, Choi SJ, Oh W, Yang YS (2012) Safety and feasibility of countering neurological impairment by intravenous administration of autologous cord blood in cerebral palsy. J Transl Med 10:58. doi:10.1186/1479-5876-10-58
Mancias-Guerra C, Marroquin-Escamilla AR, Gonzalez-Llano O, Villarreal-Martinez L, Jaime-Perez JC, Garcia-Rodriguez F, Valdes-Burnes SL, Rodriguez-Romo LN, Barrera-Morales DC, Sanchez-Hernandez JJ, Cantu-Rodriguez OG, Gutierrez-Aguirre CH, Gomez-De Leon A, Elizondo-Riojas G, Salazar-Riojas R, Gomez-Almaguer D (2014) Safety and tolerability of intrathecal delivery of autologous bone marrow nucleated cells in children with cerebral palsy: an open-label phase I trial. Cytotherapy 16(6):810–820. doi:10.1016/j.jcyt.2014.01.008
Wang X, Cheng H, Hua R, Yang J, Dai G, Zhang Z, Wang R, Qin C, An Y (2013) Effects of bone marrow mesenchymal stromal cells on gross motor function measure scores of children with cerebral palsy: a preliminary clinical study. Cytotherapy 15(12):1549–1562. doi:10.1016/j.jcyt.2013.06.001
Chang YS, Ahn SY, Yoo HS, Sung SI, Choi SJ, Oh WI, Park WS (2014) Mesenchymal stem cells for bronchopulmonary dysplasia: phase 1 dose-escalation clinical trial. J Pediatr 164(5):966–972. doi:10.1016/j.jpeds.2013.12.011, e966
Pawelec K, Gladysz D, Demkow U, Boruczkowski D (2015) Stem cell experiments moves into clinic: new hope for children with bronchopulmonary dysplasia. Adv Exp Med Biol 839:47–53. doi:10.1007/5584_2014_27
Min K, Song J, Kang JY, Ko J, Ryu JS, Kang MS, Jang SJ, Kim SH, Oh D, Kim MK, Kim SS, Kim M (2013) Umbilical cord blood therapy potentiated with erythropoietin for children with cerebral palsy: a double-blind, randomized, placebo-controlled trial. Stem Cells 31(3):581–591. doi:10.1002/stem.1304
Cotten CM, Murtha AP, Goldberg RN, Grotegut CA, Smith PB, Goldstein RF, Fisher KA, Gustafson KE, Waters-Pick B, Swamy GK, Rattray B, Tan S, Kurtzberg J (2014) Feasibility of autologous cord blood cells for infants with hypoxic-ischemic encephalopathy. J Pediatr 164(5):973–979. doi:10.1016/j.jpeds.2013.11.036, e971
Ploemacher WJCRaRE (2003) Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia 17(1):160–170
Messerli M, Wagner A, Sager R, Mueller M, Baumann M, Surbek DV, Schoeberlein A (2013) Stem cells from umbilical cord Wharton's jelly from preterm birth have neuroglial differentiation potential. Reprod Sci 20(12):1455–1464. doi:10.1177/1933719113488443
Donega V, Nijboer CH, van Velthoven CT, Youssef SA, de Bruin A, van Bel F, Kavelaars A, Heijnen CJ (2015) Assessment of long-term safety and efficacy of intranasal mesenchymal stem cell treatment for neonatal brain injury in the mouse. Pediatric research. doi:10.1038/pr.2015.145
Liu L, Mao Q, Chu S, Mounayar M, Abdi R, Fodor W, Padbury JF, De Paepe ME (2014) Intranasal versus intraperitoneal delivery of human umbilical cord tissue-derived cultured mesenchymal stromal cells in a murine model of neonatal lung injury. Am J Pathol 184(12):3344–3358. doi:10.1016/j.ajpath.2014.08.010
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
All authors have made substantive intellectual contributions to this manuscript. MM and BK designed, drafted, and wrote the manuscript. TW, AS, AG, and DS revised and provided critical and important intellectual content. All authors have given final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Mueller, M., Wolfs, T.G.A., Schoeberlein, A. et al. Mesenchymal stem/stromal cells—a key mediator for regeneration after perinatal morbidity?. Mol Cell Pediatr 3, 6 (2016). https://doi.org/10.1186/s40348-016-0034-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40348-016-0034-x