Abstract
Background
The genus Salvia is a rich source of structurally diverse terpenoids. Different species of the Salvia have been used in folk medicine of Iran and therefore attracted the attention of researchers for exploring their chemical constituents. In a project directed at structurally interesting bioactive metabolites from Iranian Lamiaceae, we studied Salvia rhytidea.
Results
Fractionation of the petroleum ether extract of the root of S. rhytidea led to the isolation of a new 20-nor-abietane diterpenoid (1), together with seven known compounds, comprising five abietane diterpenoids (2–6), and two rearranged abietanes (7, 8). Their structures were established by a combination of 1D and 2D NMR.
Conclusions
Our results showed that the root of S. rhytidea could be considered as a new and rich source of different types of abietane and rearranged abietane diterpenoids.
Similar content being viewed by others
Background
The genus Salvia is a rich source of structurally diverse terpenoids (Kintzios 2000; Moridi Farimani et al. 2013). Among these, numerous diterpenoids with promising bioactivities, such as antileishmanial, antitumor, antimicrobial, antifungal properties, have been reported from Salvia species (Ebrahimi et al. 2013; Tan et al. 2002; Akaberi et al. 2015; Moridi Farimani and Miran 2014; Ulubelen 2003; Jassbi et al. 2006). The most abundant diterpenoids in the genus are abietanes and rearranged abietanes (Wu et al. 2012). The genus Salvia is represented in the Iranian flora by 61 species, of which 17 are endemic (Jamzad et al. 2012). Salvia rhytidea Benth is an endemic species that grows widely in the eastern parts of Iran (Rechinger 1987). In our efforts to discover new and potentially bioactive secondary metabolites from Iranian Salvia species (Moridi Farimani and Mazarei 2014; Ebrahimi et al. 2014; Moridi Farimani et al. 2012; Bahadori et al. 2015; Moridi Farimani et al. 2008), we investigated the petroleum ether extract of the root of S. rhytidea. Here we report the isolation and structure elucidation of 1-deoxo aurocadiol (1), as a new 20-nor-abietane diterpenoid. In addition, the abietane diterpenoids ferruginol (2), taxodione (3), arucadiol (4), deoxyneocryptotanshinone (5), and 7α- Ethoxyroyleanone (6), and rearranged abietanes microstegiol (7) and 12-hydroxysapriparaquinone (8) were isolated and are described here for S. rhytidea for the first time.
Results and discussion
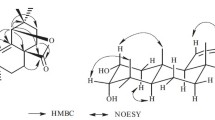
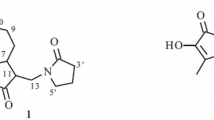
Compound 1 (Fig. 1) was isolated as an orange, amorphous solid. The IR spectrum showed absorptions of hydroxy (3475 cm−1) and olefinic (1610 cm−1) functionalities. The 13C NMR spectrum showed 19 carbon resonances, which were identified with the aid of HSQC and DEPTQ spectra as four methyl, three methylene, four methine, and eight quaternary carbons. The 13C NMR spectrum showed signals indicative of ten aromatic carbons. The 1H NMR spectrum showed resonances of two methyl singlets at δ H 1.22 (s, 6H). Resonances of two additional methyl groups at δ H 1.14 (d, J = 6.9 Hz) and 1.19 (d, J = 6.9 Hz), together with a signal at δ H 2.90 (sept, J = 6.9 Hz) indicated the presence of an isopropyl moiety. Signals at δ H 7.02 (d, J = 7.0 Hz) and 7.43 (d, J = 7.0 Hz) were indicative of two aromatic protons with ortho-position to each other. Another aromatic methine signal appeared as a singlet at δ H 6.78. Therefore, the structural features were reminiscent of a nor-abietane diterpenoid containing two aromatic rings. The NMR data of 1 showed great similarity to those of arucadiol isolated from S. argentea (Michavila et al. 1986) and S. miltiorrhiza (Ginda et al. 1988). Inspection of the 13C NMR spectra showed the lack of a carbonyl group in compound 1 but the presence of an additional methylene group at δ C 27.6. The chemical shifts of C-2, C-5, and C-7 were observed at δ C 18.5, 148.8, and 126.5, respectively, with upfield shifts of ca. 18, 10, and 11 ppm relative to arucadiol, while the chemical shift of C-3, C-6, C-9, and C-10 were observed at δ C 38.0, 129.8, 128.4, and 134.6 with downfield shift of ca. 3, 10, 9, and 10 ppm, respectively. These observations suggested the replacement of the C-1 carbonyl with a methylene group. HMBC correlations (Fig. 2) between H-1 (δ H 2.78, 2H, t), and C-2 (δ C 18.5), C-5 (δ C 148.8), C-10 (δ C 134.6), and C-9 (δ C 128.4), and COSY correlation between H-1 and H-2 (δ H 1.72, 2H, m) confirmed the location of the methylene group. Unambiguous assignments of NMR data were achieved by a combination of COSY, HMQC, and HMBC experiments. Compound 1 was therefore established structurally as 1-deoxo-arucadiol.
