Abstract
Background
Biologging technology has enhanced our understanding of the ecology of marine animals and has been central to identifying how oceanographic conditions drive patterns in their distribution and behavior. Among these environmental influences, there is increasing recognition of the impact of dissolved oxygen on the distribution of marine animals. Understanding of the impact of oxygen on vertical and horizontal movements would be advanced by contemporaneous in situ measurements of dissolved oxygen from animal-borne sensors instead of relying on environmental data that may not have appropriate spatial or temporal resolution. Here, we demonstrate the capabilities of dissolved oxygen pop-up satellite archival tags (DO-PATs) by presenting the results from calibration experiments and trial deployments of two prototype tags on bluntnose sixgill sharks (Hexanchus griseus).
Results
The DO-PATs provided fast, accurate, and stable measurements in calibration trials and demonstrated high correlation with vertical profiles obtained via traditional ship-borne oceanographic instruments. Deployments on bluntnose sixgill sharks recorded oxygen saturations as low as 9.4 % and effectively captured the oceanography of the region when compared with World Ocean Atlas 2013 values.
Conclusions
This is the first study to use an animal-borne device to autonomously measure and record in situ dissolved oxygen saturation from non-air-breathing marine animals. The DO-PATs maintained consistency over time and yielded measurements equivalent to industry standards for environmental sampling. Acquiring contemporaneous in situ measurements of dissolved oxygen saturation alongside temperature and depth data will greatly improve our ability to investigate the spatial ecology of marine animals and make informed predictions of the impacts of global climate change. The information returned from DO-PATs is relevant not only to the study of the ecology of marine animals but will also become a useful new tool for investigating the physical structure of the oceans.
Similar content being viewed by others
Background
Advances in biologging and biotelemetry technologies have enhanced our understanding of the ecology of marine animals and have been central to identifying how oceanographic features influence their distribution and behavior [1–3]. Given the changes in ocean conditions that are predicted to occur in coming decades, understanding the interplay between physical and biological phenomena is becoming increasingly important [3, 4]. Data obtained from biologging devices (tags) attached to free-ranging animals have the significant advantage that they are recorded at a scale and resolution that are pertinent to and contemporaneous with the movement and behavior of the tagged animal [2, 5]. Moreover, data collected by modern biologging sensors are often equivalent to industry standards for environmental sampling [2, 3, 5].
It is becoming increasingly clear that dissolved oxygen concentrations have a significant impact on the distribution of marine animals—especially in the vertical dimension [6–11]. However, even some species with high metabolic demands seem to have remarkable abilities to penetrate waters with low oxygen concentrations [12–15]. Our understanding of the impacts of oxygen concentrations on species distribution and of how animals deal with hypoxic conditions would be significantly advanced by contemporaneous in situ measurements of dissolved oxygen obtained from animal-borne sensors [2, 13, 16, 17]. Currently, dissolved oxygen properties of waters inhabited by tracked animals are obtained from ship-based measurements [12, 15, 18], autonomous measuring platforms [19], and model-derived climatological data [7, 13, 14, 20], which may not have appropriate spatial or temporal resolution. Therefore, animal-borne sensors have the potential to reveal fine-scale habitat selection strategies that would not otherwise be apparent. Enhanced information on how behaviors are influenced by water column dissolved oxygen profiles will also help define possible barriers to movement and improve predictions of potential shifts in distribution associated with climate change [7, 21]. In addition, the concept of ‘animals as oceanographers’ has gained considerable traction and incorporating oxygen-sensing abilities into tags carried by fishes would represent a significant step forward in using free-ranging animals to collect physical oceanographic data. Here, we present the results of the first successful deployment of fish-borne tags capable of measuring and recording in situ dissolved oxygen saturations.
Over the past decade, pop-up satellite archival tag (PAT) technologies have facilitated an enhanced understanding of spatial ecology and environmental preferences for a number of large marine animals [11, 22, 23]. Here we demonstrate the capabilities of a prototype dissolved oxygen pop-up satellite archival tag (DO-PAT) by presenting the results from calibration experiments and trial deployments on bluntnose sixgill sharks (Hexanchus griseus). The bluntnose sixgill shark is a vertically migrating species that represents an ideal platform for testing the ability of animal-borne tags to sample dissolved oxygen gradients over a wide range of depths and temperatures [18]. Our results indicate that the information returned from DO-PATs is capable not only of advancing the study of the physiological ecology of marine animals but also of becoming a useful new tool for investigating the physical structure of the oceans [24–27].
