Abstract
Background
Purified terephthalic acid (PTA) wastewater from a petrochemical complex was utilized as a fuel in the anode of a microbial fuel cell (MFC). Effects of two important parameters including different dilutions of the PTA wastewater and pH on the performance of the MFC were investigated.
Methods
The MFC used was a membrane-less single chamber consisted of a stainless steel mesh as anode electrode and a carbon cloth as cathode electrode. Both power density and current density were calculated based on the projected surface area of the cathode electrode. Power density curve method was used to specify maximum power density and internal resistance of the MFC.
Results
Using 10-times, 4-times and 2-times diluted wastewater as well as the raw wastewater resulted in the maximum power density of 10.5, 43.3, 55.5 and 65.6 mW m−2, respectively. The difference between the power densities at two successive concentrations of the wastewater was considerable in the ohmic resistance zone. It was also observed that voltage vs. initial wastewater concentration follows a Monod-type equation at a specific external resistance in the ohmic zone.
MFC performance at three different pH values (5.5, 7.0 and 8.5) was evaluated. The power generated at pH 8.5 was higher for 40% and 66% than that for pH 7.0 and pH 5.4, respectively.
Conclusions
The best performance of the examined MFC for industrial applications is achievable using the raw wastewater and under alkaline or neutralized condition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microbial fuel cell (MFC) is a device that converts biochemically released energy from bacterial catalysis of organic and inorganic materials into electrical energy. In MFCs, electricity generation and wastewater treatment can occur, simultaneously. Therefore, MFCs are considered as one of the potential solutions to overcome the crises of energy shortage and environmental pollution. On the other hand, many challenges are still remained for commercialization of MFC technology. Designing a cost-effective system with high power generation is one of the most important challenges. For example, in order to achieve a high performance, expensive materials such as platinum as cathodic catalyst, carbon cloth as cathode or anode electrode, and also a suitable membrane such as nafion are inevitably necessary. Accordingly, more studies are required to find efficient materials both in terms of cost and power production [1].
In recent years, this technology has been studied by many researchers. Generally, different parameters including both operational and designing factors affect the MFC performance. Nature of the substrate, substrate concentration [2], temperature [3], microorganisms’ species, alkalinity of anode and cathode chambers [4], external resistance [5] and residence time [6] are among the operational parameters. Anode and cathode material [7], type of membrane [8] and MFC architecture are among the designing parameters.
A wide range of different wastewaters have been examined in MFCs. For instance, domestic wastewater [3,9], landfill leachate [6], coking wastewater [2], confectionery wastewater [8] and cassava mill wastewater [10] can be mentioned. However, wastewaters from petrochemical industries have not been receiving much attention due to complications in their biodegradatiuon. Purified terephthalic acid (PTA) wastewater with a high strength organic content is generated during the production process. PTA is a raw material for manufacturing of many petrochemical products such as polyester textile fibers, polyethylene terephthalate bottles and polyester films. As much as 3–10 m3 of such a wastewater is usually generated per one ton of PTA as product [11].
In our previous work, we studied the feasibility of utilizing PTA wastewater in a MFC for the first time [12]. In the present work, we investigate the effect of two main characteristics of the wastewater i.e. organic load and pH value that significantly influence the power generation. In the present research, we investigated the following issues:
-
1)
Effect of wastewater concentration on power generation
-
2)
Correlation between voltage and wastewater concentration
-
3)
Effect of different pH values on power generation
Materials and methods
Wastewater and microorganisms
PTA wastewater was obtained from the PTA production plant of Shahid Tondgoyan Petrochemical Company, Mahshahr, Iran. It was kept at 4°C until use. This wastewater had the pH of 4.45 and pollution load of 8000 mg COD L−1. It consisted of following components with given concentrations (mg L−1): acetic acid (AA); 9850, benzoic acid (BA); 318, phthalic acid (PA); 400, terephthalic acid (TA); 389, p-toluic acid (p-Tol); 273, nitrate; 2234.7, and phosphate; 48.
Microorganisms, used in this research were obtained from the sludge of an up-flow anaerobic contact filter existing in the treatment plant of the abovementioned petrochemical company.