Ferruginol (2) is a well known abietane diterpenoid which was isolated from different Salvia species such as S. syriaca and S. sclarea (Ulubelen et al. 2000, 1994). Antileishmanial (Tan et al. 2002), antimicrobial (Ulubelen et al. 1999), cytotoxic (Moujir et al. 1996; Fronza et al. 2011), antihypertensive (Ulubelen et al. 1994), and anticholinesterase (Topcu et al. 2013) activities were reported for ferruginol and the mechanism of its antioxidant properties was also investigated (Saijo et al. 2015).
Taxodione (3) is a diterpenoid with quinone methide skeleton which was reported from different genus like Taxudium, Clerodendrum, and Salvia (Machumi et al. 2010; Kolak et al. 2009; Kusumoto et al. 2009). Different biological properties have been reported for this compound, including antibacterial (Yang et al. 2001), antioxidant (Kolak et al. 2009), antitermitic (Kusumoto et al. 2009), antifeedant (Acosta et al. 2008), antifungal (Topçu and Gören 2007), and anticholinesterase activities (Topcu et al. 2013). Moreover, cytotoxic and tumor inhibitory properties of taxodione have been investigated in in situ and in vivo experiments (Kupchan et al. 1969; Ulubelen et al. 1999; Abou Dahab et al. 2007). The mechanism of action of taxodione for its cytotoxic properties was investigated in several articles with focus on its DNA binding and DNA damaging character (Zaghloul et al. 2008), and its enzyme inhibitory action (Hanson et al. 1970).
Arucadiol (miltiodiol) (4) was previously extracted from S. argentea (Michavila et al. 1986), S. miltiorrhiza (Ginda et al. 1988), S. prionitis (Yong 1995), and S. apiana (González et al. 1992). Cytotoxic properties have been reported for arucadiol (Fronza et al. 2011).
Deoxyneocryptotanshinone (5) is a para-quinone abitanne diterpenod with cytotoxic activity which was isolated from S. miltiorrhiza for the first time (Ikeshiro et al. 1991).
7α-Ethoxyroyleanone (6) has been reported from S. lavandulaefolia, S. lanigra, and Peltodon longipes and its cytotoxic and antioxidant effects were investigated (Fronza et al. 2011; Shaheen et al. 2011; Michavila et al. 1985; Burmistrova et al. 2013).
Microstegiol (7) is a rearranged abietane with a seven-member ring skeleton. This compound has solely been reported from Salvia genus so far (Topcu et al. 2013; Ulubelen et al. 1992). It has been shown to have mild antibacterial effects (Topçu and Gören 2007).
12-hydroxysapriparaquinone (8), a rearranged 4,5-seco-abietane diterpenoid, previously isolated from S. limbata (Topcu et al. 1996).
