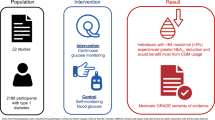
Abstract
Aims/hypothesis
Continuous glucose monitoring (CGM) is increasingly used in the treatment of type 2 diabetes, but the effects on glycaemic control are unclear. The aim of this systematic review and meta-analysis is to provide a comprehensive overview of the effect of CGM on glycaemic control in adults with type 2 diabetes.
Methods
We performed a systematic review using Embase, MEDLINE, Web of Science, Scopus and ClinicalTrials.gov from inception until 2 May 2023. We included RCTs investigating real-time CGM (rtCGM) or intermittently scanned CGM (isCGM) compared with self-monitoring of blood glucose (SMBG) in adults with type 2 diabetes. Studies with an intervention duration <6 weeks or investigating professional CGM, a combination of CGM and additional glucose-lowering treatment strategies or GlucoWatch were not eligible. Change in HbA1c and the CGM metrics time in range (TIR), time below range (TBR), time above range (TAR) and glycaemic variability were extracted. We evaluated the risk of bias using the Cochrane risk-of-bias tool version 2. Data were synthesised by performing a meta-analysis. We also explored the effects of CGM on severe hypoglycaemia and micro- and macrovascular complications.
Results
We found 12 RCTs comprising 1248 participants, with eight investigating rtCGM and four isCGM. Compared with SMBG, CGM use (rtCGM or isCGM) led to a mean difference (MD) in HbA1c of −3.43 mmol/mol (−0.31%; 95% CI −4.75, −2.11, p<0.00001, I2=15%; moderate certainty). This effect was comparable in studies that included individuals using insulin with or without oral agents (MD −3.27 mmol/mol [−0.30%]; 95% CI −6.22, −0.31, p=0.03, I2=55%), and individuals using oral agents only (MD −3.22 mmol/mol [−0.29%]; 95% CI −5.39, −1.05, p=0.004, I2=0%). Use of rtCGM showed a trend towards a larger effect (MD −3.95 mmol/mol [−0.36%]; 95% CI −5.46 to −2.44, p<0.00001, I2=0%) than use of isCGM (MD −1.79 mmol/mol [−0.16%]; 95% CI −5.28, 1.69, p=0.31, I2=64%). CGM was also associated with an increase in TIR (+6.36%; 95% CI +2.48, +10.24, p=0.001, I2=9%) and a decrease in TBR (−0.66%; 95% CI −1.21, −0.12, p=0.02, I2=45%), TAR (−5.86%; 95% CI −10.88, −0.84, p=0.02, I2=37%) and glycaemic variability (−1.47%; 95% CI −2.94, −0.01, p=0.05, I2=0%). Three studies reported one or more events of severe hypoglycaemia and macrovascular complications. In comparison with SMBG, CGM use led to a non-statistically significant difference in the incidence of severe hypoglycaemia (RR 0.66, 95% CI 0.15, 3.00, p=0.57, I2=0%) and macrovascular complications (RR 1.54, 95% CI 0.42, 5.72, p=0.52, I2=29%). No trials reported data on microvascular complications.
Conclusions/interpretation
CGM use compared with SMBG is associated with improvements in glycaemic control in adults with type 2 diabetes. However, all studies were open label. In addition, outcome data on incident severe hypoglycaemia and incident microvascular and macrovascular complications were scarce.
Registration
This systematic review was registered on PROSPERO (ID CRD42023418005).
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.

Introduction
Optimising glycaemic control is a keystone in the management of type 2 diabetes [1]. Fingerstick-based self-monitoring of blood glucose (SMBG) has been the most used method for measuring daily glucose levels [2]. However, this method does not provide continuous data about glucose levels, and, thus, may miss asymptomatic hypo- or hyperglycaemia and does not provide information about the direction of change in glucose levels. Furthermore, SMBG can be painful and increases disease burden [3].