Methods
Dissolved oxygen pop-up satellite archival tag (DO-PAT)
The DO-PAT consists of a pop–up satellite archival tag (Mk10-PAT, Wildlife Computers, Redmond, WA, USA) equipped with a micro dissolved oxygen probe (Loligo Systems, Tjele, Denmark) (see Additional file 1). The DO-PAT is 170 mm in length with a central tag body diameter of 20 mm attached to a 60-mm float at its widest point and the micro dissolved oxygen probe extends 16 mm from the float with a diameter of 12 mm. The tag has a total weight of 85 g in air. The DO-PAT measures and archives pressure, temperature, and dissolved oxygen saturation at a user-designated interval. For these deployments, we sampled these parameters at a rate of once per second. The micro dissolved oxygen probe comprises a small (12 mm × 27 mm), temperature self-compensating galvanic cell oxygen sensor. The galvanic cell consists of a metal cathode suitable for the electrolytic reduction of oxygen, a lead anode, and electrolyte solution. The galvanic cell and electrolyte solution are isolated from the environment by a thin, semipermeable membrane. As dissolved oxygen diffuses across the membrane, the sensor utilizes the linear relationship between oxygen saturation and electric current passing between the cathode and anode. Percent oxygen saturation is the amount of dissolved oxygen compared to the theoretical 100 % saturation for any given temperature and depth. Therefore, a thermistor for temperature compensation is included in the electrode circuitry. Salinity also has an effect on oxygen saturation but to a lesser extent. Salinity in Hawaii fluctuates by approximately 1 PSU from the surface to 1000 m (Hawaii Ocean Time-series, HOT [28]) and therefore has a negligible effect in the present study. A 10-bit analog-to-digital converter encodes the sensor output voltage (mV) with values scaled from 0 to 1023. These output values were stored in the data archive of the tag. Sensor output values obtained from the tag were subsequently converted to percent oxygen saturation using a linear calibration derived from a 100 % oxygen saturated environment. The sensor has a manufacturer-specified measuring range of 0–200 % with accuracy within ±1 % of the measured value. The membrane and electrolyte solution were replaced prior to each use and the micro dissolved oxygen probes were calibrated before deployment and after recovery.
Calibration experiments
Calibration of dissolved oxygen saturation measured by two DO-PATs was conducted by comparing their performance with a YSI 6600 V2 data sonde (YSI Incorporated, Yellow Springs, OH, USA) in a sealed water table. Dissolved oxygen saturation was controlled by bubbling of nitrogen or oxygen gas at a constant temperature (27.9 ± 0.2 °C) and salinity (34.84 ± 0.06 ppt). Potential sensor drift was determined by placing the DO-PATs and the YSI data sonde in an open circuit, flow-through water table over a 13-day period. Oxygen saturations recorded by the DO-PATs and the sonde were compared to determine if there was any systematic bias or drift in the micro dissolved oxygen sensor. The effect of orientation on the oxygen probe was evaluated by altering the sensor angle (0°, 90°, and 180°) relative to the direction of flow.
To evaluate performance in the field, the DO-PATs were attached to a conductivity-temperature-depth (CTD) recorder (SBE 17plus V2, Sea-Bird Electronics, Bellevue, WA, USA) equipped with a SBE 43 dissolved oxygen sensor (Sea-Bird Electronics, Bellevue, WA, USA). Two vertical water column CTD profiles to 150 and 500 m of depth were conducted off Honolulu Harbor, Oahu, Hawaii (21.28°N, 157.87°W). Since dissolved oxygen measurements need to be taken during transit through a vertical oxygen and temperature gradient, the response time for the sensor to come into equilibrium with the ambient environment is a critical factor. To assess tag response time and evaluate whether corrections should be applied to oxygen saturation data subsequently acquired from free-ranging animals, measurements from both DO-PATs were synchronized with the CTD and then observed over successive 1-s time offsets relative to the CTD for a range of 0–60 s. The DO-PATs and CTD were compared by taking the absolute difference in oxygen saturations measured at a range of depths.