Experimental
General experimental procedures
NMR spectra were recorded at a target temperature of 18 °C on a Bruker Avance III 500 MHz spectrometer operating at 500.13 MHz for 1H and 125.77 MHz for 13C. 2D NMR experiments (1H-1H COSY, HSQC, HMBC, NOESY) were performed using Bruker microprograms. CDCl3 was purchased from Armar Chemicals. TLC was performed on silica gel (Merck, Kieselgel 60, F254, 0.25 mm) phase. Column chromatography (CC) was carried out using silica gel (70–230 mesh, Merck). Flash column chromatography (FCC) was performed on silica gel (230–400 mesh, Merck).
Plant material
The roots of Salvia rhytidea were collected from Taftan Mountain, 28°36′ N and 61°4′ E, in the Baluchistan of Iran at an altitude of 2497 m, in autumn 2012. The plant was authenticated by Dr. Valizadeh and a voucher specimen (no. 4938) was deposited in the Herbarium of the School of Biology (Dr. Akhani Herbarium), University of Tehran.
Extraction and purification
The air-dried roots of S. rhytidea (2100 g) were crushed and extracted with petroleum ether (bp. 40–60 °C) at room temperature (25 °C) for 5 days. The extract was concentrated in vacuo, to afford 12.6 g dark gummy residue. It was subjected to a silica gel column chromatography using petroleum ether (3 L) as eluting solvent, increasing the polarity with a mixture of dichloromethane: acetone (90:10, 5 L), to yield nine fractions (1–9) (Fig. 3). Fraction 3 was subjected to silica gel flash column chromatography using petroleum ether-diethyl ether (98:2, 2.5 L), as mobile phase. Four subfractions (3a–3d) were collected. Subfraction 3b was recrystallized from Me2CO to afford 8 (8 mg). Fraction 5 was separated on a silica gel flash column with a gradient of petroleum ether-EtOAc (100:0, 0.5 L; 90:10, 0.3 L, 80:20, 0.3 L; 70:30, 0.3 L; 60:40, 0.2 L; 50:50, 0.2 L) as eluent, to yield 8 subfractions (5a–5h). Subfraction 5d was purified by preparative TLC [developed with acetone-EtOAc-petroleum ether (2:2:96)] to afford 1 (4 mg), 5 (5 mg), and 6 (5 mg). Subfraction 5e was separated over a silica gel flash column, eluting with petroleum ether-CH2Cl2 (85:15, 1 L) followed by increasing concentrations of CH2Cl2 (up to 100 %), to afford 20 subfractions (5e1–5e20). Compounds 7 (10 mg) and 2 (7 mg) were recrystallized from subfractions 5e5 and 5e8, respectively. Subfraction 5e2 was purified by another silica gel column to yield 3 (5 mg). Subfraction 5e18 was also separated on another silica gel column to yield 4 (3 mg).
1-Deoxoarucadiol (1)
1H-NMR (500 MHz, in CDCl3): δ 1.14 (3H, d, J = 6.9 Hz, Me-16), 1.19 (3H, d, J = 6.9 Hz, Me-17), 1.22 (6H, s, Me-18 and Me-19), 1.58 (2H, m, H-3), 1.72 (2H, m, H-2), 2.78 (2H, t, J = 6.3 Hz, H-1), 2.90 (1H, sept., J = 6.9 Hz, H-15), 6.78 (1H, s, H-14), 7.02 (1H, d, J = 7.0 Hz, H-7), 7.43 (1H, d, J = 7.0 Hz); 13C-NMR (125 MHz, in CDCl3): 18.5 (C-2), 20.6 (C-16 and C-17), 27.6 (C-1), 31.8 (C-18 and C-19), 32.4 (C-15), 34.0 (C-4), 38.0 (C-3), 126.5 (C-7), 128.4 (C-9), 129.8 (C-6), 131.6 (C-14), 134.6 (C-10), 139.7 (C-13), 140.4 (C-8), 148.8 (C-5), 152.9 (C-11), 161.2 (C-12).