The development of continuous glucose monitoring (CGM), either intermittently scanned CGM (isCGM) systems or real-time CGM (rtCGM) systems, has enabled the monitoring of glucose levels without fingersticks. CGM consists of a subcutaneous sensor that monitors interstitial glucose levels, which approximates blood glucose levels [3]. CGM thereby allows for direct observation of glycaemic excursions and daily glucose profiles that can inform therapy decisions and possibly adjust behaviours [4, 5]. Recent guidelines have recommended CGM use in individuals with type 2 diabetes treated with insulin [1]. However, the extent to which CGM improves glycaemic control in type 2 diabetes is unclear. Furthermore, it is unknown whether any such beneficial effect is different among individuals treated with or without insulin.
Glycaemic control is most often quantified by measurement of HbA1c levels [4], which reflects average glucose over the last 2–3 months. CGM provides additional parameters of glycaemic control, including time in range (TIR), time below range (TBR) and time above range (TAR). These parameters provide information about glucose control on a daily basis, and are increasingly used in clinical research and daily care [1, 4].
To date, there have been seven systematic reviews investigating the effect of CGM on glycaemic control in type 2 diabetes [6,7,8,9,10,11,12]. However, these reviews only included a limited number of RCTs, i.e. six studies or fewer, or included studies with a mixed population of both individuals with type 1 diabetes and individuals with type 2 diabetes. Furthermore, previous reviews could not conclude on whether the effect of CGM was different in individuals treated with or without insulin, and most reviews did not investigate the effect of CGM use on the sensor-derived glycaemic parameters TIR, TBR and TAR [8]. Also, no review evaluated the effect on the occurrence of severe hypoglycaemia or development of diabetes-related complications [6].
Therefore, the primary aim of this systematic review and meta-analysis was to give an up-to-date comprehensive overview of the effect of CGM (rtCGM or isCGM) compared with SMBG on glycaemic control, as quantified by HbA1c, in adults with type 2 diabetes treated with or without insulin. Secondary aims were to evaluate the effect of CGM use compared with SMBG on TIR, TAR, TBR, glycaemic variability, incident severe hypoglycaemia and incident diabetes-related micro- and macrovascular complications.
Methods
This systematic review was registered on PROSPERO (ID CRD42023418005) and is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement [13]. The PRISMA checklist is provided in electronic supplementary material (ESM) Table 1.
Data sources and searches
We searched Embase, MEDLINE (via PubMed), Web of Science, Scopus and ClinicalTrials.gov for relevant articles using a combination of the terms ‘diabetes mellitus type 2’, ‘continuous glucose monitoring’ and ‘HbA1c’ from inception until 2 May 2023. Details of the search are provided in ESM Table 2. Additionally, we did a manual search by reviewing the reference lists of all relevant articles identified and prior reviews and meta-analyses to identify any remaining articles. Two reviewers (MJ and TACMV) independently performed screening of titles/abstracts using Rayyan [14], and assessed the full texts for eligibility. Any discrepancies were discussed and resolved by a third reviewer (TTvS).
Study selection
Studies were eligible if they compared CGM (rtCGM or isCGM) to SMBG (or isCGM when rtCGM was the main intervention) and reported HbA1c as an outcome measure. We included RCTs with a minimum intervention period of 6 weeks of consecutive or intermittent use of CGM among adults with type 2 diabetes (irrespective of diabetes treatment) in an outpatient setting. We excluded studies with pregnant women or individuals with type 1 diabetes, studies that investigated GlucoWatch [15] or a professional CGM (pCGM) device (e.g. Abbott Freestyle Libre Pro IQ or Dexcom G6 Pro) or an intervention that consisted of CGM combined with an additional glucose-lowering treatment strategy.