Field trials with sharks
Following calibration testing, the DO-PATs were deployed on two bluntnose sixgill sharks (HG1, HG2) in September and November 2014. Sharks were captured using demersal longlines set off Kaneohe Bay, Oahu, Hawaii (21.46°N, 157.80°W) (for additional details of longline fishing methods see Holland et al. [29]). The DO-PATs were attached to the sharks by a 15-cm segment of 136 kg monofilament line (300 lb test Extra-hard Hi-catch, Momoi Manufacturing, Kobe, Japan) coated with heat shrink tubing connected to a urethane dart (47 mm × 13 mm) inserted into the dorsal musculature and were programmed to detach from the study animal and float to the surface after a period of 90 h. Once at the surface, DO-PATs make repeated transmissions to the Argos satellite system that allows the position of the tag to be estimated. These same Argos transmissions were used as a homing beacon to facilitate physical recovery of the tags. The comparatively short 90-h deployments were chosen to minimize the potential distance traveled by the tagged animals and thereby maximize chances of tag recovery for post hoc analysis and calibration. Following recovery, the archived environmental data (recorded at 1-s intervals) were used to construct time series profiles of depth, temperature, and dissolved oxygen saturation. There was some noise associated with the oxygen data from the first tag deployed (probably due to an intermittently faulty sensor component), which required the application of a local regression (LOESS) smoothing algorithm with a 5-s window to the oxygen saturation data using MATLAB (The Mathworks Inc., Natick, MA, USA). This method reduces the influence of outliers by using a weighted linear least-squares average with a second-degree polynomial model. This algorithm was not needed for the data from the second tag deployed. Vertical profiles of oxygen saturation were generated for each 90-h deployment by averaging all of the values recorded for each 1 m depth bin. These profiles were compared with data from the nearest World Ocean Atlas 2013 (WOA13) [30] sampling site (21.5°N, 157.5°W—see Additional file 2). The WOA13 provides global, 1° gridded, objectively analyzed climatological fields of environmental parameters. However, due to the limited availability of local seasonal and monthly WOA13 climatological data, we compared the DO-PAT data with WOA13 annual percent oxygen saturation (statistical mean) across WOA13 standard depth levels from the surface to maximum depth encountered by the sharks.
Results
Dissolved oxygen calibration
In sealed water table tests, the DO-PAT and YSI data sonde oxygen saturation measurements were very similar and displayed a strong linear relationship. Correlation analysis resulted in coefficient values (r) ranging from 0.994 to 0.998; slopes ranged from 0.99 to 1.02; intercepts ranged from −1.60 to 2.55. Furthermore, there was no evidence of any systematic bias or drift in the DO-PAT oxygen saturation data over a period of 13 days (see Additional file 3). The mean difference (±standard deviation) between DO-PAT and YSI data sonde oxygen saturation data over time was 0.3 ± 4.4 %. In addition, sensor orientation had no effect on oxygen measurements except when rotated 180° relative to the direction of flow (i.e., ‘looking backwards’). This resulted in a decrease of percent oxygen saturation of 2.7 ± 0.6 % relative to the YSI data sonde. Since DO-PATs are towed behind the animal with the sensor facing forward, this effect does not present a problem for field studies (see Additional file 1).
The oxygen saturation measurements from the DO-PAT compared well with the CTD vertical profiles (Fig. 1). The mean absolute difference between the DO-PATs and the CTD was minimal (2.4 ± 1.0 %), approximately consistent with the manufacturer’s reported accuracy of ±1 %. Furthermore, a comparison of oxygen saturation recorded from the CTD and DO-PATs demonstrated no time lag characteristic for the micro dissolved oxygen probe over a range of delay offsets from 0 to 60 s.
Field deployments on sharks
Both DO-PATs were retrieved after their scheduled 90-h deployments, providing a total of 180 h of archival data from two bluntnose sixgill sharks (Table 1). The pop-up locations were 36.84 and 18.65 km from the points of release. Atypical vertical behavior occurred for a period of several hours following release before both animals established a regular diel vertical migration behavior pattern during which they encountered a wide range of physical water properties. For purposes of illustration, we show the results from the second deployment (HG2), which ranged between daytime depths of 687 m and nighttime depths of 274 m (Fig. 2). During these behaviors, in situ measurements from the tags indicated that this shark inhabited waters with oxygen saturations as low as 9.4 % (Fig. 3).
The LOESS smoothing algorithm effectively reduced the noise from the first deployment (HG1), resulting in the removal of outliers corresponding to <1 % of the entire archival oxygen record. The in situ data collected by the sharks were used to construct water column oxygen profiles between the surface and approximately 700 m depth. However, because the sharks did not move shallower than 200 m during the 90 h deployments, the oxygen values for depths shallower than 200 m were obtained from measurements made during the sharks’ initial descents following release and during the ascent of the tags when they detached from the animals and floated to the surface. The vertical profiles of oxygen saturation derived from DO-PATs were very similar to the vertical dissolved oxygen stratification recorded by the WOA13 (Fig. 4). Dissimilarities between the DO-PAT and WOA13 station data are to be expected due to the differences in time scale and the temporal separation between when the DO-PAT and WOA13 profiles were generated. Post-deployment calibrations of oxygen saturation demonstrated no systematic drift in oxygen sensor performance. We therefore conclude that the DO-PATs accurately recorded oxygen saturations at depths between the surface and 700 m for the duration of their deployments.
