Abstract
Background
Weanling pigs, with immature immune system and physiological function, usually experience post-weaning diarrhea. This study determined the effects of dietary Clostridium butyricum supplementation on growth performance, diarrhea, and immunity of weaned pigs challenged with lipopolysaccharide (LPS).
Methods
In Experiment (Exp.) 1, 144 weaned piglets were weaned at 21 d and randomly assigned to six groups, with six replicates per group and four pigs per replicate, receiving a control diet (CON) or diet supplemented with antibiotics (AB) or C. butyricum (CB) (0.1%, 0.2%, 0.4%, or 0.8%), respectively. All diets in Exp. 1 were a highly digestible basal diet, with 3,000 mg/kg zinc oxide supplied in the first 2 wk only. In Exp. 2, 180 piglets were weaned at 21 d and randomly assigned to five groups, with six replicates per group and six pigs per replicate, receiving CON, AB, or CB (0.2%, 0.4%, or 0.6%) diets. The digestibility of diets was lower than those in Exp. 1, and did not include zinc oxide. At 36 d of Exp. 2, 12 piglets were selected from each of the CON and 0.4% CB groups, six piglets were intraperitoneally injected with LPS (50 μg/kg body weight) and the other six piglets with normal saline; animals were killed at 4 h after injection to collect blood, intestine, and digesta samples for biochemical analysis.
Results
In Exp. 1, CB and AB diets had no effect on growth performance of piglets. In Exp. 2, 0.4% CB decreased feed-gain ratio (P < 0.1), diarrhea score (P < 0.05), and increased duodenal, jejunal, and ileal villus height and jejunal villus height/crypt depth (P < 0.05). The 0.4% CB decreased the plasma tumor necrosis factor (TNF) α (P < 0.05) but increased ileal mucosa IL-10 and TLR2 mRNA expression (P < 0.05). Furthermore, 0.4% CB altered the microbial profile, with Bacillus and Ruminococcaceae UGG-003 at genus level and Lactobacillus casei and Parasutterella secunda at species level were higher than CON in colonic content (P < 0.05).
Conclusions
Dietary C. butyricum supplementation had positive effects on growth of weaned piglets with less digestible diets. There was a tendency to reduce the feed-gain ratio, which could reduce feed costs in pig production. Moreover, C. butyricum decreased post-weaning diarrhea by improving the intestinal morphology, intestinal microflora profile, and immune function.
Similar content being viewed by others
Background
Stress associated with early weaning usually results in depressed feed intake, growth retardation, and post-weaning diarrhea of piglets [1,2,3]. The sub-therapeutic use of antibiotics as growth promoters has long been recognized as an effective means for the mitigation of weanling stress. Numerous studies have reported that the sub-therapeutic use of antibiotics in diets can promote growth performance and control gastrointestinal infections of weaned piglets [4,5,6]. However, the ban on use of antibiotics in feed has largely resulted from the emergence of resistant bacteria and the potential for producing drug residues in animal products [7, 8]. Therefore, increasing attention has focused on alternatives to sub-therapeutic antibiotics. The effects of the diet formulation on intestinal development could therefore be critical during the earlier weaning stages.
Direct-fed microbials can improve the growth performance, intestinal health (e.g. intestinal morphology), intestinal microecogical equilibrium, and immunity of piglets [9, 10]. Clostridium butyricum can produce butyric acid, and so provide energy for intestinal epithelium and adjust intestinal pH, and maintain the intestinal environment [11]. Previous studies indicated that addition of C. butyricum to feed can improve growth performance [12,13,14], balance intestinal microflora [13], improve intestinal morphology [12], and stimulate the immune system through reducing the expression of pro-inflammatory factors [13, 15, 16]. However, there have been few studies on responses of weaned pigs to C. butyricum under lipopolysaccharide (LPS) challenge.
Therefore, this study was conducted to determine the effects of dietary C. butyricum supplementation on growth performance, intestinal development, and immune response of weaned piglets with LPS challenge.
Methods
Animals and diets
The protocol of this study was approved by the Animal Care and Use Committee of Animal Nutrition Institute, Sichuan Agricultural University, and was carried out in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals. In Experiment (Exp.) 1,144 crossbred piglets (Duroc × Landrace × Yorkshire, 7.01 ± 0.03 kg body weight [BW]) were weaned at 21 d of age and randomly assigned to six groups for 28 d, with six replicates per group and four pigs per replicate, receiving a control diet (CON) or diet supplemented with antibiotics (AB) or C. butyricum (CB) (0.1%, 0.2%, 0.4%, or 0.8%). All diets were a highly digestible basal diet included highly digestible carbohydrate ingredients (e.g. extruded corn, extruded rice and whey powder) and low anti-nutritional factors protein ingredients (e.g. extruded soybean, spray-dried plasma protein, fishmeal), with 3,000 mg/kg zinc oxide (ZnO) supplied in the first 2 wk only. In Exp. 2, 180 crossbred piglets (Duroc × Landrace × Yorkshire, 6.89 ± 0.02 kg BW) were weaned at 21 d of age and randomly assigned to five groups for 35 d, with six replicates per group and six pigs per replicate, receiving CON, AB, or CB (0.2%, 0.4%, or 0.6%) diets. All diets in Exp. 2 used the same less digestible basal diet without high ZnO. The basal diets of Exp. 2 included lower ratio of high digestibility carbohydrate ingredients and low anti-nutritional factors protein ingredients than Exp. 1, and it did not use rice. Levels of nutrients were provided by the basal diet met the requirements of nutrient requirements of swine (2012). The AB group was supplemented at 1 g/kg diet with (per kg of diet) 75 mg of chlortetracycline and 20 mg of enramycin. The C. butyricum strain provided by Chengdu Yukang Technology Co. Ltd. was Clostridium butyricum UCN-12, supplemented at 108 CFU/kg. The formulation of basal diets for phase 1 (1–14 d of trial) and 2 (15–28 d) of Exp. 1 are shown in Table 1, and for phase 1 (1–21 d) and 2 (22–35 d) of Exp. 2 in Table 2.