Data extraction and quality assessment
Two reviewers (MJ and TACMV) independently extracted data from the included full-text articles using a standardised form. Disagreements were resolved by consensus or a third reviewer (TTvS). We extracted data on the study authors, year of publication, study design and follow-up duration, attrition rate, intervention type and duration, comparator type, inclusion and exclusion criteria, baseline characteristics (age, sex, diabetes duration and ethnicity), baseline insulin use and use of oral glucose-lowering drugs. In addition, we retrieved information on HbA1c, TIR, TBR, TAR and glycaemic variability (defined as coefficient of variation [CV]) at baseline and at the endpoint. Finally, data on the incidence of severe hypoglycaemia (as defined in the original publication) and the incidence of microvascular complications (retinopathy, nephropathy and neuropathy) or macrovascular complications (myocardial infarction, cardiovascular death, cerebrovascular disease or peripheral artery disease) at the endpoint. Authors were contacted in case of any missing information.
We used the Cochrane risk-of-bias tool version 2 (RoB 2) to assess risk of bias of the included trials [16]. This tool includes five domains: randomisation process, deviations from intended interventions, missing outcome data, measurement of the outcome and selection of the reported results. The quality of each RCT was assessed independently by two reviewers (MJ and TACMV). Any disagreements were resolved by consensus with a third reviewer (TTvS). Studies were rated as having high, moderate or low risk of bias. We labelled trials as low risk of bias if all five domains were scored as low risk of bias.
Data synthesis and analysis
The primary outcome was mean difference in HbA1c (% and mmol/mol) from baseline to study end and corresponding 95% CI. Secondary outcomes were TIR (percentage of time glucose was between 3.9–10 mmol/l [70–180 mg/dl]), TBR (percentage of time glucose <3.9 mmol/l [<70 mg/dl]), TAR (percentage of time glucose was >10 mmol/l [>180 mg/dl]), glycaemic variability (CV [%]), incident severe hypoglycaemia (RR and 95% CI), incident microvascular (retinopathy, nephropathy and neuropathy) and macrovascular complications (myocardial infarction, cardiovascular death, cerebrovascular disease and peripheral artery disease). When TIR, TBR or TAR were described as hours and minutes, values were converted to percentage of time. For glycaemic outcomes we extracted the mean change between groups from baseline to endpoint and the SD. Incident severe hypoglycaemia and complications outcomes were analysed as RR and corresponding 95% CI.
A meta-analysis with a pooled estimates and random effects model was performed in Review Manager version 5.4 [17, 18]. A p value of <0.05 was considered statistically significant. Heterogeneity was assessed using I2 and χ2. I2 values of 0% to 40%, 30% to 60%, 50% to 90% and 75% to 100% were interpreted as low, moderate, substantial and considerable heterogeneity, respectively [18]. Funnel plots were visually inspected and the Egger test was used to assess publication bias [19]. Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) was used to estimate the certainty of evidence [20].
For the primary outcome, we performed a predefined subgroup analysis considering CGM type (comparing rtCGM to isCGM, isCGM to SMBG, and rtCGM to SMBG). If possible, the main analysis was repeated stratified according to insulin use, median baseline HbA1c, median age, median diabetes duration, median intervention duration, the presence of micro- or macrovascular complications at baseline, sex (male vs female) and background glucose-lowering therapy (insulin users, oral agents users only, and mix of insulin and oral agent users).
Results
Search results
The initial search resulted in 8000 articles (ESM Fig. 1). After removal of duplicates, 3994 articles were screened for title and abstract, of which 3971 articles were excluded. Articles were mainly excluded due to incorrect study design (e.g. observational study or no relevant research question) (n=2111). The full texts of 23 studies were assessed, of which 11 were excluded because of incorrect study design (n=5), use of a device other than rtCGM or isCGM (n=4), study duplicate (n=1) or not studying individuals with type 2 diabetes (n=1). Finally, 12 RCTs were included. Corresponding authors of the publications of all 12 RCTs were contacted to obtain any missing data, and the investigators of five RCTs [21,22,23,24,25] provided additional data.