Discussion
This is the first study to use an animal-borne device to autonomously measure and record in situ dissolved oxygen from non-air-breathing marine animals and the first to do so using PAT technology. Priede et al. [31, 32] were the first to use a biotelemetry device for measuring ambient dissolved oxygen from free-ranging fish (sea trout, Salmo trutta) but these were acoustic transmitters that required a “chase boat” to follow the animal and record the transmitted data. Svendsen et al. [16] evaluated the performance of a modern acoustic dissolved oxygen transmitter equipped with the same micro dissolved oxygen sensor utilized in this study. Overall, their results demonstrated that the device was highly accurate over temperatures ranging from 10 to 30 °C and supported the use of the micro dissolved oxygen sensors in biotelemetry. Lefevre et al. [33] expanded on this by instrumenting striped catfish (Pangasianodon hypophthalmus) with acoustic dissolved oxygen transmitters (Thelma Biotel, Trondheim, Norway) in a small-scale, aquaculture pond.
Bailleul et al. [17] recently presented oxygen profiles from tags deployed on southern elephant seals (Mirounga leonina) and acquired using oxygen optodes (Aanderaa Data Instruments AS, Nesttun, Norway) incorporated into Argos CTD-SRDL tags (Argos-linked Conductivity-Temperature-Depth-Satellite Relayed Data Loggers, Sea Mammal Research Unit, University of St. Andrews, Scotland). Oxygen concentrations (μmol l−1) measured by the seals were consistent with those obtained from a concurrent ship survey. However, there was an offset of measurements in the upper mixed layer and after a period of 30 days there was temporal drift of the oxygen sensors. The offset of measurements in the mixed layer was most likely a result of the slow response time of the optode’s thermistor [17, 34–36].
The galvanic cell oxygen probe utilized in this study is ideally designed for biologging applications where small size, durability, and negligible power supply are crucial. In addition, the oxygen probe provided fast, accurate, and stable readings and were easily maintained. The DO-PATs reliably tracked changes in dissolved oxygen saturation during calibration trials and CTD vertical profiles. Comparison of these data with the requirements of the Argo-Oxygen program reveals that the accuracy of the sensors is within the recommended range [35]. Importantly, comparisons with WOA13 values demonstrate that the tags effectively captured the oceanography of the region in terms of oxygen profiles between the surface and depths of up to 700 m. The current results from two bluntnose sixgill sharks empirically demonstrated their ability to inhabit waters with very low oxygen content, which is consistent with the findings of Comfort and Weng [18] using data ascertained from HOT and World Ocean Database 2009 [37]. Because oxygen saturations can now be recorded contemporaneously with the behavior of the tagged animal, we believe this technology will lead to many future insights into the interplay between dissolved oxygen and the physiological ecology of marine animals—especially if accelerometry data are collected in synchrony with the oxygen data [3, 15].
In this study, measurements of dissolved oxygen saturation recorded by the DO-PAT were only available when the tag was physically recovered. This approach allowed post-recovery recalibration to assess the reliability of the collected data [24, 38]. However, tags capable of recording in situ oxygen saturations will probably find their maximum utility by transmitting environmental data remotely from free-ranging animals that are not recaptured or when the tag is not otherwise physically recovered. This scenario would allow data to be collected from a wider range of species, for longer deployment periods and from remote locations [5, 17, 26, 38, 39]. To accomplish this, future iterations of oxygen-sensing tags will need to utilize well-established data compression technologies for Argos transmission [39, 40] that will allow telemetry of oxygen profiles paired with light-based [41, 42] or GPS-based [43] geolocation estimates. The limited rate of data transfer through Argos satellites and tag energy constraints may not allow all data points to be transmitted [44]. Therefore, a subset of representative depth points with corresponding temperatures and dissolved oxygen saturations may need to be selected from the high-resolution archival record. Profiles could be selected for transmission using predefined depths or a broken-stick point selection algorithm [45, 46]. Since calibration coefficients may change during longer deployments, it may be advantageous to transmit the raw output values from the oxygen sensor so that the conversion to percent oxygen saturation can be calculated post hoc. Future integration of in situ salinity measurements [47] together with pressure, temperature, and dissolved oxygen saturation would allow on-board computation of actual dissolved oxygen concentrations.