Pigs had free access to feed and water. Feed intake and fecal score of each pen was recorded daily. The severity of diarrhea was quantified by using the previous fecal consistency scoring method (fecal scoring: 0, normal; 1, soft feces; 2, mild diarrhea; and 3, severe diarrhea) [17]. Pigs were examined daily to ensure the record, if necessary, therapy of pigs suffering from diseases. Throughout the study, individual piglet BW per pen was measured at 0, 21, and 35 d. In Exp. 2, at 36 d of trial, 12 piglets were selected from each of the CON and 0.4% CB groups, then six piglets were intraperitoneally injected with LPS (50 μg/kg BW) and the othe six piglets with saline. Feed was removed before the injection, and the rectal temperature of each piglet was recorded at 0, 2, and 4 h after injection. The LPS (Escherichia coli L2880, Sigma-Aldrich, Los Angeles, CA, USA) was dissolved in sterile saline (9 g/L) to make LPS solution (400 mg/L). Dosage of LPS injection and the time to kill piglets were as previously described [18].
Sample collection
At 0, 2, and 4 h after injecting LPS or saline, blood samples were collected from the anterior vena cava into heparinized tubes, centrifuged (3,000 r/min at 4 °C for 10 min) and stored at − 20 °C until analysis [19]. The abdominal cavity was opened after being euthanized with an intravenous injection of pentobarbital sodium (50 mg/kg BW). The middle portion (~ 2 cm) of each segment of the small intestine (duodenum, jejunum, and ileum) was sampled and fixed in phosphate-buffered paraformaldehyde for histological measurements as previously described [3]. Ileum segments (10 cm in length) were opened longitudinally and the contents flushed with ice-cold sterile saline. Ileal mucosa and colonic content samples were quickly collected as described previously [20, 21], mucosa was collected by scraping using a sterile glass microscope slide at 4 °C, rapidly frozen in liquid nitrogen and stored at − 80 °C for analysis. Freshly collected contents from the proximal colon were put into sterile Eppendorf tubes and immediately stored at − 80 °C for analyses.
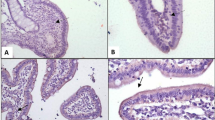
Intestinal morphology analysis
Intestinal segments were removed from fixative solution and then dehydrated with increasing concentrations of ethanol and chloroform. The segments were processed with paraffin, and two transverse tissue samples were cut from each segment using a microtome. These parts of the tissue samples were dehydrated, embedded together in paraffin wax, and sectioned at 5 μm. One transverse tissue sample of each segment was transferred to a slide and stained with hematoxylin and eosin. Villus height (VH) and crypt depth (CD) were determined as we described previously [3]. Briefly, 10 intact, well-oriented crypt-villi units per sample were randomly selected and measured. The VH was measured from the tip of the villi to the base between individual villi, and CD measurements were taken from the valley between individual villi to the basal membrane.
Cytokine mRNA abundance analysis
Ileal mucosa samples were used to determine the expression of genes: TLR2, TLR4, NF-κB, tumor necrosis factor α (TNF-α), interleukin 6 (IL-6), and IL-10. Total RNA was extracted from about 50 mg of frozen samples using the RNAiso Plus reagent (TaKaRa Bio, Inc., Dalian, China) according to the manufacturer’s specifications. The RNA concentration in the samples was determined using a DU-800 nucleic and protein detector (Beckman Coulter Inc., Fullerton, CA) at an optical density (OD value) of 260 nm; an OD260:OD280 ratio ranging between 1.8 and 2.0 was considered acceptable. The complementary DNA (cDNA) was then synthesized using a reverse transcription kit (TaKaRa Bio, Inc.) following the manufacturer’s instructions. Primers were synthesized by Invitrogen (Chengdu, China). Real-time PCR was performed on an ABI-7900HT instrument (Bio-Rad, Hercules, CA, USA) to quantify TLR2, TLR4, NF-κB, TNF-α, IL-6 and IL-10 mRNA expression with a commercial SYBR Green kit (TaKaRa Bio, Inc.). The reference gene β-actin was amplified for each sample to verify the presence of cDNA and as an internal control to calculate the relative level of target gene expression using the 2−ΔΔCT method [22]. Relative mRNA expression level of each target gene was normalized to the CON group. Primer sequences are shown in Table 3.