Characteristics of included trials
The 12 RCTs were published between 2008 and 2023 and included a total of 1248 participants. The sample size ranged from 25 to 224 participants (Table 1). Participants (43.3% female) had a mean age of 58.9 years and a mean diabetes duration of 14.7 years. The baseline HbA1c ranged from a mean of 61.1 mmol/mol (7.83%) to 77.9 mmol/mol (9.27%) (Table 1). Eleven trials had a two-arm open-label parallel group design [21,22,23,24,25,26,27,28,29,30,31]. One trial had a three-arm parallel group design [32], including arms with 1 week of CGM use at baseline only (first arm), 1 week of CGM use at both baseline and at the end of the study period after 12 weeks (second arm) and a control arm that did not use CGM (third arm) [32]. We only included data of the intervention arm with CGM use at both the start and study endpoint and control. Eight trials compared rtCGM to SMBG [22, 26,27,28,29,30,31,32] and four trials compared isCGM to SMBG [21, 23,24,25]. No studies compared rtCGM to isCGM. The intervention duration ranged from 10 to 34 weeks (Table 1). Five trials had an extended follow-up period of 24 to 52 weeks [21, 24, 29, 30, 32]. In seven trials, CGM was used continuously [21,22,23,24,25,26, 28], whereas five trials used intermittent wearing of the CGM ranging from two cycles of 2 days to continuous use [27, 29,30,31,32]. As the primary outcome, 10 trials had HbA1c [22,23,24, 26,27,28,29,30,31,32], one trial had TIR [21] and another trial had treatment satisfaction [25]. Four trials included participants on insulin therapy only [22, 23, 25, 28], three trials included participants on oral glucose-lowering medication only [24, 29, 32] and five trials included participants on both insulin and/or oral glucose-lowering medication [21, 26, 27, 30, 31].
Risk of bias
Four trials [21, 22, 24, 28] had an overall low risk of bias, whereas eight trials [23, 25,26,27, 29,30,31,32] had some concerns (ESM Fig. 2). As for the domain of ‘randomisation process’, three trials [25, 29, 30] were graded with some concerns due to lack of information about the randomisation and allocation concealment process. All trials were graded with low risk of bias for the domains ‘deviation from the intended interventions’, ‘missing outcome data’ and ‘measurement of the outcome’. As for the domain of ‘selection of the reported results’, seven trials [23, 25,26,27, 29, 31, 32] were graded with some concerns due to missing trial protocols and statistical analysis plan.
Primary outcome
Results of all included 12 trials [21,22,23,24,25,26,27,28,29,30,31,32] were pooled in the primary meta-analysis. The mean change in HbA1c level was −3.43 mmol/mol (−0.31%; 95% CI −4.75, −2.11, p<0.00001) in favour of the CGM group (Fig. 1). Heterogeneity was low (I2=15%) and we found no evidence of publication bias (ESM Fig. 3; Egger test, p=0.29). Using GRADE criteria, the HbA1c outcome was graded with moderate certainty (Table 2).
Subgroup analysis
In the eight trials on rtCGM (n=720) [22, 26,27,28,29,30,31,32], the pooled mean change in HbA1c level was −3.95 mmol/mol (−0.36%) (95% CI −5.46, −2.44, p<0.00001, I2=0%) in favour of the rtCGM group. In the four trials on isCGM (n=528) [21, 23,24,25], we found a non-significant mean change in HbA1c level of −1.79 mmol/mol (−0.16%; 95% CI −5.28, 1.69, p=0.31) in favour of the isCGM group with substantial heterogeneity (I2=64%).
Trials including participants all using insulin [22, 23, 25, 28] showed a mean change in HbA1c level of −3.27 mmol/mol (−0.30%; 95% CI −6.22, −0.31, p=0.03, I2=55%), trials including users of oral agents only [24, 29, 32] showed a mean change of −3.22 mmol/mol (−0.29%; 95% CI −5.39, −1.05, p=0.004, I2=0%) and studies including users of both insulin or oral agents [21, 26, 27, 30, 31] showed a mean change of −3.65 mmol/mol (−0.33%; 95% CI −6.14, −1.15, p=0.004, I2=21%) (Fig. 2). Subgroup analyses for baseline HbA1c, age, diabetes duration and intervention duration showed comparable change in HbA1c level in each subgroup (ESM Fig. 4–7). Subgroup analyses for sex, the presence of microvascular and macrovascular complications or background glucose-lowering therapy other than insulin at baseline were not possible due to lack of data.
