Abstract
Background
Impaired metabolic response such as blood glucose fast fluctuations may be hypothesized in binge eating disorder (BED) and food addiction (FA) by virtue of the repetitive consumption of highly processed food. Conversely, rapid changes in plasma glucose (i.e., hypoglycemia) may trigger craving for the same food products. The investigation of early glycemic disturbances in BED and FA could enhance the understanding of the metabolic mechanisms involved in the maintenance of the disorders. Present study investigated hypoglycemia events during a 5-h-long oral glucose tolerance test (OGTT) in people with BED, FA, and the comorbid phenotype. Further, the association between the severity of eating psychopathology and the variability in hypoglycaemia events was explored.
Methods
Two-hundred participants with high weight and no diabetes completed the extended OGTT and were screened for BED, FA, BED-FA, or no-BED/FA. The four groups were compared in hypoglycemia events, OGTT-derived measures, and eating psychopathology. The association between predictors (eating psychopathology), confounders (demographics, metabolic features), and the outcomes (hypoglycemia, early/late hypoglycemia, severe hypoglycemia, reactive hypoglycemia) was examined through logistic regression.
Results
Hypoglycemia in general, and reactive hypoglycemia were highly frequent (79% and 28% of the sample, respectively). Hypoglycemia events (< 70 mg/dL) were equally experienced among groups, whilst severe hypoglycemia (< 54 mg/dL) was more frequent in BED at the late stage of OGTT (5 h; χ2 = 1.120, p = .011). The FA and BED groups exhibited significantly higher number of reactive hypoglycemia (χ2 = 13.898, p = .003), in different times by diagnosis (FA: 210′–240′; BED: at the 270′). FA severity was the only predictor of early and reactive hypoglycemia.
Conclusions
People with BED or FA are prone to experiencing reactive hypoglycemia; FA severity may predict early and symptomatic hypoglycemia events. This can further reinforce disordered eating behaviours by promoting addictive responses, both biologically and behaviourally. These results inform professionals dealing with eating disorders about the need to refer patients for metabolic evaluation. On the other hand, clinicians dealing with obesity should screen for and address BED and FA in patients seeking care for weight loss.
Plain English summary
Impairment in blood glucose control may be attended in binge eating disorder (BED) and food addiction (FA), two distinct eating disorders which are characterized by the recurrent consumption of highly palatable food rich in high-glucose index carbohydrates. Conversely, rapid changes in blood glucose, such as hypoglycemia, may intensify craving for high-calorie products, thus reinforcing pathological eating behaviours. This study investigated the presence of hypoglycemia events in people suffering from BED, FA, both, or no eating disorder, and explored whether the severity of eating behaviours correlated with a higher probability of having hypoglycemia. Results showed that people with BED and FA experienced more episodes of symptomatic hypoglycemia than those with obesity but no eating disorder. The severity of binge eating was associated with more severe hypoglycemia events, indicated by lower plasma glucose values. Lastly, people with severe FA were more prone to experiencing early post-meal hypoglycemia accompanied by symptoms. These results inform professionals dealing with eating disorders about the need to refer patients for metabolic evaluation. On the other hand, clinicians dealing with obesity should screen for and address BED and FA in patients seeking care for weight loss.
Similar content being viewed by others
Background
Binge eating disorder (BED) is characterized by recurrent episodes of eating large amount of food in a short amount of time with the subjective feeling of losing control, at least three of the associated features (i.e., high speed, embarrassment while eating, eating when not physically hungry, eating until uncomfortably full, and guilt, or disgust associated with the episodes), and without any compensatory behaviour taking place to counterweigh the food intake [1]. Research that has examined BED from a psychopathological perspective has highlighted that episodes of binge eating are consequential to a state of negative affect, and that the emotional state tends to improve shortly after the binge episode [2, 3].
Food addiction (FA), whom BED has shown to have high co-occurrence and share symptomatology with [4,5,6], refers to a chronic and relapsing condition characterized by uncontrolled eating in order to achieve pleasure or relieve emotional or physical distress [7, 8]. FA is thought to have neurobiological underpinnings involving the circuitry of reward, and shares clinical and neurobiological similarities with substance use disorders [9, 10].
Beyond that, it is important to acknowledge that food contains various nutrients that could potentially act as triggers of eating behaviours on their own [11]. In FA, the type of food consumed is typically highly processed and contains either macronutrients or pure high-glycemic index carbohydrates [11, 12].
From a metabolic point of view, impaired metabolic response in blood glucose fluctuations may be hypothesized in FA, but also in BED, by virtue of the high-glycemic index carbohydrates content of binge eating episodes [13,14,15]. As a result, insulin secretion and/or targeted efficacy may alter and fail to maintain euglycemia. Plasma glucose levels play a key role in the short-term daily regulation of the feelings of hunger, satiety, and, therefore, eating habits [16]. It is well known that unfavourable changes in the level of blood glucose and the presence of hypoglycemia episodes may, in turn, promote craving for high-calorie products [14]. Reactive hypoglycemia, defined as plasma glucose ≤ 70 mg/dL (≤ 3.9 mmol/L) within 2–5 h after a meal, is characterized by symptoms such as hands tremor, sweating, palpitations, disorientation, impaired vision, fainting [19]. The hypoglycemia-related stimulation of appetite evoking hormones is then responsible for the drive to eating behaviours [15]. From this perspective, the clinical presentation of reactive hypoglycemia may resemble some of the symptoms which trigger binge-eating episodes and/or FA episodes. On the other hand, recent findings suggest that nearly half of individuals with overweight and obesity experience hypoglycemia without significant symptoms except hunger, and that the frequency of snacking is unrelated to hypoglycemia symptoms. Authors hypothesized that hunger may be induced at a higher threshold than neurogenic symptoms, and that individuals experiencing asymptomatic hypoglycemia might unconsciously prevent symptoms by snacking [20].
De facto, either the characteristics of food consumed, or the behavioural aspect of losing control overeating and craving, raise the question, for BED and FA, if what has traditionally been viewed as a coping mechanism for emotional regulation may have a metabolic counterpart, and that chemical or metabolic mechanisms may be at play, rather than solely neurobehavioral factors [12].