MFC assembly and operation
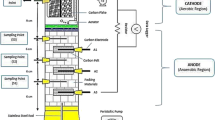
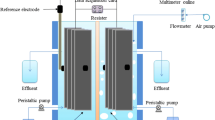
A typical membrane-less single chamber MFC as described previously [13] was used in this study (Figure 1). A stainless steel mesh (30 × 60 cm2) and a carbon cloth (30% w/w wet proofed (type B-1B, E-TEK) using platinum as catalyst (0.5 mg Pt cm−2) and four diffusion layers with projected area of 0.785 cm2) were used as anode and cathode electrodes, respectively. Content of the MFC was mixed gently by a magnetic stirrer to reach a uniform concentration.
Analytical methods and calculations
To measure the voltage, a digital multimeter (m58217, Mastech) was used at specific time intervals. Current was calculated based on Ohm’s law as I = V/R, where, I (mA), V (mV) and R (Ω) stand for current, voltage and external resistance, respectively. To calculate the current density, i = I/A was used where, A (m2) is the projected surface area of the cathode. Power density (P) was calculated using the following equation: P = IV/A. Reactions occurred in the cathode were considered as the basis to calculate current density and power density. To derive the polarization curve, the external resistance was changed from 400 KΩ to 300 Ω and the voltage was measured. Power density curve method was used to obtain maximum power density and internal resistance of the MFC [1]. Chemical oxygen demand (COD), nitrate, phosphate and pH were measured according to standard methods. The concentration of BA, PA, TA, p-Tol and AA was measured by a high performance liquid chromatography (HPLC, Agilent Technologies 1200 series, US) under isocratic conditions at the ambient temperature [12].
Results and discussion
Power production at different concentrations of wastewater
Effect of substrates’ concentration required for the microbial activity in the anode chamber was investigated. The substrate as fuel was supplied in the following order, consecutively: 10-times diluted wastewater (C1), 4-times diluted wastewater (C2), 2-times diluted wastewater (C3) and the raw wastewater (C4). Figure 2 shows power density curves of the MFC at different concentrations of the wastewater. Maximum power density was 10.5, 43.3, 55.5 and 65.6 mW m−2 for C1, C2, C3 and C4, respectively. Internal resistance of the MFC was 5.6 kΩ for all concentrations except for C3 that was equal to 3.2 kΩ.
Maximum power densities of C3, C2 and C1 wastewaters were 84%, 66% and 16% of the maximum power density of the raw wastewater. Therefore, the maximum power density increased when more concentrated wastewater was used.
Current generated in a MFC is limited by two factors: (i) oxidization rate of substrate by bacteria and (ii) rate of electrons transfer to the electrode surface [1]. Substrate oxidation rate depends on the concentration of substrate which is assumed usually as a first order reaction. Therefore, it is expected to observe a higher current and power at higher concentrations. However, other factors such as mass transfer and biofilm layer thickness can suppress power production. The influence of such factors can be studied when the substrate concentration is high enough where oxidation rate is not limited. This observation has been further explained in the next section.
Power differences at different external resistances between two successive concentrations
Increase in the power density due to employing more concentrated wastewater in the anode, was correlated to the external resistance. Figure 3 indicates the difference between the power densities at two successive concentrations of wastewater at definite external resistances. The maximum power density difference was 32.7, 11.9 and 18.3 mW m−2 between C2 - C1, C3 - C2, and C4 - C3, respectively.
According to the polarization curve, mass transfer resistance zone and activation loss zone are observable at low external resistances and large external resistances, respectively. All three curves follow the same trend. The power density difference was negligible at higher external resistances while the cell operated in the activation loss zone. This is the same at very low external resistances when the cell operates in the mass transfer zone. This shows that the substrate concentration has a minor effect in these zones. Accordingly, if a MFC is working with an external resistance which leads to operating, either in the activation loss zone or the mass transfer zone, increasing the substrate concentration would not have a significant effect on its performance. In contrary, a considerable power density difference was observed in the ohmic resistance zone. The power density difference reached a maximum value at an external resistance equal or close to the internal resistance of the MFC. Therefore, according to these observations, the maximum power production is reachable when the external resistance is near the internal resistance. Besides, the major positive effect of concentration increase is visible when the MFC works in the ohmic zone.