Secondary outcomes
For TIR and TAR as the outcome, eight trials (n=937 participants) [21,22,23,24, 26, 28, 29, 32] could be included in a pooled analysis, for TBR nine trials (n=956 participants) [21,22,23,24, 26,27,28,29, 32], and for glycaemic variability (CV) five trials (n=658 participants) [22,23,24, 28, 32]. The mean change for TIR was +6.36% (95% CI +2.48, +10.24, p=0.001, I2=9%), for TBR −0.66% (95% CI −1.21, −0.12, p=0.02, I2=45%), for TAR −5.86% (95% CI −10.88, −0.84, p=0.02, I2=37%) (Fig. 3) and for glycaemic variability −1.47% (95% CI −2.94, −0.01, p=0.05, I2=0%), all in favour of the CGM group (ESM Fig. 8).
Nine studies [21,22,23,24,25, 28, 29, 31, 32] reported on severe hypoglycaemia, yet only three studies [21, 23, 28] reported one or more events in one of the groups and contributed to the meta-analysis. There was no statistically significant difference in the incidence of severe hypoglycaemia (RR 0.66, 95% CI 0.15, 3.00, p=0.57, I2=0%). In addition, based on three trials [21, 23, 28], there was no statistically significant difference in the incidence of macrovascular complications (RR 1.54, 95% CI 0.42, 5.72, p=0.52, I2=29%) in the CGM group compared with SMBG (ESM Fig. 9–10). No trials reported outcome data on microvascular complications.
Using GRADE criteria, the outcomes TIR and TAR were graded with moderate certainty. The outcomes TBR, glycaemic variability and severe hypoglycaemia were graded with low certainty, whereas outcome macrovascular complications was graded with very low certainty. Outcomes were downgraded partly because of inconsistency and the low number of events resulting in imprecision (Table 2).
Discussion
This systematic review and meta-analysis on the effect of CGM use (rtCGM or isCGM) on glycaemic control in adults with type 2 diabetes showed a modest reduction of −3.43 mmol/mol (−0.31%) in HbA1c. This effect was comparable among users of insulin and other oral agents. Furthermore, CGM was associated with a +6.36% increase in TIR and a decrease of −0.66% in TBR, −5.86% in TAR and −1.47% in glycaemic variability.
Our results are in accordance with previous systematic reviews and meta-analyses, which found a significant reduction in HbA1c ranging from −7.65 mmol/mol (−0.70%) to −2.73 mmol/mol (−0.25%) in individuals with type 2 diabetes [6,7,8,9,10, 12]. Moreover, our result is comparable with a systematic review showing a reduction of −2.46 mmol/mol (0.23%) in HbA1c with CGM use compared with SMBG in individuals with type 1 diabetes [33]. Our review extends previous reviews [6,7,8,9,10,11] because we could include an additional six RCTs including 589 participants, which allowed us to calculate a more precise effect size with higher statistical power. This also allowed us to evaluate the effect of CGM use in users of insulin and oral agents, according to CGM type (rtCGM and isCGM) and in relevant subgroups.
We found a reduction in HbA1c that was comparable between studies including both users of insulin and other oral agents. Current guidelines suggest CGM as a therapy strategy only in individuals with type 2 diabetes who use insulin [1]. Our findings, however, might support the efficacy of CGM in individuals with type 2 diabetes, irrespective of glucose-lowering therapy. CGM use may improve dosing of any glucose-lowering therapy (insulin and other oral agents) and/or stimulate a healthy lifestyle, and this may explain its beneficial effects on glycaemic control compared with SMBG [1].
In our analyses, we found a trend towards a larger reduction in HbA1c in studies investigating rtCGM rather than those investigating isCGM. This is in accordance with a previous systematic review that reported, in a subgroup analysis, a non-significant change in HbA1c in both type 1 diabetes or type 2 diabetes [11]. This finding might suggest that real-time techniques might provide additional benefit compared with techniques requiring intermittent scanning. However, no study directly compared rtCGM to isCGM in type 2 diabetes and this issue, therefore, requires further study.