The DO-PATs used in this study maintained consistency over time and made measurements that are equivalent to industry standards for environmental sampling. If sensors (i.e., tags) are not recovered, they cannot be recalibrated and hence require long-term stability [26]. In absence of regular ship-based measurements to check for sensor drift over time, the problems of possible long-term sensor drift may be obviated if the animals occasionally enter the upper mixed layer of the ocean where oxygen saturation is typically close to equilibrium with the atmosphere (i.e., 100 % saturation). If this is the case, appropriate corrections could be made to data recorded after these “recalibration” events. However, in more dynamic environments physical and biological processes may cause departures from equilibrium resulting in cycles of under- and supersaturation. An alternative is to look for oxygen trends at great depths, where we expect fairly stable values in oceanic regions with weak horizontal environmental gradients [48, 49]. For example, an Autonomous Profiling Explorer float (APEX, Teledyne Webb Research, North Falmouth, MA, USA) equipped with a SBE IDO electrochemical dissolved oxygen sensor (formerly SBE 43, Sea-Bird Electronics, Bellevue, WA, USA) deployed near a HOT site demonstrated very little sensor drift over a period of 3 years near the sea surface and at 2000 m [35]. The IDO sensor operates on the same principle as the galvanic cell used in the current experiments; thereby, demonstrating the promise of long-term stability of remote measurements of dissolved oxygen across a large vertical gradient.
Future laboratory and field experiments will evaluate sensor drift over longer deployments and other factors such as biofouling [50] and electrode ‘fatigue’ may have to be addressed in order to maximize the longevity of deployments [17, 36]. Lefevre et al. [33] used only the first 4–5 days of oxygen data due to the development of biofilm over the sensor membrane but in the current study, there was no biofilm on the oxygen probe membrane after each of the 90-h deployments on free-ranging sharks or when the tags were placed in an unfiltered, open circuit, flow-through water table for a period of 13 days. Whereas long-term deployments would be desirable for incorporation of data into oceanographic databases, from the perspective of the behavioral ecology of marine fishes, many important questions can be answered with deployments ranging from days to weeks.
We believe that the results from the current experiments indicate that DO-PATs attached to marine animals can be used to gather detailed oceanographic information of high temporal and spatial resolution. Furthermore, animal-borne in situ measurements of dissolved oxygen would overcome the spatial, temporal and cost limitations of using traditional ship-borne measurements in support of biologging studies [4, 26, 51]. Because the in situ data are recorded contemporaneously and at scales appropriate to the animal’s behavior and at locations chosen by the animal, these tags have the potential of revealing the circumstances under which oxygen limits distribution and whether animals have evolved mechanisms to exploit fine-scale features in the oxygen structure of the oceans [26, 52].
The inclusion of dissolved oxygen saturation measurements from animal-borne sensors could also play an important role in the study of global climate change [38]. Climate models driven by global warming conditions predict an overall decline in oceanic dissolved oxygen concentration and a consequent expansion of the oxygen minimum zone (OMZ) as a result of greater stratification, reduced ventilation below the thermocline, and decreased solubility at higher temperatures [53–55]. Currently, our limited knowledge of physiological and behavioral responses of individual species hinders modeling efforts on the effects of declining global oxygen or expanding OMZs on ecosystems [10]. For example, shoaling of the OMZ upper boundary may result in an expansion of habitat for hypoxia-tolerant species and a resulting vertical compression of habitat for those that reside above the OMZ [6, 9, 10, 53]. Vertical habitat compression will probably impact species with high oxygen requirements such as billfishes, tunas, and sharks [6, 8, 9]. Furthermore, the impacts of temperature and dissolved oxygen concentrations on marine organisms are inextricably linked [6] and reemphasizes the value of DO-PATs that obtain measurements of dissolved oxygen saturation alongside temperature and depth data. These measurements will greatly improve our ability to investigate the spatial ecology of marine animals in light of global climate change.
Conclusion
This study is the first to demonstrate that DO-PATs are able to autonomously collect and record dissolved oxygen data of a quality suitable for oceanographic studies. As such, this technology has the potential to play a major role in improving ocean-observing capabilities [5, 26, 44]. Oxygen-sensing tags such as those described here can enable major advances in understanding the distribution, behavior, and physiological ecology of marine animals in relation to their dynamic physical environment [2, 5, 27].
References
Rutz C, Hays GC. New frontiers in biologging science. Biol Lett. 2009;5(3):289–92.
Bograd SJ, Block BA, Costa DP, Godley BJ. Biologging technologies: new tools for conservation. Introd Endanger Species Res. 2010;10:1–7.
Payne NL, Taylor MD, Watanabe YY, Semmens JM. From physiology to physics: are we recognizing the flexibility of biologging tools? J Exp Biol. 2014;217(3):317–22.