Ferruginol (2)
1H-NMR (500 MHz, in CDCl3); δ 0.92 and 0.95 (each 3H, s, Me-18 and Me-19), 1.17 (3H, s, Me-20), 1.22 (1H, m, H-3), 1.25 and 1.26 (each 3H, d, J = 7.0 Hz, Me-16 and Me-17), 1.33 (1H, d, J = 12.0 and 2.2 Hz, H-5), 1.37 (1H, m, H-1),1.48 (1H, m, H-3) 1.58 (1H, dt, J = 13.9 and 3.4 Hz, H-2), 1.68 (1H, brd, J = 12.9 Hz, H-6), 1.72 (1H, m, H-2), 1.88 (1H, tdd, J = 13.2, 7.4, and 1.8 Hz, H-6), 2.14 (1H, brd, J = 12.5 Hz, H-1), 2.80 (1H, dd, J = 11.4 and 7.4 Hz, H-7), 2.88 (1H, ddd, J = 11.4 and 7.4 Hz, H-7), 3.20 (1H, sept., J = 6.9 Hz, H-15), 5.34 (s, OH), 6.69 (1H, s, H-11), 6.86 (1H, s, H-14), 13C-NMR (125 MHz, in CDCl3): δ 20. 1(C-2), 20.4 (C-6), 22.7 (C-19), 23.7 (C-16), 23.8 (C-17), 26.2 (C-20), 27.7 (C-15), 31.0 (C-7), 33.4 (C-18), 34.5 (C-4), 38.8 (C-10), 40.0 (C-1), 42.8 (C-3), 51.5 (C-5), 111.8 (C-11), 127.5 (C-14), 127.8 (C-8), 132.5 (C-13), 149.5 (C-9), 151.8 (C- 12).
Taxodione (3)
1H-NMR (500 MHz, in CDCl3): δ 1.18 and 1.21 (each 3H, d, J = 6.9 Hz, Me-16 and Me-17), 1.15, 1.30 and 1.30 (each 3H, s, Me-18, Me-19 and Me-20), 1.20 and 1.44 (1H, m, H-3), 1.67 and 1.86 (each 1H, m, H-2), 2.64 (1H, brs, H-5), 2.97 (1H, ddd, J = 10.7, 3.6 and 1.3 Hz, H-1), 3.10 (1H, dsept., J = 6.9 and 0.8 Hz, H-15), 6.26 (1H, s, H-7), 6.91 (1H, brs, H-14), 7.66 (s, 11-OH); 13C-NMR (125 MHz, in CDCl3): δ 19.3 (C-2), 22.7 (C-20), 22.7 (C-16), 22.8 (C-17), 22.8 (C-19), 28.1 (C- 15), 33.0 (C-4), 33.6 (C-18), 38.3 (C-1), 42.7 (C-3), 43.5 (C-10), 63.1 (C-5), 126.7 (C-9), 135.1 (C-7), 137.5 (C-14), 140.0 (C-8), 145.1 (C-11), 146.2 (C- 13), 182.7 (C-12), 201.7 (C-6).
Arucadiol (4)
1H-NMR (500 MHz, in CDCl3); δ 1.37 (6H, d, J = 6.9 Hz Me-16 and Me-17), 1.47 (6H, s, Me-18 and Me-19), 2.09 (2H, t, J = 6.8 Hz, H-3), 2.94 (2H, t, J = 6.8 Hz, H-2), 3.47 (1H, sept., J = 6.9 Hz, H-15), 6.89 (brs, 11-OH), 7.28 (1H, s, H-14), 7.33 (1H, d, J = 8.6 Hz, H-6), 7.95 (1H, d, J = 8.6 Hz, H-7), 10.73 (s, 11-OH), 13C-NMR (125 MHz, in CDCl3): δ 22.0 (C-16 and C-17), 27.4 (C-15), 29.6 (C-18 and C-19), 35.5 (C-3), 36.2 (C-2), 118.5 (C-14), 120.0 (C-11), 120.1 (C-6), 126.7 (C-10), 127.7 (C-9), 128.4 (C-8), 136.6 (C-13), 137.8 (C-7), 148.2 (C-12), 158.3 (C-5), 204.3 (C-1).