An understanding of the metabolic correlates of BED and FA may aid the general conceptualization of the constructs, including the identification of factors responsible for perpetuating the pathological eating behaviours. Literature on the metabolic correlates of BED revealed a straightforward association with obesity, type 2 diabetes, and metabolic syndrome [21,22,23,24]. The research on the metabolic correlates of FA is still in its infancy [25]. Recent studies indicate that FA is common among people with obesity (estimated prevalence ranging from 25 to 42%) [26,27,28] and is associated with unfavourable outcomes in weight loss management [29]. The prevalence of FA may be even higher in patients undergoing bariatric surgery (57.8%) [30]. While strong support for the association between FA and type 2 diabetes has been recently provided [31], further evidence is required to make definitive conclusions regarding the metabolic-related complications of this disorder [24, 26].
To our knowledge, hypoglycemia, as marker of early onset of glucose metabolism impairment, has not been investigated so far in patients suffering from BED, FA, or in the comorbid phenotype. Present study investigated hypoglycemia events during 5-h-long oral glucose tolerance test (OGTT) in patients with obesity and without diabetes, according to BED, FA, and the comorbid phenotype. It further examined the role of eating psychopathology severity in explaining the variability in hypoglycemia events, when adjusting for demographics, anthropometric, and metabolic features.
Methods
Participants
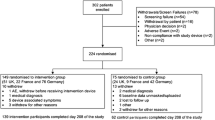
Participants were recruited among people admitted to the diagnostic and therapeutic network of care for obesity (PDTA Obesità) of the University Hospital of Catanzaro (Italy) from May 2017 to December 2022. The PDTA Obesità is a dedicated pathway of care for obesity networked by an interdisciplinary team of specialists (internal medicine, psychiatry, and surgery). Eligibility was met if: (a) men and women aged between 18 and 65 years; (b) body mass index (BMI) ≥ 30 kg/m2; (c) consent to execute an extended oral glucose tolerance test (300 min); (d) ability to answer self-reported questionnaires autonomously; and (e) valid informed consent to participate. Conversely, (a) pregnancy, having recently given birth or breast feeding, (b) diagnosis of type 2 diabetes, (c) substance use disorder, and (d) ongoing pharmacological treatment or medical conditions with a clear influence on glucose metabolism (e.g., previous gastrointestinal surgery, peptic ulcer disease, hormonal disorders, known inflammatory diseases, oncological diseases, infectious disorders, corticosteroids, chemotherapy, antipsychotics, mood stabilizers, antidepressants, or biologic drugs) were considered exclusion criteria. Eligible patients were fully informed about the aim and the procedures of the study, and that participation was voluntary and free from any compensation. The study was approved by the institutional review boards (Local Ethical Committee, n. 53/2013), and all the procedures were performed in accordance with the principles of the Declaration of Helsinki [34]. A total of 236 eligible patients agreed to participate. Out of the initial sample, 14 participants consented but did not show up at the psychiatric examination, and 22 did not fulfil psychometric questionnaires (attrition rate 14%). Out of the final sample (N = 200), 20 participants dropped out over time during the oral glucose load (see supplementary material for further details on drop-out analysis).
Procedures
All the metabolic evaluations were run the first day of the admission to the hospital during the visit with the specialist in internal medicine; information relating to previous/ongoing medical conditions and pharmacotherapy was collected in that occasion. After accounting for the eligibility, patients were asked for participation and addressed to complete the psychiatric evaluation (second visit) within one week.
Metabolic evaluation
Anthropometrics, fasting and OGTT-derived plasma glucose and insulin, and dynamic measures of insulin secretion/sensitivity/resistance were extracted for this study. Anthropometric evaluation included height and weight, wearing light indoor clothing and no shoes; then BMI was calculated.
The extended OGTT was performed at 8:00 a.m., after 12-h fasting. Plasma glucose and insulin were tested at baseline, and after 30′, 60′, 90′, 120′, 150′, 180′, 210′, 240′, 270′, and 300′ of oral ingestion of a glucose load containing the equivalent of 75 g anhydrous glucose dissolved in water. During the OGTT, patients were guaranteed a relaxing environment and asked to report any symptoms of malaise. The presence of disabling symptoms or symptoms related to hypoglycemia (e.g., hands tremor, sweating, palpitations, hunger, disorientation, impaired vision, fainting) were followed by glucometer examination. All symptomatic hypoglycemia resulted in discontinuation of the procedure, and a carbohydrate meal was offered. Reactive hypoglycemia was diagnosed by documentation of the Whipple’s triad (i.e., symptoms/signs consistent with hypoglycemia, low plasma glucose concentration, resolution of symptoms with carbohydrates intake) [35]. Hypoglycemia events were registered and then classified by severity (hypoglycemia or severe hypoglycemia for plasma glucose, respectively, ≤ 70 mg/dL (3.9 mmol/L) and ≤ 54 mg/dL (3 mmol/)) [35], time (early or late hypoglycemia occurring, respectively, before and after the 180th minute), and the presence of neuroglycopenic/neurogenic symptoms (i.e., reactive hypoglycemia) for the analysis.
Glucose tolerance profile (GTP) and fasting and OGTT-derived measures of insulin response and sensitivity were calculated from the glucose load. Participants were classified by their glucose tolerance as having normal glucose tolerance (NGT) (fasting plasma glucose < 100 mg/dL and 2 h plasma glucose < 140 mg/dL), impaired fasting glucose (IFG) (fasting plasma glucose 100–125 mg/dl and 2 h plasma glucose < 140 mg/dL), and impaired glucose tolerance (IGT) (fasting plasma glucose < 126 mg/dl and 2 h plasma glucose 140–199 mg/dL) [36]. Early glucose-stimulated insulin response was calculated as the insulinogenic index (Ð plasma insulin (0–30 min)/ Ð plasma glucose (0–30 min) [37]. The homeostatic model assessment insulin resistance index (HOMA-IR) was calculated as fasting plasma insulin (pmol/l) × fasting plasma glucose (mmol/l)/135 [38]. The β cells function and the liver and peripheral tissues sensitivity to insulin were evaluated by Matsuda dynamic index proposed by Matsuda and De Fronzo (10 000/ (fasting plasma insulin × fasting plasma glucose × mean plasma insulin × mean plasma glucose) [39].