Hooker SK, Biuw M, McConnell BJ, Miller PJO, Sparling CE. Bio-logging science: logging and relaying physical and biological data using animal-attached tags. Deep Sea Res Part II. 2007;54(3):177–82.
Costa DP, Block BA, Bograd S, Fedak MA, Gunn JS. TOPP as a marine life observatory: using electronic tags to monitor the movements, behaviour and habitats of marine vertebrates. Proc OceanObs. 2010;9:21–5.
Prince ED, Goodyear CP. Hypoxia-based habitat compression of tropical pelagic fishes. Fish Oceanogr. 2006;15(6):451–64.
Nasby-Lucas N, Dewar H, Lam CH, Goldman KJ, Domeier ML. White shark offshore habitat: a behavioral and environmental characterization of the eastern Pacific shared offshore foraging area. PLoS One. 2009;4(12):e8163.
Ekau W, Auel H, Pörtner HO, Gilbert D. Impacts of hypoxia on the structure and processes in pelagic communities (zooplankton, macro-invertebrates and fish). Biogeosciences. 2010;7(5):1669–99.
Stramma L, Prince ED, Schmidtko S, Luo J, Hoolihan JP, Visbeck M, et al. Expansion of oxygen minimum zones may reduce available habitat for tropical pelagic fishes. Nat Clim Change. 2012;2(1):33–7.
Gilly WF, Beman JM, Litvin SY, Robison BH. Oceanographic and biological effects of shoaling of the oxygen minimum zone. Ann Rev Mar Sci. 2013;5:393–420.
Braun CD, Kaplan MB, Horodysky AZ, Llopiz JK. Satellite telemetry reveals physical processes driving billfish behavior. Anim Biotelem. 2015;3(1):1–16.
Jorgensen SJ, Klimley AP, Muhlia-Melo AF. Scalloped hammerhead shark Sphyrna lewini, utilizes deep-water, hypoxic zone in the Gulf of California. J Fish Biol. 2009;74(7):1682–7.
Dewar H, Prince ED, Musyl MK, Brill RW, Sepulveda C, Luo J, et al. Movements and behaviors of swordfish in the Atlantic and Pacific Oceans examined using pop-up satellite archival tags. Fish Oceanogr. 2011;20(3):219–41.
Abecassis M, Dewar H, Hawn D, Polovina J. Modeling swordfish daytime vertical habitat in the North Pacific Ocean from pop-up archival tags. Mar Ecol Prog Ser. 2012;452:219–36.
Gilly WF, Zeidberg LD, Booth JA, Stewart JS, Marshall G, Abernathy K, et al. Locomotion and behavior of Humboldt squid, Dosidicus gigas, in relation to natural hypoxia in the Gulf of California, Mexico. J Exp Biol. 2012;215(18):3175–90.
Svendsen JC, Aarestrup K, Steffensen JF, Herskin J. A novel acoustic dissolved oxygen transmitter for fish telemetry. Mar Technol Soc J. 2006;40(1):103–8.
Bailleul F, Vacquie-Garcia J, Guinet C. Dissolved oxygen sensor in animal-borne instruments: an innovation for monitoring the health of oceans and investigating the functioning of marine ecosystems. PLoS One. 2015;10(7):e0132681.
Comfort CM, Weng KC. Vertical habitat and behaviour of the bluntnose sixgill shark in Hawaii. Deep Sea Res Part II. 2015;115:116–26.
Haulsee DE, Breece MW, Miller DC, Wetherbee BM, Fox DA, Oliver MJ. Habitat selection of a coastal shark species estimated from an autonomous underwater vehicle. Mar Ecol Prog Ser. 2015;528:277–88.
Lam CH, Kiefer DA, Domeier ML. Habitat characterization for striped marlin in the Pacific Ocean. Fish Res. 2015;166:80–91.
Hazen EL, Jorgensen S, Rykaczewski RR, Bograd SJ, Foley DG, Jonsen ID, et al. Predicted habitat shifts of Pacific top predators in a changing climate. Nat Clim Change. 2012;3(3):234–8.
Block BA, Jonsen ID, Jorgensen SJ, Winship AJ, Shaffer SA, Bograd SJ, et al. Tracking apex marine predator movements in a dynamic ocean. Nature. 2011;475(7354):86–90.
Hammerschlag N, Gallagher AJ, Lazarre DM. A review of shark satellite tagging studies. J Exp Mar Biol Ecol. 2011;398(1):1–8.
Boehlert GW, Costa DP, Crocker DE, Green P, O’Brien T, Levitus S, et al. Autonomous pinniped environmental samplers: using instrumented animals as oceanographic data collectors. J Atmos Oceanic Technol. 2001;18(11):1882–93.