Generated voltage versus wastewater concentration in ohmic zone
In this section, the behavior of MFC in the ohmic zone is explored. The generated voltage at different concentration of wastewater for a certain external resistance (in the Ohmic zone) is exhibited in Figure 4. It was observed that the correlation between voltage and concentration is so that at higher concentrations, a little increase occurs in the voltage generation. For example, there is no significant difference between the produced, voltage when C3 or C4 was applied. One can say that the concentration increase effect is limited even in the ohmic zone which might be as a result of concentration inhibition that halts bacteria metabolism.
The voltages vs. concentration curves suggest a Monod-type equation as follows:
where, V (mV) and V max (mV) stand for voltage and maximum voltage, respectively; S (mg L-1) represents COD of substrates and K s (mg L−1) is the half-saturation constant. V max and K s were calculated for each curve as presented in Table 1.
Maximum achievable voltage versus external resistance follows a linear equation:
Not to be neglected that the above equation is valid only when the MFC operates at the ohmic zone.
Power production at different pH values
pH has a significant effect on the activity of bacteria in terms of removal efficiency and energy production. In order to study the influence of pH, the MFC was fed with 10- times diluted wastewater at three different pH values including 8.5, 7.0 and 5.4, periodically. These pH values were selected based on the optimal range of the pH reported for methane-producing bacteria. It has been observed that these bacteria are active in the pH range of 6.3-7.8 [14]. Presence of methane producers is very possible in our system. The power density curves for different pH values are shown in Figure 5. It was observed that the maximum power density was 12.5, 7.5 and 4.3 mW m−2 for the pH values of 8.5, 7.0 and 5.4, respectively.
In general, the higher the pH value, the higher the power density. The produced power at pH 8.5 was higher for 40% and 66% than that for pH 7.0 and pH 5.4, respectively. This observation is consistent with other previous studies [15,16].
Apparently, acidogenic bacteria are active in pH 5.5. Under this condition, hydrogen production would be the dominant mechanism which overcomes the pollutants degradation and a decreased removal rate is expected compared to the neutral or alkaline conditions [14]. Due to the low removal rate, fewer electrons are released and the power production is lowered, consequently. At pH 7.0, methane gas production is the dominant metabolic pathway. This would lead to a less number of released electrons that can contribute in electricity generation and a lower power density is observed, eventually. The increase in power density production at pH 8.5 might be due to the lower activity of methanogenic and acidogenic bacteria. As a result, the electrons released in the oxidation process of the substrates would contribute significantly in electricity generation. However, further studies are required to clarify the occurrence of these phenomena, more precisely.
It can be concluded from the trend of power production at different pH values that alkaline condition provides a favorable situation for the growth of electrogenic bacteria. Previous studies have shown that the electrochemical interaction of bacteria significantly increases under alkaline conditions [15,16], which ultimately leads to a higher power production.
Conclusion
The main purpose of this research was to provide more information and insight into the MFC operation that can pave the way towards practical utilization of MFC technology for the application of real wastewater. Bioelectricity generation using purified terephthalic acid wastewater from a petrochemical plant was successfully conducted in a single chamber microbial fuel cell with a stainless steel mesh as anode electrode.
The influence of wastewater concentration on the MFC performance showed that using the raw wastewater with the concentration of 8000 mg COD L−1 results in the highest power density (65.6 mW m−2). Our observations suggest that the best performance is achievable when the MFC operates at the ohmic zone and has an external resistance which is equal to the internal resistance of the cell.
The voltage against different initial concentrations of the wastewater in the ohmic zone followed a Monod-type equation. This observation implies that the concentration increase has a positive effect of electricity generation but it cannot exceed a maximum value.
Performance of the MFC at different pH values was investigated and the highest power density was observed under alkaline condition (pH 8.5) due to inactivation of acidogenic and methanogenic bacteria in favor of more activity for electrogenic bacteria.