Psychiatric evaluation
Psychiatrists with adequate training in the field of eating disorders interviewed the participants during the week after the metabolic evaluation. The structured clinical interview for the DSM-5 (SCID-5) [40] was performed to diagnose BED. Given the diagnosis of FA is not formally recognized in the DSM, diagnostic criteria for a probable diagnosis have mainly been extrapolated from the DSM criteria for substance use disorders, as suggested by the common pathogenetic and clinical background for behavioural and drug addictions [41]. The diagnosis of FA was then performed basing on the specific and validated psychometric questionnaire (see below). Participants then completed the following self-reported questionnaires:
-
The Binge Eating Scale (BES) is a 16 items questionnaire which assess the severity of behaviours, feelings, and cognitions associated with BED [42,43,44]. A total BES score < 17 indicates unlikely BED (“mild”), a 17–27 score possible BED (“moderate”) and values > 27 probable BED (“severe”). Cronbach’s alpha in the present study was 0.89.
-
The Yale Food Addiction Scale (YFAS 2.0) questionnaire consists of 35 items that assess addiction-like eating behaviours across 11 criteria (e.g., overeating, desire to cut down, time spent, craving, related impairment, risky use, tolerance, and withdrawal) over the past 12 months [45, 46]. The sum of the endorsed symptoms yields a total score (0–11), and a severity level (mild: 2–3 symptoms, moderate:4–5 symptoms, severe > 6 symptoms). It is important to note that the presence of impairment/distress criteria is essential for diagnosing FA, and only the severity level scoring system ensures the presence of this criteria. Thus, the severity level was used for the FA diagnosis confirmation and analysis. Internal consistency (Kuder–Richardson) for this study was 0.86.
According to the presence/absence of the BED/FA diagnosis, the total sample was divided into 4 groups (BED, FA, BED-FA, no-BED/FA).
Statistical analysis
Dataset manipulation and statistical analyses were performed using the Statistical Package for the Social Sciences for Mac (SPSS; ver. 25). To detect and address for potential outliers within the variables of interest, Mahalanobis' distance and the winsorizing method were employed. After accommodation, skewed variables were identified through Shapiro–Wilk test and natural log-transformed (IGI). Groups were compared in the variables of interest through χ2 and multivariate analysis adjusting for potential confounders (BMI, sex, and age), as appropriate. For significant results the effect size (ŋ2 values of 0.2, 0.6, 1.2 and > 1.2 considered, respectively, slight, small, moderate, and large effect) [47] and Bonferroni post-hoc analyses were run. Time-to-event analysis (Kaplan–Meier), accounting for censoring, was run to assess the probability for reactive hypoglycemia; log-rank test was used to test significance among groups. Logistic regression served to examine associations between BED/FA severity and the outcomes. Specifically, five regression models were run for the outcomes (hypoglycemia, early/late hypoglycemia, severe hypoglycemia, and reactive hypoglycemia), entering simultaneously predictors (BES severity, YFAS severity), and confounders (age, sex, BMI, GTP, HOMA-IR). To handle type I errors, the Bonferroni correction method was applied within families of tests. Drop-out analysis was performed to address for possible attrition bias. Significant coefficients were considered for corrected p values (specific corrected significance levels in tables).
Results
Sample description is shown in Table 1. Groups showed similar age and BMI. Female were overrepresented in all groups, with higher percentages in the BED and BED-FA groups (χ2 = 10.272, p = 0.016). Significant differences by diagnostic group emerged in fasting plasma glucose (F = 4.365, p = 0.006), fasting plasma insulin (F = 4.296, p = 0.007), and HOMA-IR (F = 6.386, p < 0.001), with the BED-FA group scoring the highest value in fasting plasma glucose and insulin and the BED group in fasting plasma insulin and HOMA-IR (Table 1). Differences in metabolic variables were also explained by the effect of age (fasting plasma glucose and insulin, insulinogenic index), BMI (fasting plasma insulin, Matsuda index, HOMA-IR), sex (fasting plasma insulin, HOMA-IR), and the combined effect of diagnosis*sex (fasting plasma glucose and insulin, HOMA-IR) (statistics of the multivariate analysis in Table 1). NGT was more frequent in all groups (with BED scoring the highest prevalence, FA the lowest); IFG was more frequent in BED, IGT in FA, and the combined IFG-IGT in the BED-FA group (χ2 = 22.090, p = 0.009).
Table 2 shows frequencies and percentages of hypoglycemia events. A flow-chart describing the interruptions of the OGTT due to hypoglycemia events or drop-out is embed within the supplementary material (Additional file 1: Figure S1). Drop-out analysis revealed no effect of drop-out on the variables of interest (Additional file 1: Table S1). A total of 158 participants (79%) experienced hypoglycemia events during the OGTT, with the highest incidence in the FA group (87.5%, p = ns). Hypoglycemia was significantly more frequent in the no BED/FA and BED-FA group at the 270′ (χ2 = 10.732, p = 0.013), although significance lost after multiple comparison correction (p < 0.008). Severe hypoglycemia (plasma glucose < 54 mg/dL) was more frequently endorsed in the late stages of the glucose load, with the BED group experiencing severe hypoglycemia at the 300′ (χ2 = 1.120, p = 0.011).
Reactive hypoglycemia was documented in 28% (N = 56) of the sample, globally considered. By groups, the FA and BED groups experienced significantly higher number of episodes compared to other groups (χ2 = 13.898, p = 0.003; FA, BED > no-BED/FA, BED-FA); by time, reactive hypoglycemia was more frequently experienced between the 210′–240′ in the FA group, and at the 270′ in the BED group (significance lost after multiple comparison correction). Time-to-event analysis showed significant lower time to reactive hypoglycemia in the group with FA with respect to the other groups (Mantel Cox Log-Rank test; χ2 = 13.952, p = 0.003; Fig. 1).
Results from the logistic regression analysis are shown in Table 3. When entering all the variables, no predictors associated with hypoglycemia events, and late hypoglycemia. Early hypoglycemia was significantly associated with severe FA, with participants suffering from severe FA experiencing 14 times more likely early hypoglycemia (p = 0.001). Severe hypoglycemia was significantly associated with BMI, although significance was beyond the set corrected p-value (0.007). Lastly, FA severity was associated with reactive hypoglycemia, although this associations did not remain significant after Bonferroni correction (0.008 vs 0.007).
Discussion
Present study investigated hypoglycemia events during extended OGTT in people seeking care for weight loss and suffering from BED, FA, and the comorbid phenotype. It further aimed to explore the extent to which the severity of eating psychopathology accounts for the variability in hypoglycemia events, when adjusting for demographics, anthropometrics, and metabolic features as confounders.