The mean reduction in HbA1c of −3.43 mmol/mol (−0.31%) was relatively modest, but the effect was consistent across all included studies, indicating the robustness of the study findings. Furthermore, the effect was consistent across studies with younger and older individuals, short and long diabetes duration and higher or lower HbA1c at baseline. Also, consistent beneficial effects of CGM were found on other markers of glycaemic control, i.e. TIR, TBR, TAR and glycaemic variability [1]. The beneficial effect on TIR (+6.36%) was more than the current consensus of 5% minimal clinical relevant difference in TIR [34].
We found a non-significant decrease in the incidence of severe hypoglycaemia. This was, however, based on only three studies with a small number of events (eight events in total). Most studies that assessed severe hypoglycaemia reported no events in both groups. Therefore, we likely had insufficient power to detect a difference in incidence of severe hypoglycaemia. Furthermore, only three trials assessed macrovascular complications, with most events detected in one study [21]. This study was performed in people who had experienced a recent myocardial infarction. Thus, our aggregated results for macrovascular complications have limited generalisability and should be interpreted with caution. A reduction in HbA1c would likely translate to lower rates of diabetes-related complications in the long term, but this cannot be substantiated in this data.
Strengths of our analysis include the comprehensive overview of the effect of both rtCGM and isCGM on glycaemic control in adults with type 2 diabetes with different glucose-lowering therapies, and the analysis of multiple relevant glycaemic outcome parameters. In addition, our study findings are consistent with, and extend the results of, a recent meta-analysis [12]. We did a more recent search and identified one additional RCT [21]. Furthermore, we were able to do a large range of prespecified subgroup analyses. Limitations include the fact that we did not include quality of life as outcome. However, previous trials have demonstrated that CGM use in type 2 diabetes is associated with beneficial effects on quality of life compared with SMBG [25, 32]. In contrast to studies done in individuals with type 1 diabetes [33] we did not find different effects of glucose sensor use according to baseline HbA1c levels. However, we may not have had sufficient power to detect any differences related to baseline HbA1c, because all studies except one [24] had a baseline HbA1c value of 64 mmol/mol (8%) or higher. In addition, all RCTs were open label, the study duration of included RCTs was relatively short (maximum 52 weeks) and no or only limited data were available on incident severe hypoglycaemia and incident microvascular and macrovascular complications.
In conclusion, this systematic review and meta-analysis showed an improvement in HbA1c and other parameters of glycaemic control related to CGM use (rtCGM or isCGM) in adults with type 2 diabetes. Future studies are needed to compare the effect on glycaemic control of rtCGM to isCGM and assess the effect of CGM use on incident micro- and macrovascular complications.
Abbreviations
- CGM:
-
Continuous glucose monitoring
- CV:
-
Coefficient of variation
- GRADE:
-
Grading of Recommendations, Assessment, Development, and Evaluations
- isCGM:
-
Intermittently scanned continuous glucose monitoring
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-analysis
- SMBG:
-
Self-monitoring of blood glucose
- rtCGM:
-
Real-time continuous glucose monitoring
- TAR:
-
Time above range
- TBR:
-
Time below range
- TIR:
-
Time in range
References
Davies MJ, Aroda VR, Collins BS et al (2022) Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 65:1925–1966. https://doi.org/10.1007/s00125-022-05787-2
Czupryniak L, Barkai L, Bolgarska S et al (2014) Self-monitoring of blood glucose in diabetes: from evidence to clinical reality in Central and Eastern Europe - recommendations from the international Central-Eastern European expert group. Diabetes Technol Ther 16:460–475. https://doi.org/10.1089/dia.2013.0302