References
Logan BE. Microbial Fuel Cells. NewJersy: John Wiley & Sons; 2008.
Huang L, Yang X, Quan X, Chen J, Yang F. A microbial fuel cell–electro-oxidation system for coking wastewater treatment and bioelectricity generation. J Chem Technol Biot. 2010;85:621–7.
Ahn Y, Logan BE. Effectiveness of domestic wastewater treatment using microbial fuel cells at ambient and mesophilic temperatures. Bioresour Technol. 2010;101:469–75.
Zhuang L, Zhou S, Li Y, Yuan Y. Enhanced performance of air-cathode two-chamber microbial fuel cells with high-pH anode and low-pH cathode. Bioresour Technol. 2010;101:3514–9.
Lyon DY, Buret F, Vogel TM, Monier J-M. Is resistance futile? Changing external resistance does not improve microbial fuel cell performance. Bioelectrochemistry. 2010;78:2–7.
Greenman J, Gálvez A, Giusti L, Ieropoulos I. Electricity from landfill leachate using microbial fuel cells: Comparison with a biological aerated filter. Enzyme Microb Technol. 2009;44:112–9.
Popov AL, Kim JR, Dinsdale RM, Esteves SR, Guwy AJ. The effect of physico-chemically immobilized methylene blue and neutral red on the anode of microbial fuel cell. Biotechnol Bioprocess Eng. 2012;17:361–70.
Sun J, Hu Y, Bi Z, Cao Y. Improved performance of air-cathode single-chamber microbial fuel cell for wastewater treatment using microfiltration membranes and multiple sludge inoculation. J Power Sources. 2009;187:471–9.
Rodrigo MA, Ca˜nizares P, Lobato J, Paz CSa R, Linares JJ. Production of electricity from the treatment of urban waste water using a microbial fuel cell. J Power Sources. 2007;169:198–204.
Kaewkannetra P, Chiwes W, Chiu TY. Treatment of cassava mill wastewater and production of electricity through microbial fuel cell technology. Fuel. 2011;90:2746–50.
Joung JY, Lee HW, Choi H, Lee MW, Park JM. Influences of organic loading disturbances on the performance of anaerobic filter process to treat purified terephthalic acid wastewater. Bioresour Technol. 2009;100:2457–61.
Marashi SKF, Kariminia H-R, Savizi ISP. Bimodal electricity generation and aromatic compounds removal from purified terephthalic acid plant wastewater in a microbial fuel cell. Biotechnol Lett. 2013;35:197–203.
Marashi, SKF, Kariminia, H-R, Electricity generation from petrochemical wastewater using a membrane-less single chamber microbial fuel cell. Renewable Energy and Distributed Generation (ICREDG), 2012 Second Iranian Conference on (pp. 23-27). IEEE. doi:10.1109/ICREDG.2012.6190462
Zhu G-F, Wua P, Wei Q-S, Lin J-y, Gao Y-L, Liu H-N. Biohydrogen production from purified terephthalic acid (PTA) processing wastewater by anaerobic fermentation using mixed microbial communities. Int J Hydrogen Energy. 2010;35:8350–6.
Behera M, Jana PS, More TT, Ghangrekar MM. Rice mill wastewater treatment in microbial fuel cells fabricated using proton exchange membrane and earthen pot at different pH. Bioelectrochemistry. 2010;79:228–33.
Yuan Y, Zhao B, Zhou S, Zhong S, Zhuang L. Electrocatalytic activity of anodic biofilm responses to pH changes in microbial fuel cells. Bioresour Technol. 2011;102:6887–91.
Acknowledgements
Authors wish to thank the research office of Sharif University of Technology for the financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SKFM carried out the experiments, analyzed the data and drafted the manuscript. HRK designed the study, participated in data analysis and reviewed the article. Both authors read and approved the final manuscript.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Foad Marashi, S.K., Kariminia, HR. Performance of a single chamber microbial fuel cell at different organic loads and pH values using purified terephthalic acid wastewater. J Environ Health Sci Engineer 13, 27 (2015). https://doi.org/10.1186/s40201-015-0179-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40201-015-0179-x