Hypoglycemia is defined as a fall in plasma glucose level beyond the minimum considered of 70 mg/dL (3.8 mmol) [19]. Reactive hypoglycemia, more specifically, refers to hypoglycemia events associated with neurogenic or neuroglycopenic symptoms, which typically revert with carbohydrates intake [48]. Although reactive hypoglycemia may not associate with metabolic disturbances in people with normal weight [49], some evidence suggests it associates with glucose metabolism impairment in its early stage, and, consequently, with increased risk of late diabetes [50, 51]. On the other hand, it is reported that patients suffering from obesity exhibit higher rate of reactive hypoglycemia after oral glucose load than normal weight [52], suggesting that this population is further exposed to develop metabolic complications.
Hypoglycemia, globally considered, was detected in up to 80% of participants. Reactive hypoglycemia, on the other hand, was experienced from 15 to 54% of the sample. These frequencies were unexpectedly high, especially considering that participants had no diabetes and were not exposed to any medical condition or drug affecting glucose metabolism. One study previously investigated reactive hypoglycemia after 4-h long OGTT according to different BMI, founding a prevalence up to 45% in patients with obesity [52]. This result should be read considering that Xue and colleagues adopted a very stringent cut-off (< 55 mg/dL) for the hypoglycemia event, accounting for a smaller prevalence. Some evidence states that autonomic symptoms of hypoglycemia usually occur for plasma glucose < 58 mg/dL [53], and that a strict cut-off is of clinical interest for outcome implications [54]. However, we opted for a more conservative cut-off considering that reactive hypoglycemia related symptoms may present with higher glucose values under the minimum considered of 70 mg/dL, agreeing with other studies on hypoglycemia and other medical conditions [49]. We further considered that intermediate glucose levels (50–70 mg/dL) still have potential to stimulate hunger, with evidence suggesting an association between subthreshold hypoglycemia and the frequency of snacking in daily life [20]. Another factor that may have accounted for the higher reactive hypoglycemia rates, is that the present investigation was conducted on the extended OGTT, which is double the time of a regular oral glucose load (2 h), and one hour longer than Xue and colleagues evaluated [57].
Hypoglycemia, independent of the severity or related symptoms, was more frequent in the late stages of the glucose load for all groups, confirming previous findings of more frequent hypoglycemia at the 4th hour in patients with obesity [52]. This trend was also confirmed considering reactive hypoglycemia, where eating psychopathology did show an effect. To this extent, patients suffering from only FA and BED reported significantly higher frequency of reactive episodes than the other groups, in different times (between the 210′–240′, and at the 270′, respectively). BED was also associated with more severe events at the same timing, although results were not significant after correction for multiple comparisons.
Different factors may account for these results. First, a plausible effect of insulin resistance and hyperinsulinemia should be considered. De facto, all groups exhibited HOMA-IR values suggestive of insulin resistance [58]. The group with BED exhibited the highest plasma insulin and insulin resistance among the groups, confirming previous findings [59,60,61,62]. Nevertheless, this mechanism alone does not fully elucidate why the group with FA, whilst reporting the lowest plasma insulin and insulin resistance, still exhibits higher frequencies of reactive hypoglycemia with respect to the other groups. To date, evidence for FA is still scarce and heterogeneous in supporting the association with early disruption in glycemia control [55]. Alternatively, disrupted first phase in insulin secretion has been proposed as a plausible mechanism underlying post-prandial hypoglycemia [66]. According to these hypotheses, low insulin secretion in the first phase would result in high plasma glucose, leading to late and excessive second-phase insulin secretion. We are not able to evaluate if disturbances in the early/late insulin response may play a role, but relatively high and sustained insulin secretion might explain delayed hypoglycemia in FA and BED. An excess of the incretin effect should also be considered [67]. Beyond the effect on insulin secretion in response to glucose ingestion, the glucagon-like peptide-1 (GLP-1) is also responsible for suppressing glucagon response and has been associated with reactive hypoglycemia [68]. Four studies evaluated GLP-1 in binge eating and food addiction, with null findings [69,70,71,72]. On the other hand, some promising results emerged from interventional studies using the GLP-1 agonists for the treatment of binge eating and uncontrolled eating, suggesting a positive modulation of craving and food related reward activation [73]. When discussing hormones that are known to contribute to glucose homeostasis and food intake modulation, some other candidates have been recently studied in disordered eating behaviours, binge eating, and food addiction (e.g., ghrelin, leptin, nesfatin-1) [73, 74]. Their potential impact on the correlation between disordered eating behaviours and reactive hypoglycemia should be considered and investigated.
Finally, a recent study evaluated reactive hypoglycemia, snacking habits, and obesity in relation to the index of glucose effectiveness (i.e., the ability to increase peripheral glucose uptake independent of insulin) [75]. Results from this study suggested that either low or high glucose effectiveness relates to hypoglycemia; in the first case, hypoglycemia would result from post-prandial hyperglycemia and hyperinsulinemia (insulin-dependent hypoglycemia). In the second case, high glucose effectiveness would facilitate glucose disposal, predisposing to insulin-independent hypoglycemia. Present results support hyperinsulinemia, insulin resistance, and more severe hypoglycemia at 5 h in BED. On the other hand, patients with FA exhibited higher reactive hypoglycemia frequency, and the severity of FA was associated with early hypoglycemia. Different glucose effectiveness in these two groups could explain the diverse hypoglycemia profiles (insulin-dependent for BED, insulin-independent for FA); however, data about complete insulin load were not available for this analysis, preventing from arguing if this is the case for FA and BED population. By adding new insight on one more hypothesis below the association between FA, BED, and hypoglycemia, Kishimoto and Ohashi also found increased odds of snaking habits according to the lowest and highest percentiles of glucose effectiveness [75].
This is particularly important when dealing with the hypothesis that metabolic profile, hereby in the construct of hypoglycemia, should be considered a risk factor for maintaining the disordered eating behaviours. Previous findings documented the strength to which hypoglycemia and glucose fluctuations promote the motivation for high dense and caloric foods, by modulating limbic/striatal brain regions [76] and triggering addictive responses, biologically and behaviourally [12]. There is also some evidence that insulin interacts with dopamine signalling in the brain areas of reward [77]. Conversely, the consumption of high glycemic index foods is associated with a further surge in glucose and insulin secretion, which may promote subsequent hypoglycemia [78]. Therefore, based on our findings, we could speculate that reactive hypoglycemia, supported by glucose and insulin fluctuations, may reinforce food intake and disordered eating behaviours (e.g., binge eating, food addiction) which in turn may exacerbate reactive hypoglycemia. This is of pivotal importance for both BED and FA, but mostly for the last one, where the craving and addiction-like behavioural profile is more pronounced [6].