Plasma pro-inflammatory cytokine concentration analysis
Plasma TNF-α and IL-6 concentrations were measured using the ELISA kits suitable for porcine TNF-α and IL-6 (Nanjing JianCheng Bioengineering Institute Inc.), respectively, according to the manufacture’s protocol. Plasma concentrations of TNF-α and IL-6 were calculated from the standard curve and expressed as ng/L.
Short chain fatty acid (SCFA) analysis
Colonic SCFAs (acetic, propionic, butyric) were assayed using gas chromatography with a modification of the previous method [3]. Briefly, 1 g of digesta samples was weighed into a 5-mL centrifuge tube and 2 mL of deionized water was added. After the tube was capped, the content vortex-mixed for 30 s, left to stand for 30 min at 4 °C, and then centrifuged (5,000 r/min at 4 °C) for 10 min. The supernatant (1 mL) was removed by aspiration into another 5-mL centrifuge tube, 0.2 mL of 25% metaphosphate and 23.3 μL of 210 mmol/L cortonic acid were added, and this was vortex-mixed for 30 s and left to stand for 30 min. Next, the contents were centrifuged (1,000 r/min at 4 °C) for 10 min. Then 0.3 mL of the supernatant was removed to another 2-mL tube, 0.9 mL carbinol was added, and this was vortex-mixed for 30 s and centrifuged at 1,000 r/min. The supernatant was filtered using a 0.22-μm membrane for gas chromatography analysis.
16S rRNA analysis of bacteria
The total genomic DNA of colonic digesta was extracted using a QIAamp DNA stool Mini Kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer’s instructions. Before sequencing, the concentration and purity of the extracted genomic DNA were measured. Integrity of extracted genomic DNA was determined with electrophoresis on a 1% (w/v) agarose gel. Primer sequencing and bioinformatics analysis were performed by Novogene (Beijing, China) on the Illumina HiSeq platform, using the paired-end sequenced. The V3-V4 region of the bacterial 16S rRNA gene was amplified to comprehensively define the bacterial composition and abundance by PCR using bacterial universal primers. The resulting sequences were clustered into operational taxonomic units (OTUs) using Uparse (Uparse v7.0.1001) at 97% sequence identity. Significant differences were determined through further alpha diversity and beta diversity analyses.
Statistical analysis
Data were analyzed using SAS (version 9.4; SAS Inst. Inc., Cary, NC, USA). Growth performance of pigs was analyzed using one-way ANOVA to compare the BW, average daily gain (ADG), average daily feed intake (ADFI), feed-gain ratio (F/G), and diarrhea score. The pen was recognized as a statistical unit for the growth performance of pigs. The selected piglet in each pen was taken as an experimental unit for the parameters related to intestinal and immunological function in the LPS challenge study. The parameters related to the inflammatory cytokines in plasma were analyzed by repeated measures analysis with time for the LPS challenge study. For pigs challenged by LPS, data were analyzed using the MIXED procedure, according to the following model:
where Yijk is the analyzed variable, μ is the mean, αi is the effect of CB (i = 1 or 2), βj is the effect of LPS (j = 1 or 2), γk is the effect of time (k = 1, 2, or 3), (αβ)ij is the interaction between CB and LPS, (αγ)ik is the interaction between CB and time, (βγ)jk is the interaction between LPS and time, εijk is the residual error, and (αβγ)ijk is the interaction among CB, LPS, and time. Bacteria population data were log-transformed to ensure normal distribution. Values were means with their standard error (SE). Differences were considered significant at P < 0.05; when P > 0.05 but P < 0.1, differences were considered to indicate a trend toward significance. When main effects or interactive effects were significant, the means were compared using the least significant difference method with P < 0.05 indicating significance.
Results
Growth performance
In Exp. 1, supplementation of CB and AB had no significant effect on growth performance of piglets compared with CON (Table 4). In Exp. 2, 0.4% CB had a tendency to reduce the feed-gain ratio than CON (P < 0.1). The 0.4% CB had a lower diarrhea score than CON during the first 3 wk and all period (P < 0.05). There were no significant differences in BW, ADG, ADFI, or F/G between the 0.4% CB and AB treatments (Table 5).
Changes of rectal temperature
Compared with the non-challenged piglets, the rectal temperature of LPS-challenged piglets increased significantly at 2 and 4 h (Fig. 1).
Intestinal morphology
The 0.4% CB significantly increased duodenal, jejunal and ileal VH and jejunal VH/CD (P < 0.05); whereas VH, CD and VH/CD were not affected by LPS or the CB × LPS interaction. Duodenal VH and VH/CD in the CB + LPS were higher (P < 0.05) than those in the CON + LPS; jejunal VH and VH/CD were higher (P < 0.05) in the CB – LPS than in the CON – LPS; and ileal VH was higher (P < 0.05) in the CB + LPS than in the CON + LPS (Table 6).
Plasma TNF-α and IL-6 concentrations, and ileum mRNA expression
Plasma TNF-α concentration was affected by LPS challenge (P < 0.1) and CB × LPS interaction (P < 0.05); plasma IL-6 concentration was affected by LPS (P < 0.1). Plasma TNF-α concentration averaged across time was higher (P < 0.05) in the CON + LPS than the CON – LPS treatment, but no difference was observed between the CB – LPS and CB + LPS treatments (Table 7).