Deoxyneocryptotanshinone (5)
1H-NMR (500 MHz, in CDCl3): δ 1.29 (6H, d, J = 7.0 Hz, Me-16 and Me-17), 1.30 (6H, s, Me-18 and Me-19), 1.79 (4H, m, H-2 and H-3), 3.20 (2H, t, J = 6.4 Hz), 3.37 (1H, sept., J = 6.5 Hz, H-15), 7.71 (1H, d, J = 8.2 Hz, H-6), 7.97 (1H, d, J = 8.2 Hz, H-7), 7.82 (1H, s, 12-OH); 13C-NMR (125 MHz, in CDCl3): 19.2 (C-2), 19.9 (C-16 and C-17), 24.5 (C-15), 30.0 (C-1), 31.8 (C-18 and C-19), 37.8 (C-3), 123.8 (C-13), 125.1 (C-7), 126.5 (C-9), 132.8 (C-8), 133.4 (C-6), 140.7 (C-4), 152.5 (C-10), 153.3 (C-12), 183.4 (C-11), 184.6 (C-14).
7α-Ethoxyroyleanone (6)
1H-NMR (500 MHz, in CDCl3): δ 0.84 and 0.87 (each 3H, s, Me-18 and Me-19), 1.11 (3H, t, J = 5.9 Hz, Me-2′), 1.12 and 1.15 (each 3H, d, J = 7.1 Hz, Me-16 and Me-17), 1.13 (3H, s, Me-20), 1.13 (1H, overlap, H-1), 1.17 (1H, overlap, H-3), 1.29 (1H, m, H-6), 1.39 (1H, brd, J = 13.1 Hz, H-3), 1.47 (1H, dt, J = 14.0, 3.5 Hz, H-2), 1.57 (1H, d, J = 12.8 Hz, H-8), 1.64 (1H, m, H-2), 1.93 (1H, d, J = 14.1 Hz, H-14), 2.60 (1H, brd, J = 13.1 Hz, H-1), 3.10 (1H, sept., J = 7.1 Hz, H-15), 3.61 (2H, m, H-1′), 4.35 (1H, dd, J = 3.2 and 1.6 Hz, H-7); 13C-NMR (125 MHz, in CDCl3): 15.5 (C-2′), 18.5 (C-2), 18.7 (C-16 and C-17), 18.8 (C-20), 21.5 (C-19), 22.8 (C-6), 23.4 (C-15), 32.5 (C-18), 33.0 (C-4), 35.2 (C-1), 38.9 (C-10), 40.5 (C-5), 45.2 (C-5), 65.0 (C-1′), 68.6 (C-7), 124.8 (C-13), 141.2 (C-8), 146.9 (C-9), 150.2 (C-12), 184.0 (C-11), 186.1 (C-14).
Microstegiol (7)
1H-NMR (500 MHz, in CDCl3): δ 0.80 and 0.81 (each 3H, s, Me-18 and Me-19), 1.17 and 1.22 (each 3H, d. J = 6.9 Hz, Me-16 and Me-17), 1.27 and 2.4 (each 1H, m, H-3), 1.42 and 1.69 (each 1H, m, H-2), 2.33 (3H, s, H-20), 2.80 (1H, ddd, J = 14.0, 6.2 and 2.3 Hz, H-1), 3.03 (1H, dsept, J = 6.7 and 0.7 Hz, H-15), 3.61 (1H, dt, J = 2.1 and 13.1 Hz, H-1), 4.53 (s, 11-OH), 6.91 and 7.07 (1H, d, J = 7.6 Hz, H-7 and H-6), 6.97 (1H, d, J = 0.5 Hz, H-14); 13C-NMR (125 MHz, in CDCl3): 21.1 (C-16), 21.4 (C-20), 21.5 (C-18), 22.1 (C-17), 23.5 (C-2), 26.9 (C-1), 27.1 (C-15), 28.0 (C-19), 39.0 (C-4), 42.9 (C-3), 84.3 (C-11), 126.7 (C-7), 129.1 (C-8), 130.1 (C-6), 137.3 (C-5), 139.3 (C-9), 140.4 (C-14), 141.0 (C-13), 143.3 (C-10), 206.18 (C-12).