It is important to mention that 65% experienced hypoglycemia without reporting related symptoms. The occurrence of hypoglycemia-like symptoms answers to individual and biological factors (e.g., decrease sympathetic response) [79]. Interestingly, there is consensus about autonomic symptoms activating for plasma glucose around 50 mg/dL, while hunger input starts within higher plasma glucose concentrations. Recent evidence found that subclinical reactive hypoglycemia associates with dysfunctional eating behaviours independent of the Whipple’s triad, arguing that “snacking begets snacking through subclinical hypoglycemia” [20]. This might be the case for BED, whom hypoglycemia events were less frequently accompanied by symptoms with respect to patients with FA. Notwithstanding the potentiality of such results, the research scenario on subclinical reactive hypoglycemia is still in its infancy, preventing any direct application in real-word clinical settings. To date, the evaluation and management of hypoglycemia are recommended exclusively for patients meeting the Whipple’s triad criteria (thus, symptomatic) [35], and the definition of reactive hypoglycemia itself does not include asymptomatic episodes [67]. Further evidence is essential to comprehensively assess the long-term clinical impact of subclinical reactive hypoglycemia before revising the concept and translating subclinical reactive hypoglycemia research into clinical practice.
Lastly, studies evaluating the co-occurrence of BED and FA have suggested the this phenotype may represent a more severe form of BED [26], and is associated with worse eating and general psychopathology with respect to simple obesity [80, 81]. In the present study, the comorbid phenotype did not exhibit more hypoglycemia or reactive hypoglycemia events. On the other hand, this group exhibited higher levels of fasting plasma glucose compared to the other groups, higher levels of fasting plasma insulin compared to all groups except those with BED alone, and a more frequent occurrence of impaired fasting glucose plus impaired glucose tolerance (pre-diabetes). It is plausible that hypoglycemia occurs less frequently in this group due to the progression to a later stage of the disorder and a more disrupted glycemic homeostasis. Nonetheless, further investigation is imperative to thoroughly explore and substantiate this hypothesis.
To the best of our knowledge, this is the first evidence so far on the evaluation and characterization of hypoglycemia in people suffering from obesity, without diabetes, and according to BED and FA.
However, present results should be interpreted cautiously considering some limitations. First, the cross-sectional design prevents to evaluate causal relationships between eating psychopathology and hypoglycemia events. Longitudinal studies or intervention studies would provide more robust evidence. Second, the study examined hypoglycemia events during a single testing session which may not capture the full range of metabolic responses and fluctuations in the real-world setting. Although no gold standard is actually recognised for the diagnosis of reactive hypoglycemia [67], and the OGTT has been the most widely used methodology in this field, we acknowledge it is associated with the over-estimation of hypoglycemia incidence [82], preventing any critical translation of results to clinical practice. Real time assessment of glucose variability through continuous glucose monitoring, together with the record of daily nutritional intake, could provide a more comprehensive understanding of the metabolic dynamics and expand actual knowledge about the influence that eating disorders and disordered eating behaviours exert on the metabolic profile. Third, the lack of a healthy normal-weight control group may hinder the ability to compare the metabolic response and hypoglycemia events specifically associated with these conditions. Including healthy subjects with normal weight would allow for better differentiation and interpretation of the results. Finally, while the very strong clinical and biological evidence supports the existence of FA as a distinct clinical phenomenon to be taken into consideration for its long-term complications, FA is not yet incorporated in the current diagnostic systems (i.e., DSM-5 or ICD-11), and lacks well-validated diagnostic criteria. Within this study, the diagnosis of FA relied on the results of the YFAS-2.0 questionnaire, consistent with the literature so far conducted on the topic. Although a recent debate consensus has emerged in support of the Yale Food Addiction Scale as a valid tool to evaluate addictive-like eating behaviors [83], present results certainly require contextualizing the diagnostic constraints.
On the other, it should be considered that the extended OGTT is not formally included in the diagnostic protocol for the diagnosis and treatment of obesity. Accordingly, the major strength of present study is the sample size consistent with the relative low application of the procedure. Further, the sample included male participants, which are usually underrepresented in study on eating disorders.
Conclusions
Taken together, present results suggest that the frequency of reactive hypoglycemia is higher in patients suffering from obesity and BED or FA and that the severity of food addiction influences early and symptomatic events, independent of metabolic, anthropometric, and demographic features. Hypothetical underlying mechanisms do not fit in explaining this phenomenon in the two groups, needing further investigations on the metabolic biomarkers associated with the glucose homeostasis. At the same time, our findings add to the understanding of the complex relationship between eating psychopathology, metabolic response, and addictive processes.
BED and FA are often associated with obesity, type 2 diabetes, and metabolic syndrome. From the perspective of clinician dealing with eating disorders, these results inform about the need to investigate hypoglycemia and hypoglycemia-related symptoms within the medical assessment. Hypoglycemia may represent an early risk factor for future metabolic complications and need to be assessed and addressed in the specific setting. Moreover, hypoglycemia has the potential to maintain disordered eating behaviours, affecting treatment outcomes.
On the other hand, eating disorders associated with obesity are often underdiagnosed and not addressed in obesity care settings, partly explaining failure of obesity interventions. BED and FA deserve a precise identification and need to be addressed to tailor nutritional and pharmacological treatments. For prevention, early detection, and tailored treatment, a multidisciplinary approach is then necessary.
Abbreviations
- BED:
-
Binge eating disorder
- FA:
-
Food addiction
- OGTT:
-
Oral glucose tolerance test
- BMI:
-
Body mass index
- GTP:
-
Glucose tolerance profile
- NGT:
-
Normal glucose tolerance
- IFG:
-
Impaired fasting glucose
- IFG:
-
Impaired glucose tolerance
- HOMA-IR:
-
Homeostatic model assessment insulin resistance index
- IGI:
-
Insulinogenic index
- DSM:
-
Diagnostic and statistical manual of mental disorders
- BES:
-
Binge eating scale
- YFAS:
-
Yale food addiction scale
References
American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®). 5th ed. American Psychiatric Pub; 2013.