The 0.4% CB increased ileum mRNA relative expression of TLR2 and IL-10 (P < 0.05). The LPS decreased the mRNA relative expression of IL-10 (P < 0.05), and there was a significant CB × LPS interaction (P < 0.05) (Fig. 2).
SCFA concentrations
The acetate concentration in colonic content was affected by LPS challenge (P < 0.1). Concentrations of acetate, propionic acid, and butyric acid in colonic content were not affected by CB and CB × LPS (P > 0.05) (Table 8).
Microbial community in colonic content
DNA sequence data and OTU clustering
A total of 1,712,770 effective tags were obtained from four groups, with an average of 71,365 ± 1,437 per sample. Further study of the species diversity of the samples and species annotated on the representative sequence of OTUs. A total of 20,102 OTUs were found in the four groups, with an average of 838 ± 17 per sample (Fig. 3).
Alpha diversity of microbial community in colonic content
Many indexes that represented alpha diversity of microbial community (Table 9), in addition to the observed-species and ACE, were higher in the CB than in the CON (P < 0.1), indicating significantly greater species richness.
Change of relative abundance at phylum and genus levels
A total of 24 phyla were shared by piglets from all groups, and seven bacteria had relative abundance exceeding 1% in at least one sample: Firmicutes, Bacteroidetes, Proteobacteria, Spirochaetes, Tenericutes, Actinobacteria, and Euryarchaeota. The top 10 phyla are shown in Fig. 4a and relative abundances of the top 10 genera are show in Fig. 4b. There were no significant differences for the top 10 at phylum and genus levels among all groups.
Analysis of different species among groups
The abundance of Fusicatenibacter at genus level was higher in the CB.C than in the CON.C (Fig. 5). The abundances of Lactobacillus casei and Parasutterella secunda at species level were higher in the CB than CON (Fig. 6). The t-test show that greater abundance of Bacillaceae at family level in the CB than the CON (Fig. 7) and abundances of Bacillus and Ruminococcaceae UGG-003 at genus level were higher in the CB than the CON; however, abundance of Peptococcus at genus level was lower in the CB than the CON (Fig. 8).
The box graph of significant differences among species. The cross line represents two groups with significant differences, and no cross line indicates that there is no difference between the two groups. “*” indicates significant differences between the two groups. Piglets in CB treatment challenged with LPS (CB.C) and not challenged with LPS (CB.NC). Piglets in CON treatment challenged with LPS (CON.C) and not challenged with LPS (CON.NC)
The abundance of species at species level. The cross line represents two groups with significant differences, and no cross line indicates that there is no difference between the two groups. “*” indicates significant differences between the two groups. Piglets in CB treatment challenged with LPS (CB.C) and not challenged with LPS (CB.NC). Piglets in CON treatment challenged with LPS (CON.C) and not challenged with LPS (CON.NC)
The species of significant differences at family level. The left picture shows the diversity of species abundance, each of which indicates the mean value of species with significant differences in the abundance between groups. The right picture shows the difference confidence between groups. The most left-hand point of each circle represents the lower limit of the 95% confidence interval of mean difference, and the most right end point of the circle represents the upper limit of mean difference and 95% confidence interval. The center of the circle represents the difference of the mean. The group represented by the circle color is a group with high mean value. The right end of the display results was the P-value of significance test for the corresponding species between groups. Piglets in CB treatment challenged with LPS (CB.C) and not challenged with LPS (CB.NC). Piglets in CON treatment challenged with LPS (CON.C) and not challenged with LPS (CON.NC)
The species of significant differences at genus level. The left picture shows the diversity of species abundance, each of which indicates the mean value of species with significant differences in the abundance between groups. The right picture shows the difference confidence between groups. The most left-hand point of each circle represents the lower limit of the 95% confidence interval of mean difference, and the most right end point of the circle represents the upper limit of mean difference and 95% confidence interval. The center of the circle represents the difference of the mean. The group represented by the circle color is a group with high mean value. The right end of the display results was the P-value of significance test for the corresponding species between groups. Piglets in CB treatment challenged with LPS (CB.C) and not challenged with LPS (CB.NC). Piglets in CON treatment challenged with LPS (CON.C) and not challenged with LPS (CON.NC)
Discussion
Many reports have shown that C. butyricum can promote growth performance and improve nutrient utilization [13, 14, 16, 23], but other studies have no effect on growth performance [24]. Consistent with previous studies, dietary C. butyricum supplementation decreased diarrhea score in Exp. 2, but the supplementation had no effect on growth performance and diarrhea score in Exp. 1. This discrepancy might be related to diet type, such as the different percentages of highly digestible ingredients between the Exps 1 and 2. In the diet formulation of Exp. 1, we attempted to maximize the inclusion of various highly digestible carbohydrate ingredients and reduce anti-nutritional factors. This was because high quality protein sources and a high digestibility of carbohydrate sources were necessary for weaned pigs, to avoid the negative effects associated with post-weaning performance. Previous work suggested that ZnO and antibiotics are beneficial to growth and decrease diarrhea [6, 25]. The purpose of using ZnO was to prevent severe diarrhea of piglets in Exp. 1, and resulted in no significant severe diarrhea among groups; the growth performance did not differ significantly between AB and CB groups. The C. butyricum reduced diarrhea for low digestibility diets without antibiotics and ZnO in Exp. 2, which would be very beneficial to reduce costs in commercial production. This study showed that 0.4% CB improved feed efficiency and decreased diarrhea score compared with CON, and with no significant difference to AB, showing that C. butyricum had positive effects and similar growth-promoting effects to antibiotics with the less digestible diet. Previous studies found that dietary supplementation with direct-fed microbials could reduce the frequency of post-weaning diarrhea in piglets, reduce diarrhea severity, and provide greater growth rate and feed efficiency [26]. Oral administration of C. butyricum as a direct-fed microbial is gaining importance in treating and improving animal performance [15].