Hooker SK, Boyd IL. Salinity sensors on seals: use of marine predators to carry CTD data loggers. Deep Sea Res Part I. 2003;50(7):927–39.
Fedak MA. Marine animals as platforms for oceanographic sampling: a “win/win” situation for biology and operational oceanography. Mem Natl Inst Polar Res. 2004;58:133–47.
Block BA. Physiological ecology in the 21st century: advancements in biologging science. Integr Comp Biol. 2005;45(2):305–20.
Karl DM, Lukas R. The Hawaii Ocean Time-series (HOT) program: background, rationale and field implementation. Deep Sea Res Part II. 1996;43(2):129–56.
Holland KN, Wetherbee BM, Lowe CG, Meyer CG. Movements of tiger sharks (Galeocerdo cuvier) in coastal Hawaiian waters. Mar Biol. 1999;134(4):665–73.
Garcia HE, Locarnini RA, Boyer TP, Antonov JI, Baranova OK, Zweng MM et al. World Ocean Atlas 2013, vol 3: dissolved oxygen, apparent oxygen utilization, and oxygen saturation. In: Levitus S, editor. NOAA Atlas NESDIS. 2014. p. 27.
Priede IG, Solbé JFDLG, Nott JE. Short communication: an acoustic oxygen telemetry transmitter for the study of exposure of fish to variations in environmental dissolved oxygen. J Exp Biol. 1988;140:563–7.
Priede IG, Solbé JFDLG, Nott JE, O’Grady KT, Cragg‐Hine D. Behaviour of adult Atlantic salmon, Salmo salar L., in the estuary of the River Ribble in relation to variations in dissolved oxygen and tidal flow. J Fish Biol. 1988;33(sA):133–9.
Lefevre S, Huong DTT, Ha NTK, Wang T, Phuong NT, Bayley M. A telemetry study of swimming depth and oxygen level in a Pangasius pond in the Mekong Delta. Aquaculture. 2011;315(3):410–3.
Tengberg A, Hovdenes J, Andersson JH, Brocandel O, Diaz R, Hebert D, et al. Evaluation of a lifetime-based optode to measure oxygen in aquatic systems. Limnol Oceanogr Methods. 2006;4:7–17.
Gruber N, Doney SC, Emerson SR, Gilbert D, Kobayashi T, Körtzinger A et al. The ARGO-oxygen program—a white paper to promote the addition of oxygen sensors to the international Argo float program. 2007. p. 1–60.
Coppola L, Salvetat F, Delauney L, Machoczek D, Karstensen J, Sparnocchia S et al. White paper on dissolved oxygen measurements: scientific needs and sensors accuracy. 2012. p. 1–22.
Boyer TP, Antonov JI, Baranova OK, Garcia HE, Johnson DR, Locarnini RA, et al. World Ocean database 2009. In: Levitus S, editor. NOAA Atlas NESDIS. Washington, D.C.: US Government Printing Office; 2009. p. 216.
McMahon CR, Autret E, Houghton JD, Lovell P, Myers AE, Hays GC. Animal-borne sensors successfully capture the real-time thermal properties of ocean basins. Limnol Oceanogr Methods. 2005;3(9):392–8.
Gunn J, Block B. Advances in acoustic, archival, and satellite tagging of tunas. In: Block BA, Stevens ED, editors. Tuna: physiology, ecology and evolution: fish physiology. San Diego, California: Academic Press; 2001. p. 167–224.
Block BA, Dewar H, Farwell C, Prince ED. A new satellite technology for tracking the movements of Atlantic bluefin tuna. Proc Natl Acad Sci. 1998;95(16):9384–9.
Musyl MK, Brill RW, Curran DS, Gunn JS, Hartog JR, Hill RD, et al. Ability of archival tags to provide estimates of geographical position based on light intensity. In: Sibert JR, Nielsen JL, editors. Electronic tagging and tracking in marine fisheries. Springer: Netherlands; 2001. p. 343–67.
Teo SL, Boustany A, Blackwell S, Walli A, Weng KC, Block BA. Validation of geolocation estimates based on light level and sea surface temperature from electronic tags. Mar Ecol Prog Ser. 2004;283:81–98.
Evans K, Baer H, Bryant E, Holland M, Rupley T, Wilcox C. Resolving estimation of movement in a vertically migrating pelagic fish: does GPS provide a solution? J Exp Mar Biol Ecol. 2011;398(1–2):9–17.
Boehme L, Lovell P, Biuw M, Roquet F, Nicholson J, Thorpe SE, et al. Technical note: animal-borne CTD-satellite relay data loggers for real-time oceanographic data collection. Ocean Sci. 2009;5(4):685–95.