Agüera Z, Paslakis G, Munguía L, Sánchez I, Granero R, Sánchez-González J, et al. Gender-related patterns of emotion regulation among patients with eating disorders. J Clin Med. 2019;8(2):161.
Burton AL, Abbott MJ. Conceptualising Binge Eating: A Review of the Theoretical and Empirical Literature. Vol. 34, Behaviour Change. Cambridge University Press; 2017. p. 168–98. https://doi.org/10.1017/bec.2017.12
Schulte EM, Grilo CM, Gearhardt AN. Shared and unique mechanisms underlying binge eating disorder and addictive disorders. Clin Psychol Rev. 2016;44:125–39.
Bąk-Sosnowska M. Differential criteria for binge eating disorder and food addiction in the context of causes and treatment of obesity. Psychiatr Pol. 2017;51(2):247–9.
di Giacomo E, Aliberti F, Pescatore F, Santorelli M, Pessina R, Placenti V, et al. Disentangling binge eating disorder and food addiction: a systematic review and meta-analysis. Eat Weight Disord. 2022;27(6):1963–70.
Meule A, Gearhardt AN. Food addiction in the light of DSM-5. Nutrients. 2014;6(9):3653–71.
Cathelain S, Brunault P, Ballon N, Réveillère C, Courtois R. Food addiction: Definition, measurement and limits of the concept, associated factors, therapeutic and clinical implications. Presse Med. 2016;45:1154–63.
Smith DG, Robbins TW. The neurobiological underpinnings of obesity and binge eating: a rationale for adopting the food addiction model. Biol Psychiatry. 2013;73(9):804–10.
Gearhardt AN, Yokum S, Orr PT, Stice E, Corbin WR, Brownell KD. Neural correlates of food addiction. Arch Gen Psychiatry. 2011;68(8):808–16.
Schulte EM, Avena NM, Gearhardt AN. Which foods may be addictive? The roles of processing, fat content, and glycemic load. PLoS ONE. 2015;10(2):e0117959.
Lennerz B, Lennerz JK. Food addiction, high-glycemic-index carbohydrates, and obesity. Clin Chem. 2018;64:64–71.
Allison S, Timmerman GM. Anatomy of a binge: food environment and characteristics of nonpurge binge episodes. Eat Behav. 2007;8(1):31–8.
Goodman EL, Breithaupt L, Watson HJ, Peat CM, Baker JH, Bulik CM, et al. Sweet taste preference in binge-eating disorder: a preliminary investigation. Eat Behav. 2018;1(28):8–15.
Raymond NC, Bartholome LT, Lee SS, Peterson RE, Raatz SK. A comparison of energy intake and food selection during laboratory binge eating episodes in obese women with and without a binge eating disorder diagnosis. Int J Eat Disord. 2007;40(1):67–71.
Rodin J, Wack J, Ferrannini E, DeFronzo RA. Effect of insulin and glucose on feeding behavior. Metabolism. 1985;34(9):826–31.
Brody S, Keller U, Degen L, Cox DJ, Schächinger H. Selective processing of food words during insulin-induced hypoglycemia in healthy humans. Psychopharmacology. 2004;173(1):217–20.
Schultes B, Peters A, Kern W, Gais S, Oltmanns KM, Fehm HL, et al. Processing of food stimuli is selectively enhanced during insulin-induced hypoglycemia in healthy men. Psychoneuroendocrinology. 2005;30(5):496–504.
Seaquist ER, Anderson J, Childs B, Cryer P, Dagogo-Jack S, Fish L, et al. Hypoglycemia and diabetes: A report of a workgroup of the american diabetes association and the endocrine society. J Clin Endocrinol Metab. 2013;98(5):1845–59.
Kishimoto I, Ohashi A. Subclinical reactive hypoglycemia is associated with higher eating and snacking frequencies in obese or overweight men without diabetes. Endocrines. 2022;3(3):530–7.
Hudson JI, Hiripi E, Pope HG, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61(3):348–58.
Udo T, Grilo CM. Prevalence and correlates of DSM-5-defined eating disorders in a nationally representative sample of U.S. adults. Biol Psychiatry. 2018;84(5):345–54.
Abraham TM, Massaro JM, Hoffmann U, Yanovski JA, Fox CS. Metabolic characterization of adults with binge eating in the general population: the Framingham Heart Study. Obesity (Silver Spring). 2014;22:2441–9.
Appolinario JC, Sichieri R, Lopes CS, Moraes CE, da Veiga GV, Freitas S, et al. Correlates and impact of DSM-5 binge eating disorder, bulimia nervosa and recurrent binge eating: a representative population survey in a middle-income country. Soc Psychiatry Psychiatr Epidemiol. 2022;57(7):1491–503.
Milano W, Carizzone F, De Biasio V, Angela Mercorio M, Francesca Milano M, Saetta B, et al. Neurobiological correlates shared between obesity, BED and food addiction. Endocr Metab Immune Disord Drug Targets. 2023;23(3):283–93.
Gearhardt AN, White MA, Masheb RM, Morgan PT, Crosby RD, Grilo CM. An examination of the food addiction construct in obese patients with binge eating disorder. Int J Eat Disord. 2012;45(5):657–63.
Pursey K, Stanwell P, Gearhardt A, Collins C, Burrows T. The prevalence of food addiction as assessed by the yale food addiction scale: a systematic review. Nutrients. 2014;6(10):4552–90.
Penzenstadler L, Soares C, Karila L, Khazaal Y. Systematic review of food addiction as measured with the Yale Food Addiction Scale: implications for the food addiction construct. Curr Neuropharmacol. 2018;17(6):526–38.
Camacho-Barcia L, Munguía L, Lucas I, de la Torre R, Salas-Salvadó J, Pintó X, et al. Metabolic, affective and neurocognitive characterization of metabolic syndrome patients with and without food addiction. Implications for weight progression. Nutrients. 2021;13(8):2779.
Guerrero Pérez F, Sánchez-González J, Sánchez I, Jiménez-Murcia S, Granero R, Simó-Servat A, et al. Food addiction and preoperative weight loss achievement in patients seeking bariatric surgery. Eur Eat Disord Rev. 2018;26(6):645–56.
Horsager C, Bruun JM, Færk E, Hagstrøm S, Lauritsen MB, Østergaard SD. Food addiction is strongly associated with type 2 diabetes. Clin Nutr. 2023;42(5):717–21.