Intestinal histomorphology had been widely used for assessing intestinal development and function [27, 28]. The decreased digestion and absorption of nutrients due to villous atrophy and crypt hypertrophy as a result of early weaning may contribute to diarrhea [29, 30]. The underlying mechanism is related to the fact that increased VH and VH/CD are directly correlated with increased epithelial turnover [31], and longer villi are linked with activation of cell mitosis, with shortening of villi and deeper crypts leading to poor nutrient absorption [32], increased secretion in the gastrointestinal tract and reduced performance [33]. Previous studies indicated that direct-fed microbials could promote intestinal development and so improve piglet health and the growth performance [9, 10, 34, 35]. Consistent with this, some studies reported that use of C. butyricum in diets for weaned piglets could improve weight gain and feed efficiency when used at an appropriate dose [36]. In our study, supplementation of C. butyricum in the diet of weaned piglets consistently increased the VH of duodenum and ileum [37], and the VH/CD significantly increased [29, 38], which indicated the better digestive and absorption capability and resulted in the decreased F/G [34].
The lower diarrhea score, for piglets receiving C. butyricum, suggested a healthier gastrointestinal environment, possibly associated with intestine development, simultaneously, changes in intestinal microbiota and immunity were also possible. Previous studies indicated that direct-fed microbials in diets can significantly improve immune response [39, 40]. In line with this, dietary supplementation with C. butyricum has promoted immune response and improved intestinal barrier function in broiler chickens, rats and ducks [16, 31, 41]. The present study, the increased body temperature and plasma TNFα and IL-6 concentrations indicated successful establishment of the immune model following LPS challenge. The current results showed that the inflammatory process might be modulated by C. butyricum, as shown by results indicating decreased pro-inflammatory cytokine TNF-α and increased IL-10 and TLR2 expressions. The molecular action mechanism of C. butyricum involved reduced inflammation and improved immune homeostasis [42]. Mucosal surfaces of the gastrointestinal tract are in continuous contact with microbes, and toll-like receptors (TLRs) mediate recognition of microbial molecules to generate immune response [43]. The C. butyricum was shown to drive secretion of MyD88-independent inflammatory cytokines via TLR2-induced NF-κB activation [15], and C. butyricum can induce IL-10 expression from intestinal macrophages through the TLR2/MyD88-mediated pathway [44] consistent with our results showing C. butyricum increasing TLR2 and IL-10 expressions. The IL-10 is one of the most potent anti-inflammatory cytokines and is required for protection in many animal models of inflammation, and it has important roles in the regulation of gut homeostasis during host defense [45, 46]. The association between IL-10 and inflammatory bowel disease has been demonstrated in both humans and in animal models [46].
The TLR/MyD88-signal pathway triggers several responses critical for maintaining host-microbial homesostasis [47]. Opportunistic invasion of host tissue by resident bacteria has serious health consequences including inflammation and sepsis. The immune system has thus evolved adaptations that work together to contain the microbiota and preserve the host-microbiota symbiotic relationship [47]. The intestinal tract harbors a complex microbial community that plays a key role in nutrition and health, and the colon is the main site of microbial colonization [45]. Failure to achieve or maintain equilibrium between a host and its microbiota has negative consequences for both intestinal and systemic health, likely resulting not only in intestinal inflammatory diseases [40], such as Crohn’s disease and ulcerative colitis, but might also contribute to “auto-immune” diseases at extra-intestinal sites [48]. This study showed that microbial richness increased in the CB compared with the CON, indicating greater stability in the gut and ability to recover from infections. Research has shown that a reduction of diversity in the gut microbiota of patients with inflammatory bowel disease [40]. The microbial richness increase in the gut might account for greater stability in the digestive tract, which enhances the ability to recover from infectious postweaning diarrhea [46]. Previous research demonstrated that consumption of C. butyricum benefited the ecosystem of the intestinal tract by increasing the populations of probiotics and reducing those of unwanted bacteria [49]; Adding C. butyricum to feed of weaned piglets can increase the content of Lactobacillus [50], and also increase the diversity of intestinal bacteria [51]. Lactobacillus casei reduced the cytokine production in vitro for specimens of intestinal tissue from patients with ileal Crohn’s disease [52]. Direct-fed microbials that contain C. butyricum can reduce both severity and duration of diarrhea in children hospitalized with acute diarrhea, and increase fecal count of Lactobacillus by improvement in diarrheal disease [52]. Bacillus is one of the a member of direct-fed microbials [53], and the increase of Bacillus in the CB indicated the beneficial effect of C. butyricum. Fusicatenibacter and Ruminococcaceae are types of fermentative bacteria in the hindgut, which can help the host obtain more energy from complex polysaccharides resistant to the action of digestive enzymes [29, 54], and increased feed efficiency might be associated with increases in Fusicatenibacter and Ruminococcaceae. Research has shown that dysbiosis in rats decreased the level of Ruminococcaceae and increased intestinal permeability [55]. The increasing of Ruminococcaceae in the CB treatment might indicate that C. butyricum decreased the dysbiosis.