Fedak MA, Lovell P, Grant SM. Two approaches to compressing and interpreting time-depth information as collected by time-depth recorders and satellite-linked data recorders. Mar Mamm Sci. 2001;17(1):94–110.
Fedak M, Lovell P, McConnell B, Hunter C. Overcoming the constraints of long range radio telemetry from animals: getting more useful data from smaller packages. Integr Comp Biol. 2002;42(1):3–10.
Luo J, Ault JS, Larkin MF, Barbieri LR. Salinity measurements from pop-up archival transmitting (PAT) tags and their application to geolocation estimation for Atlantic tarpon. Mar Ecol Prog Ser. 2008;357:101–9.
Bingham FM, Lukas R. Seasonal cycles of temperature, salinity and dissolved oxygen observed in the Hawaii Ocean Time-series. Deep Sea Res Part II. 1996;43(2):199–213.
Körtzinger A, Schimanski J, Send U. High quality oxygen measurements from profiling floats: a promising new technique. J Atmos Oceanic Technol. 2005;22(3):302–8.
Musyl MK, Domeier ML, Nasby-Lucas N, Brill RW, McNaughton LM, Swimmer JY, et al. Performance of pop-up satellite archival tags. Mar Ecol Prog Ser. 2011;433:1–28.
Keeling RE, Körtzinger A, Gruber N. Ocean deoxygenation in a warming world. Annu Rev Mar Sci. 2010;2:199–229.
Lydersen C, Nøst OA, Kovacs KM, Fedak MA. Temperature data from Norwegian and Russian waters of the northern Barents Sea collected by free-living ringed seals. J Mar Syst. 2004;46(1):99–108.
Stramma L, Johnson GC, Sprintall J, Mohrholz V. Expanding oxygen-minimum zones in the tropical oceans. Science. 2008;320(5876):655–8.
Stramma L, Schmidtko S, Levin LA, Johnson GC. Ocean oxygen minima expansions and their biological impacts. Deep Sea Res Part I. 2010;57(4):587–95.
Keller AA, Ciannelli L, Wakefield WW, Simon V, Barth JA, Pierce SD. Occurrence of demersal fishes in relation to near-bottom oxygen levels within the California Current large marine ecosystem. Fish Oceanogr. 2015;24(2):162–76.
Authors’ contributions
DMC participated in the design and coordination of the study, performed the statistical analyses and helped to draft the manuscript. KNH conceived of the study, participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We would like to thank C. Meyer, M. Royer, M. Hutchinson, J. Anderson, K. Bahr, C. Comfort, and S. Searson for their invaluable assistance with this project. We also thank Wildlife Computers for providing the prototype DO-PATs utilized in this study and for technical advice. Funding support was provided by PacIOOS (http://www.pacioos.org), which is a part of the U.S. Integrated Ocean Observing System (IOOS®), funded in part by National Oceanic and Atmospheric Administration (NOAA) Award #NA11NOS0120039. This work was carried out in accordance with the animal use protocols of the University of Hawaii (protocol #05-058).
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional files
40317_2015_88_MOESM1_ESM.pdf
Additional file 1. Dissolved oxygen pop-up satellite archival tag. DO-PAT deployed on a bluntnose sixgill shark (Hexanchus griseus). Inset: Picture of DO-PAT.
40317_2015_88_MOESM2_ESM.pdf
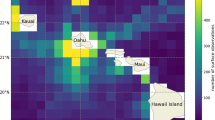
Additional file 2. Map of tagging location (square) of bluntnose sixgill sharks and pop-up locations (triangle). First DO-PAT deployment (HG1; black) straight-line distance travelled measures 36.84 km and 18.65 km for second deployment (HG2; white). Red circle denotes center value (21.5°N, 157.5°W) of nearest 1° grid cell of World Ocean Atlas 2013 oxygen data.
40317_2015_88_MOESM3_ESM.pdf
Additional file 3. DO-PAT drift analysis. (A) Comparison of oxygen saturation derived from YSI data sonde (red) and DO-PAT (blue) over 13-day period in an open-circuit, water table at 10 min sampling intervals. (B) Mean difference (±standard deviation) of DO-PAT oxygen saturation relative to YSI data sonde per day. Red dashed line denotes mean difference over entire 13-day period.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Coffey, D.M., Holland, K.N. First autonomous recording of in situ dissolved oxygen from free-ranging fish. Anim Biotelemetry 3, 47 (2015). https://doi.org/10.1186/s40317-015-0088-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40317-015-0088-x