Constant A, Moirand R, Thibault R, Val-Laillet D. Meeting of minds around food addiction: insights from addiction medicine, nutrition, psychology, and neurosciences. Nutrients. 2020;12:3564.
Römer SS, Bliokas V, Teo JT, Thomas SJ. Food addiction, hormones and blood biomarkers in humans: a systematic literature review. Appetite. 2023;183:106475.
World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA - J Am Med Assoc U.S.; 2013;2191–4.
Cryer PE, Axelrod L, Grossman AB, Heller SR, Montori VM, Seaquist ER, et al. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2009;94(3):709–28.
Genuth S, Alberti KGMM, Bennett P, Buse J, DeFronzo R, Kahn R, et al. Follow-up Report on the Diagnosis of Diabetes Mellitus. Diabetes Care. 2003;26:3160–8.
Phillips DIW, Clark PM, Hales CN, Osmond C. Understanding oral glucose tolerance: comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabet Med. 1994;11(3):286–92.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. https://doi.org/10.1007/BF00280883.
Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–70.
First MB, Williams JB, Karg RS, Spitzer RL. Structured clinical interview for DSM-5 disorders, clinician version (SCID-5-CV). Am Psychiatr Assoc. 2016;94:1.
Vasiliu O. Current status of evidence for a new diagnosis: food addiction-a literature review. Front Psychiatry. 2022;12:2545.
Gormally J, Black S, Daston S, Rardin D. The assessment of binge eating severity among obese persons. Addict Behav. 1982;7(1):47–55.
DiBernardo M, Barciulli E, Ricca V, Mannucci E, Moretti S, Cabras PL, et al. Validazione della versione italiana della Binge Eating Scale in pazienti obesi. [Binge Eating Scale in obese patients: Validation of the Italian version]. Minerva Psichiatr. 1998;39:125–30.
Imperatori C, Innamorati M, Lamis DA, Contardi A, Continisio M, Castelnuovo G, et al. Factor structure of the binge eating scale in a large sample of obese and overweight patients attending low energy diet therapy. Eur Eat Disord Rev. 2016;24(2):174–8.
Aloi M, Rania M, Rodríguez Muñoz RC, Jiménez Murcia S, Fernández-Aranda F, De Fazio P, et al. Validation of the Italian version of the Yale Food Addiction Scale 2.0 (I-YFAS 2.0) in a sample of undergraduate students. Eat Weight Disord. 2017;22(3):1.
Gearhardt AN, Corbin WR, Brownell KD. Development of the Yale Food Addiction Scale Version 2.0. Psychol Addict Behav. 2016;30(1):1.
Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, MI: Lawrence Erlbaum Associates Inc.; 1988.
Kittah NE, Vella A. Management of endocrine disease: pathogenesis and management of hypoglycemia. Eur J Endocrinol. 2017;177(1):37–47.
Hall M, Walicka M, Panczyk M, Traczyk I. Metabolic parameters in patients with suspected reactive hypoglycemia. J Pers Med. 2021;11(4):1.
Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high-risk state for diabetes development. Lancet (Lond England). 2012;379(9833):2279–90.
Brun JF, Fedou C, Mercier J. Postprandial Reactive Hypoglycemia. Sisli Etfal Hastan tip Bul. 2019;53(3):337–51.
Lv X, Fang K, Hao W, Han Y, Yang N, Yu Q. Identification of Reactive Hypoglycemia with Different Basic BMI and Its Causes by Prolonged Oral Glucose Tolerance Test. 2020. https://doi.org/10.2147/DMSO.S280084.
Mitrakou A, Ryan C, Veneman T, Mokan M, Jenssen T, Kiss I, et al. Hierarchy of glycemic thresholds for counterregulatory hormone secretion, symptoms, and cerebral dysfunction. Physiol-Endocrinol Metab. 1991;260(1):67–74. https://doi.org/10.1152/ajpendo.1991.260.1.E67.
Standards of Medical Care in Diabetes-2017: Summary of Revisions. Diabetes Care. 2017;40(Suppl 1). https://pubmed.ncbi.nlm.nih.gov/27979887/
Armaghanian N, Hetherington J, Parameswaran V, Chua EL, Markovic TP, Brand-Miller J, et al. Hypoglycemia in cystic fibrosis during an extended oral glucose tolerance test. Pediatr Pulmonol. 2020;55(12):3391–9.
Mumm H, Altinok ML, Henriksen JE, Ravn P, Glintborg D, Andersen M. Prevalence and possible mechanisms of reactive hypoglycemia in polycystic ovary syndrome. Hum Reprod. 2016;31(5):1105–12.
Lv X, Fang K, Hao W, Han Y, Yang N, Yu Q. Identification of reactive hypoglycemia with different basic BMI and its causes by prolonged oral glucose tolerance test. Diabetes Metab Syndr Obes. 2020;13:4717–26.
Tang Q, Li X, Song P, Xu L. Optimal cut-off values for the homeostasis model assessment of insulin resistance (HOMA-IR) and pre-diabetes screening: developments in research and prospects for the future. Drug Discov Ther. 2015;9(6):380–5.
Succurro E, Segura-Garcia C, Ruffo M, Caroleo M, Rania M, Aloi M, et al. Obese patients with a binge eating disorder have an unfavorable metabolic and inflammatory profile. Med (United States). 2015;94(52):1.
Chao A, Grey M, Whittemore R, Reuning-Scherer J, Grilo CM, Sinha R. Examining the mediating roles of binge eating and emotional eating in the relationships between stress and metabolic abnormalities. J Behav Med. 2016;39(2):320.
Hudson JI, Lalonde JK, Coit CE, Tsuang MT, McElroy SL, Crow SJ, et al. Longitudinal study of the diagnosis of components of the metabolic syndrome in individuals with binge-eating disorder. Am J Clin Nutr. 2010;91(6):1568–73.
Thornton LM, Watson HJ, Jangmo A, Welch E, Wiklund C, von Hausswolff-Juhlin Y, et al. Binge-eating disorder in the Swedish national registers: somatic comorbidity. Int J Eat Disord. 2017;50(1):58–65.
Nelder M, Cahill F, Zhang H, Zhai G, Gulliver W, Teng W, et al. The association between an addictive tendency toward food and metabolic characteristics in the general newfoundland population. Front Endocrinol (Lausanne). 2018;9(9):661.