Conclusions
Dietary supplementation with C. butyricum had positive effects on growth of weaned pigletswith less digestible diets. There was a tendency to reduce F/G, which could reduce feed costs in pig production. The beneficial effect may result from decreasing of post-weaning diarrhea by improving the intestinal morphology, intestinal microflora profile and immune function.
Abbreviations
- AB:
-
Antibiotic treatment
- ADFI:
-
Average day feed intake
- ADG:
-
Average day gain
- BW:
-
Body weight
- C. butyricum :
-
Clostridium butyricum
- CB:
-
C.butyricum treatment
- CB.C:
-
CB with LPS-challenged
- CD:
-
Crypt depth
- CON:
-
Control treatment
- CON.C:
-
CON with LPS-challenged
- F/G:
-
Feed-gain ratio
- IL-10:
-
Interleukin-10
- IL-6:
-
Interleukin-6
- LPS:
-
Lipopolysaccharide
- TLR2:
-
Toll-like receptor 2
- TLR4:
-
Toll-like receptor 4
- TNF-α:
-
Tumor necrosis factor-α
- VH:
-
Villus height
- VH/CD:
-
Villus height/ crypt depth
References
Moeser AJ, Klok CV, Ryan KA, Wooten JG, Little D, Cook VL, et al. Stress signaling pathways activated by weaning mediate intestinal dysfunction in the pig. Am J Physiol Gastrointest Liver Physiol. 2007;292(1):G173–81. https://doi.org/10.1152/ajpgi.00197.2006.
Castillo M, Martin-Orue SM, Nofrarias M, Manzanilla EG, Gasa J. Changes in caecal microbiota and mucosal morphology of weaned pigs. Vet Microbiol. 2007;124(3–4):239–47. https://doi.org/10.1016/j.vetmic.2007.04.026.
Gu Y, Song Y, Yin H, Lin S, Zhang X, Che L, et al. Dietary supplementation with tributyrin prevented weaned pigs from growth retardation and lethal infection via modulation of inflammatory cytokines production, ileal expression, and intestinal acetate fermentation. J Anim Sci. 2017;95(1):226–38. https://doi.org/10.2527/jas.2016.0911.
Andreas P, Farfán-López C, Rondón Y, Mora F, Rossini M, Araque H. Effect of using mannoproteins and antibiotics as growth promoters in diets for weaned piglets on performance. Rev Cient Vet. 2016;XXVI(1):26–32.
Walsh MC, Sholly DM, Hinson RB, Saddoris KL, Sutton AL, Radcliffe JS, et al. Effects of water and diet acidification with and without antibiotics on weanling pig growth and microbial shedding. J Animal Sci. 2007;85(7):1799–808.
Weber TE, Schinckel AP, Houseknecht KL, Richert BT. Evaluation of conjugated linoleic acid and dietary antibiotics as growth promotants in weanling pigs. J Anim Sci. 2001;79(10):2542.
Looft T, Allen HK, Cantarel BL, Levine UY, Bayles DO, Alt DP, et al. Bacteria, phages and pigs: the effects of in-feed antibiotics on the microbiome at different gut locations. ISME J. 2014;8(8):1566.
Aarestrup F. Get pigs off antibiotics. Nature. 2012;486(7404):465–6.
Fuller R. Probiotics in man and animals. J Appl Bacteriol. 1989;66(5):365–78.
Ghadimi D, Fölster-Holst R, De VM, Winkler P, Heller KJ, Schrezenmeir J. Effects of probiotic bacteria and their genomic DNA on TH1/TH2-cytokine production by peripheral blood mononuclear cells (PBMCs) of healthy and allergic subjects. Immunobiology. 2008;213(8):677–92.
Meimandipour A, Shuhaimi M, Soleimani AF, Azhar K, Hair-Bejo M, Kabeir BM, et al. Selected microbial groups and short-chain fatty acids profile in a simulated chicken cecum supplemented with two strains of lactobacillus. Poult Sci. 2010;89(3):470–6.
Liao XD, Ma G, Cai J, Fu Y, Yan XY, Wei XB, et al. Effects of Clostridium butyricum on growth performance, antioxidation, and immune function of broilers[J]. Poult Sci. 2015;94(4):662–7.
Yang CM, Cao GT, Ferket PR, Liu TT, Zhou L, Zhang L, et al. Effects of probiotic, Clostridium butyricum, on growth performance, immune function, and cecal microflora in broiler chickens. Poult Sci. 2012;91(9):2121.
Zhao X, Guo Y, Guo S, Tan J. Effects of Clostridium butyricum and enterococcus faecium on growth performance, lipid metabolism, and cecal microbiota of broiler chickens. Appl Microbiol Biotechnol. 2013;97(14):6477–88. https://doi.org/10.1007/s00253-013-4970-2.
Gao Q, Qi L, Wu T, Wang J. Clostridium butyricum activates TLR2-mediated MyD88-independent signaling pathway in HT-29 cells. Mol Cell Biochem. 2012;361(1–2):31–7. https://doi.org/10.1007/s11010-011-1084-y.