Edelman SV, Argento NB, Pettus J, Hirsch IB (2018) Clinical implications of real-time and intermittently scanned continuous glucose monitoring. Diabetes Care 41(11):2265–2274. https://doi.org/10.2337/dc18-1150
Battelino T, Alexander CM, Amiel SA et al (2023) Continuous glucose monitoring and metrics for clinical trials: an international consensus statement. Lancet Diabetes Endocrinol 11(1):42–57. https://doi.org/10.1016/S2213-8587(22)00319-9
Jackson MA, Ahmann A, Shah VN (2021) Type 2 diabetes and the use of real-time continuous glucose monitoring. Diabetes Technol Ther 23(S1):S27–S34. https://doi.org/10.1089/dia.2021.0007
Park C, Le QA (2018) The effectiveness of continuous glucose monitoring in patients with type 2 diabetes: a systematic review of literature and meta-analysis. Diabetes Technol Ther 20(9):613–621. https://doi.org/10.1089/dia.2018.0177
Poolsup N, Suksomboon N, Kyaw AM (2013) Systematic review and meta-analysis of the effectiveness of continuous glucose monitoring (CGM) on glucose control in diabetes. Diabetol Metab Syndr 5(1):1. https://doi.org/10.1186/1758-5996-5-39
Castellana M, Parisi C, Di Molfetta S et al (2020) Efficacy and safety of flash glucose monitoring in patients with type 1 and type 2 diabetes: a systematic review and meta-analysis. BMJ Open Diabetes Res Care 8(1):1–10. https://doi.org/10.1136/bmjdrc-2019-001092
Gandhi GY, Kovalaske M, Kudva Y et al (2011) Efficacy of continuous glucose monitoring in improving glycemic control and reducing hypoglycemia: a systematic review and meta-analysis of randomized trials. J Diabetes Sci Technol 5(4):952–965. https://doi.org/10.1177/193229681100500419
Janapala RN, Jayaraj JS, Fathima N et al (2019) Continuous glucose monitoring versus self-monitoring of blood glucose in type 2 diabetes mellitus: a systematic review with meta-analysis. Cureus 11(9):e5634. https://doi.org/10.7759/cureus.5634
Maiorino MI, Signoriello S, Maio A et al (2020) Effects of continuous glucose monitoring on metrics of glycemic control in diabetes: a systematic review with meta-analysis of randomized controlled trials. Diabetes Care 43(5):1146–1156. https://doi.org/10.2337/dc19-1459
Uhl S, Choure A, Rouse B, Loblack A, Reaven P (2023) Effectiveness of continuous glucose monitoring on metrics of glycemic control in type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab (November):1–13. https://doi.org/10.1210/clinem/dgad652
Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 6(7):e1000100. https://doi.org/10.1371/journal.pmed.1000100
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A (2016) Rayyan—a web and mobile app for systematic reviews. Syst Rev 5(1):210. https://doi.org/10.1186/s13643-016-0384-4
Tierney MJ, Tamada JA, Potts RO et al (2000) The GlucoWatch biographer: a frequent automatic and noninvasive glucose monitor. Ann Med 32(9):632–41. https://doi.org/10.3109/07853890009002034
Sterne JAC, Savović J, Page MJ et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ l4898. https://doi.org/10.1136/bmj.l4898
Review Manager Web (RevMan Web). Version 5.4.1. The Cochrane Collaboration, 2020. Available from revman.cochrane.org
Higgins JPT, Green S (2011) Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated 2022]. The Cochrane Collaboration. Available from: https://training.cochrane.org/handbook
Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. Br Med J 315(7109):629–634. https://doi.org/10.1136/bmj.315.7109.629
GRADEpro GDT (2020) GRADEpro Guideline Development Tool. In: McMaster University and Evidence Prime, Inc, Hamilton, Canada
Ajjan RA, Heller SR, Everett CC et al (2023) Multicenter randomized trial of intermittently scanned continuous glucose monitoring versus self-monitoring of blood glucose in individuals with type 2 diabetes and recent-onset acute myocardial infarction: results of the LIBERATES trial. Diabetes Care 46(2):441–449. https://doi.org/10.2337/dc22-1219
Beck RW, Riddlesworth TD, Ruedy K et al (2017) Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections. Ann Intern Med 167(6):365–374. https://doi.org/10.7326/M16-2855
Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline J-P, Rayman G (2017) Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther 8(1):55–73. https://doi.org/10.1007/s13300-016-0223-6
Wada E, Onoue T, Kobayashi T et al (2020) Flash glucose monitoring helps achieve better glycemic control than conventional self-monitoring of blood glucose in non-insulin-treated type 2 diabetes: a randomized controlled trial. BMJ Open Diabetes Res Care 8(1):10–17. https://doi.org/10.1136/bmjdrc-2019-001115
Yaron M, Roitman E, Aharon-Hananel G et al (2019) Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with type 2 diabetes. Diabetes Care 42(7):1178–1184. https://doi.org/10.2337/dc18-0166
Bergenstal RM, Mullen DM, Strock E, Johnson ML, Xi MX (2022) Randomized comparison of self-monitored blood glucose (BGM) versus continuous glucose monitoring (CGM) data to optimize glucose control in type 2 diabetes. J Diabetes Complications 36(3):108106. https://doi.org/10.1016/j.jdiacomp.2021.108106
Cosson E, Hamo-Tchatchouang E, Dufaitre-Patouraux L, Attali JR, Pariès J, Schaepelynck-Bélicar P (2009) Multicentre, randomised, controlled study of the impact of continuous sub-cutaneous glucose monitoring (GlucoDay®) on glycaemic control in type 1 and type 2 diabetes patients. Diabetes Metab 35(4):312–318. https://doi.org/10.1016/j.diabet.2009.02.006
Martens T, Beck RW, Bailey R et al (2021) Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA 325(22):2262–2272. https://doi.org/10.1001/jama.2021.7444
Price DA, Deng Q, Kipnes M, Beck SE (2021) Episodic real-time CGM use in adults with type 2 diabetes: results of a pilot randomized controlled trial. Diabetes Ther 12(7):2089–2099. https://doi.org/10.1007/s13300-021-01086-y
Vigersky RA, Fonda SJ, Chellappa M, Walker MS, Ehrhardt NM (2012) Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care 35(1):32–38. https://doi.org/10.2337/dc11-1438
Yoo HJ, An HG, Park SY et al (2008) Use of a real time continuous glucose monitoring system as a motivational device for poorly controlled type 2 diabetes. Diabetes Res Clin Pract 82(1):73–79. https://doi.org/10.1016/j.diabres.2008.06.015
Moon SJ, Kim KS, Lee WJ, Lee MY, Vigersky R, Park CY (2023) Efficacy of intermittent short-term use of a real-time continuous glucose monitoring system in non-insulin–treated patients with type 2 diabetes: a randomized controlled trial. Diabetes Obes Metab 25(1):110–120. https://doi.org/10.1111/dom.14852
Teo E, Hassan N, Tam W, Koh S (2022) Effectiveness of continuous glucose monitoring in maintaining glycaemic control among people with type 1 diabetes mellitus: a systematic review of randomised controlled trials and meta-analysis. Diabetologia 65(4):604–619. https://doi.org/10.1007/s00125-021-05648-4
Battelino T, Danne T, Bergenstal RM et al (2019) Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care 42(8):1593–1603. https://doi.org/10.2337/dci19-0028
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Acknowledgements
An altered version of the abstract was submitted to the Advanced Technologies & Treatments for Diabetes (ATTD) conference held in Florence, Italy, 6–9 March 2024.
Data availability
Data are available from the corresponding author upon request.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Authors’ relationships and activities
The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Contribution statement
All authors contributed to the systematic review protocol. MJ and TACMV conducted the systematic search, study selection, data extraction and risk assessment, with differences resolved by TTvS. MJ, TACMV and TTvS wrote the initial draft of the manuscript. All other authors contributed to the discussion and reviewed the manuscript. All authors critically revised the manuscript and approved the final version of the manuscript. TTvS and MJ are the guarantors of this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jancev, M., Vissers, T.A.C.M., Visseren, F.L.J. et al. Continuous glucose monitoring in adults with type 2 diabetes: a systematic review and meta-analysis. Diabetologia 67, 798–810 (2024). https://doi.org/10.1007/s00125-024-06107-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-024-06107-6