Yang F, Liu A, Li Y, Lai Y, Wang G, Sun C, et al. Food addiction in patients with newly diagnosed type 2 diabetes in northeast China. Front Endocrinol (Lausanne). 2017;8:1.
Stojek MM, Maples-Keller JL, Dixon HD, Umpierrez GE, Gillespie CF, Michopoulos V. Associations of childhood trauma with food addiction and insulin resistance in African-American women with diabetes mellitus. Appetite. 2019;141:1.
Postprandial AY, Hypoglycemia R. Sisli Etfal Hastan tip Bul. 2019;53(3):215–20.
Brun JF, Fedou C, Mercier J. Postprandial reactive hypoglycemia. Diabetes Metab. 2000;26:1.
Lupoli R, Cotugno M, Griffo E, Nosso G, Riccardi G, Capaldo B. Role of the entero-insular axis in the pathogenesis of idiopathic reactive hypoglycemia: a pilot study. J Clin Endocrinol Metab. 2015;100(12):4441–6.
Pedram P, Sun G. Hormonal and dietary characteristics in obese human subjects with and without food addiction. Nutrients. 2014;7(1):223–38.
Geliebter A, Hashim SA, Gluck ME. Appetite-related gut peptides, ghrelin, PYY, and GLP-1 in obese women with and without binge eating disorder (BED). Physiol Behav. 2008;94(5):696–9.
Hernandez D, Mehta N, Geliebter A. Meal-related acyl and des-acyl ghrelin and other appetite-related hormones in people with obesity and binge eating. Obesity (Silver Spring). 2019;27(4):629–35.
Bryant EJ, King NA, Falkén Y, Hellström PM, Juul Holst J, Blundell JE, et al. (2013) Relationships among tonic and episodic aspects of motivation to eat, gut peptides, and weight before and after bariatric surgery. Surg Obes Relat Dis. 2013;9(5):802–8.
Yu Y, Fernandez ID, Meng Y, Zhao W, Groth SW. Gut hormones, adipokines, and pro- and anti-inflammatory cytokines/markers in loss of control eating: a scoping review. Appetite. 2021;166:1.
Caroleo M, Carbone EA, Arcidiacono B, Greco M, Primerano A, Mirabelli M, et al. Does NUCB2/nesfatin-1 influence eating behaviors in obese patients with binge eating disorder? Toward a neurobiological pathway. Nutr. 2023;15(2):348.
Kishimoto I, Ohashi A. Lower glucose effectiveness is associated with subclinical reactive hypoglycemia, snacking habits, and obesity. Metabolites. 2023;13(2):1.
Page KA, Seo D, Belfort-DeAguiar R, Lacadie C, Dzuira J, Naik S, et al. Circulating glucose levels modulate neural control of desire for high-calorie foods in humans. J Clin Invest. 2011;121(10):4161–9.
Daws LC, Avison MJ, Robertson SD, Niswender KD, Galli A, Saunders C. Insulin signaling and addiction. Neuropharmacology. 2011;61(7):1123–8.
Kishimoto I, Ohashi A. Impact of lifestyle behaviors on postprandial hyperglycemia during continuous glucose monitoring in adult males with overweight/obesity but without diabetes. Nutrients. 2021;13(9):1.
Sprague JE, Arbeláez AM. Glucose counterregulatory responses to hypoglycemia. Pediatr Endocrinol Rev. 2011;9(1):463.
Ivezaj V, White MA, Grilo CM. Examining binge-eating disorder and food addiction in adults with overweight and obesity. Obesity. 2016;24(10):2064–9. https://doi.org/10.1002/oby.21607.
Jiménez-Murcia S, Agüera Z, Paslakis G, Munguia L, Granero R, Sánchez-González J, et al. Food addiction in eating disorders and obesity: analysis of clusters and implications for treatment. Nutrients. 2019;11(11):1.
Charles MA, Hofeldt F, Shackelford A, Waldeck N, Dodson LE, Bunker D, et al. Comparison of oral glucose tolerance tests and mixed meals in patients with apparent idiopathic postabsorptive hypoglycaemia. Absence of hypoglycemia after meals. Diabetes. 1981;30(6):1.
Gearhardt AN, Hebebrand J. The concept of “food addiction” helps inform the understanding of overeating and obesity: debate consensus. Am J Clin Nutrit. 2021;113:1.
Acknowledgements
Not applicable.
Funding
No fundings were received for this study.
Author information
Authors and Affiliations
Contributions
Conceptualization (ID 8b73531f-db56-4914–9502-4cc4d4d8ed73), Methodology (ID: f21e2be9-4e38-4ab7-8691-d6f72d5d5843), Project administration (ID: a693fe76-ea33-49ad-9dcc-5e4f3ac5f938): MR, CS-G, FA. Supervision (ID: 0c8ca7d4-06ad-4527-9cea-a8801fcb8746): CS-G, FA. Investigation (ID: 2451924d-425e-4778-9f4c-36c848ca70c2), Data curation (ID: f93e0f44-f2a4-4ea1-824a-4e0853b05c9d): MR, MC, MR, MCP, EAC, FC, IZ, MA. Formal analysis (ID: 95394cbd-4dc8-4735-b589-7e5f9e622b3f): MR, CS-G. Writing original draft (ID: 43ebbd94-98b4-42f1-866b-c930cef228ca): MR. Writing- review and editing (ID: d3aead86-f2a2-47f7-bb99-79de6421164d): MC, RdF, EAC, CS-G, PdF, FA.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Eligible patients were fully informed about the aim and the procedures of the study, and that participation was voluntary and free from any compensation. All participants gave written informed consent. The study was approved by the institutional review boards (Local Ethical Committee, n. 53/2013), and all the procedures were performed in accordance with the principles of the Declaration of Helsinki.
Consent for publication
All participants gave consent for publication.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file1: Figure S1
. Flow chart describing participants interruption of the glucose load for the events reactive hypoglycemia and attrition. Table S1. Comparison between participants completing the OGTT and drop-outs at various level of the glucose load.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rania, M., Caroleo, M., Carbone, E.A. et al. Reactive hypoglycemia in binge eating disorder, food addiction, and the comorbid phenotype: unravelling the metabolic drive to disordered eating behaviours. J Eat Disord 11, 162 (2023). https://doi.org/10.1186/s40337-023-00891-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40337-023-00891-z