Zhang L, Zhang L, Zhan X, Zeng X, Zhou L, Cao G, et al. Effects of dietary supplementation of probiotic, Clostridium butyricum, on growth performance, immune response, intestinal barrier function, and digestive enzyme activity in broiler chickens challenged with Escherichia coli K88. J Anim Sci Biotechnol. 2016;7:3. https://doi.org/10.1186/s40104-016-0061-4.
Bhandari SK, Xu B, Nyachoti CM, Giesting DW, Krause DO. Evaluation of alternatives to antibiotics using an Escherichia coli K88+ model of piglet diarrhea: effects on gut microbial ecology. J Anim Sci. 2008;86(4):836.
Wang JP, Yoo JS, Jang HD, Lee JH, Cho JH, Kim IH. Effect of dietary fermented garlic by Weissella koreensis powder on growth performance, blood characteristics, and immune response of growing pigs challenged with Escherichia coli lipopolysaccharide. J Anim Sci. 2011;89(7):2123–31. https://doi.org/10.2527/jas.2010-3186.
Liu YL, Li DF, Gong LM, Yi GF, Gaines AM, Carroll JA. Effects of fish oil supplementation on the performance and the immunological, adrenal, and somatotropic responses of weaned pigs after an Escherichia coli lipopolysaccharide challenge. J Anim Sci. 2003;81(11):2758–65. https://doi.org/10.2527/2003.81112758x.
Giang HH, Viet TQ, Ogle B, Lindberg JE. Growth performance, digestibility, gut environment and health status in weaned piglets fed a diet supplemented with potentially probiotic complexes of lactic acid bacteria. Livest Sci. 2010;129(1):95–103.
Wang H, Liu Y, Shi H, Wang X, Zhu H, Pi D, et al. Aspartate attenuates intestinal injury and inhibits TLR4 and NODs/NF-κB and p38 signaling in weaned pigs after LPS challenge. Eur J Nutr. 2017;56(4):1433–43.
Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–8.
Cao GT, Xiao YP, Yang CM, Chen AG, Liu TT, Zhou L, et al. Effects of Clostridium butyricum on growth performance, nitrogen metabolism, intestinal morphology and Cecal microflora in broiler chickens. J Anim Vet Adv. 2012;11(15):2665–71.
Zhang B, Yang X, Guo Y, Long F. Effects of dietary lipids and Clostridium butyricum on the performance and the digestive tract of broiler chickens. Arc Anim Nutr. 2011;65(4):329.
Stensland I, Kim JC, Bowring B, Collins AM, Mansfield JP, Pluske JR. A comparison of diets supplemented with a feed additive containing organic acids, Cinnamaldehyde and a Permeabilizing complex, or zinc oxide, on post-weaning Diarrhoea, selected bacterial populations, blood measures and performance in weaned pigs experimentally infected with Enterotoxigenic E. Coli. Animals. 2015;5(4):1147–68.
Vrotniakiene V, Jatkauskas J. Effects of probiotics dietary supplementation on diarrhea incidence, fecal shedding of escherichia coli and growth performance in post-weaned piglets. Vet Zootech-Lich. 2013;63(85):81–8.
Pluske JR, Hampson DJ, Williams IH. Factors influencing the structure and function of the small intestine in the weaned pig: a review. Livest Prod Sci. 1997;51(1–3):215–36.
Nyachoti CM, Kiarie E, Bhandari SK, Zhang G, Krause DO. Weaned pig responses to Escherichia coli K88 oral challenge when receiving a lysozyme supplement. J Anim Sci. 2012;90(1):252–60.
Huang C, Song P, Fan P, Hou C, Thacker P, Ma X. Dietary sodium butyrate decreases Postweaning diarrhea by modulating intestinal permeability and changing the bacterial communities in weaned piglets. J Nutr. 2015;145(12):2774–80. https://doi.org/10.3945/jn.115.217406.
O’Loughlin EV, Scott RB, Gall DG. Pathophysiology of infectious diarrhea: changes in intestinal structure and function. J Pediatr Gastroenterol Nutr. 1991;12(1):5–20.
Ichikawa H, Kuroiwa T, Inagaki A, Shineha R, Nishihira T, Satomi S, et al. Probiotic bacteria stimulate gut epithelial cell proliferation in rat. Dig Dis Sci. 1999;44(10):2119–23.
Nyachoti CM, Omogbenigun FO, Rademacher M, Blank G. Performance responses and indicators of gastrointestinal health in early-weaned pigs fed low-protein amino acid-supplemented diets. J Anim Sci. 2006;84(1):125.
Hossain MM, Begum M, Kim IH. Effect of Bacillus subtilis, Clostridium butyricum and lactobacillus acidophilus endospores on growth performance, nutrient digestibility, meat quality, relative organ weight, microbial shedding and excreta noxious gas emission in broilers. Vet Med-Czech. 2015;60(2):77–86.
Pan L, Zhao PF, Ma XK, Shang QH, Xu YT, Long SF, et al. Probiotic supplementation protects weaned pigs against enterotoxigenic Escherichia coli K88 challenge and improves performance similar to antibiotics. J Anim Sci. 2017;95(6):2627–39. https://doi.org/10.2527/jas.2016.1243.
Plaza-Diaz J, Gomez-Llorente C, Fontana L, Gil A. Modulation of immunity and inflammatory gene expression in the gut, in inflammatory diseases of the gut and in the liver by probiotics. World J Gastroenterol. 2014;20(42):15632–49. https://doi.org/10.3748/wjg.v20.i42.15632.
Italy EPoA, Feed PoSuiA. Scientific opinion on Miya〨old® (Clostridium butyricum) as a feed additive for weaned piglets, minor weaned porcine species and minor avian species. EFSA J. 2011;9(1):1951.
Liu T. Effects of glutamine and Clostridium butyricum on growth Performance, Immune Function, Small intestinal morphology and microflora in weanling piglets. Chin J Anim Nutr. 2011;23(06):998-1005.
Pang M, Qingping LU, Xia B, Zhang H. Effects of Clostridium butyricum on growth Performance, Intestinal morphology and intestinal permeability of weanling piglets. Chin J Anim Nutr. 2016;28(7):2113-21.
Walker WA. Mechanisms of action of probiotics. Clin Infect Dis. 2008;46 Suppl 2(Supplement_2):S87.
Kanai T, Mikami Y, Hayashi A. A breakthrough in probiotics: Clostridium butyricum regulates gut homeostasis and anti-inflammatory response in inflammatory bowel disease. J Gastroenterol. 2015;50(9):928–39.
Zhuang S, Jiang FB, Jia ZX, Yan R. Clostridium butyricum can be used as a potential alternative for the antibiotic in Cherry Valley ducks. J Anim Plant Sci. 2015;25(5):1227–32.
Chen ZF, Ai LY, Wang JL, Ren LL, Yu YN, Xu J, et al. Probiotics Clostridium butyricum and Bacillus subtilis ameliorate intestinal tumorigenesis. Future Microbiol. 2015;10(9):1433–45. https://doi.org/10.2217/fmb.15.66.
Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, et al. The toll-like receptor pathway establishes commensal gut colonization. Science. 2011;332(6032):974.
Hayashi A, Sato T, Kamada N, Mikami Y, Matsuoka K, Hisamatsu T, et al. A single strain of Clostridium butyricum induces intestinal IL-10-producing macrophages to suppress acute experimental colitis in mice. Cell Host Microbe. 2013;13(6):711–22. https://doi.org/10.1016/j.chom.2013.05.013.
Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453(7195):620.
Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474(7351):298–306. https://doi.org/10.1038/nature10208.
Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336(6086):1268.
Garrett WS, Gordon JI, Glimcher LH. Homeostasis and inflammation in the intestine. Cell. 2010;140(6):859–70. https://doi.org/10.1016/j.cell.2010.01.023.
Kong Q, He GQ, Jia JL, Zhu QL, Ruan H. Oral administration of Clostridium butyricum for modulating gastrointestinal microflora in mice. Curr Microbiol. 2011;62(2):512–7. https://doi.org/10.1007/s00284-010-9737-8.
Liang MZ, Li LI, Liu H. The effect of Clostridium butyricum on intestinal microflora of weaned piglets. Chin J Anim Sci. 2013;49(23):64-7.
Liu J, Jing S, Wang F, Yu X, Ling Z, Li H, et al. Neuroprotective effects of Clostridium butyricum against vascular dementia in mice via metabolic butyrate. Biomed Res Int. 2015;2015:1.
Gareau MG, Sherman PM, Walker WA. Probiotics and the gut microbiota in intestinal health and disease. Nat Rev Gastroenterol Hepatol. 2010;7(9):503–14. https://doi.org/10.1038/nrgastro.2010.117.
Gerritsen J, Smidt H, Rijkers GT, de Vos WM. Intestinal microbiota in human health and disease: the impact of probiotics. Genes Nutr. 2011;6(3):209–40. https://doi.org/10.1007/s12263-011-0229-7.
Takada T, Kurakawa T, Tsuji H, Nomoto K. Fusicatenibacter saccharivorans gen. Nov., sp. nov., isolated from human faeces. Int J Syst Evol Micr. 2013;63(Pt 10):3691.
Leclercq S, Matamoros S, Cani PD, Neyrinck AM, Jamar F, Starkel P, et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci U S A. 2014;111(42):E4485–93. https://doi.org/10.1073/pnas.1415174111.
Acknowledgements
We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Funding
This work was supported by the Program for Changjiang Scholars, Sichuan Province “135” Breeding Tackle Project (Project No. 2016NYZ0052).
Availability of data and materials
The datasets during and/or analyzed during the current study available from the corresponding authors on reasonable request.
Author information
Authors and Affiliations
Contributions
LC and DW designed the study, LC, JZ, SL, XZ and WL performed the research, LC and XJ collected the data, ZF, YL, SX, BF and JL analyzed the data, LC, DW and YL wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The protocol of this study was approved by the Animal Care and Use Committee of Animal Nutrition Institute, Sichuan Agricultural University, and was carried out in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Chen, L., Li, S., Zheng, J. et al. Effects of dietary Clostridium butyricum supplementation on growth performance, intestinal development, and immune response of weaned piglets challenged with lipopolysaccharide. J Animal Sci Biotechnol 9, 62 (2018). https://doi.org/10.1186/s40104-018-0275-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40104-018-0275-8