12-hydroxysapriparaquinone (8)
1H-NMR(500 MHz, in CDCl3): δ 1.29 (6H, d, J = 7.0 Hz, Me-16 and Me-17), 1.60 and 1.70 (each 3H, s, Me-18 and Me-19), 1.65 (2H, m, H-3), 2.15 (2H, q, J = 8.2 Hz, H-2), 2.42 (3H, s, Me-20), 3.16 (2H, brt, J = 8.2 Hz, H-1), 3.37 (1H, sept., J = 6.5 Hz, H-15), 5.27 (1H, t, J = 6.9 Hz, H-3), 7.47 (1H, d, J = 7.9 Hz, H-6), 7.83 (1H, s, 12-OH), 7.93 (1H, J = 7.9 Hz, H-7); 13C-NMR (125 MHz, in CDCl3): 17.6 (C-19), 19.9 (C-18 and C-19), 20.4 (C-20), 24.6 (C-15), 27.1 (C-1), 30.3 (C-2), 38.5 (C-3), 124.7 (C-13), 125.5 (C-7), 126.2 (C-9), 132.8 (C-8), 136.3 (C-6), 143.3 (C-5), 144.4 (C-10), 145.6 (C-4), 153.3 (C-12), 183.4 (C-11), 184.5 (C-14).
Conclusions
Our results showed that the root of S. rhytidea contained different types of abietane and rearranged abietane diterpenoids. Biological properties of some of these compounds have been reported previously. Accordingly, S. rhytidea could be considered as a new and rich source of natural agents for the treatment of cancer, malaria, and microbial strains.
References
Abou Dahab MA, El-Bahr MK, Taha HS, Habib AM, Bekheet SA, Gabr AMM, Refaat A (2007) Cytotoxic activity of Taxodium Calli Extracts on rat liver cells. J Appl Sci Res 3(12):1987–1996
Acosta MB, Villalobos MJP, Rodríguez B (2008) The antifeedant activity of natural plant products towards the larvae of Spodoptera littoralis. Span J agric Res 1:85–91
Akaberi M, Mehri S, Iranshahi M (2015) Multiple pro-apoptotic targets of abietane diterpenoids from Salvia species. Fitoterapia 100:118–132
Bahadori MB, Valizadeh H, Asghari B, Dinparast L, Moridi Farimani M, Bahadori S (2015) Chemical composition and antimicrobial, cytotoxicity, antioxidant and enzyme inhibitory activities of Salvia spinosa L. J Funct Foods 18:727–736
Burmistrova O, Simões MF, Rijo P, Quintana J, Bermejo J, Estévez F (2013) Antiproliferative activity of abietane diterpenoids against human tumor cells. J Nat Prod 76:1413–1423
Ebrahimi SN, Zimmermann S, Zaugg J, Smiesko M, Brun R, Hamburger M (2013) Abietane diterpenoids from Salvia sahendica–antiprotozoal activity and determination of their absolute configurations. Planta Med 79:150–156
Ebrahimi SN, Moridi Farimani M, Mirzania F, Soltanipoor MA, De Mieri M, Hamburger M (2014) Manoyloxide sesterterpenoids from Salvia mirzayanii. J Nat Prod 77:848–854
Fronza M, Murillo R, Ślusarczyk S, Adams M, Hamburger M, Heinzmann B, Laufer S, Merfort I (2011) In vitro cytotoxic activity of abietane diterpenes from Peltodon longipes as well as Salvia miltiorrhiza and Salvia sahendica. Bioorg med chem 19(16):4876–4881
Ginda H, Kusumi T, Ishitsuka MO, Kakisawa H, Weijie Z, Jun C, Tian GY (1988) Salviolone, a cytotoxic bisnorditerpene with a benzotropolone chromophore from a chinese drug dan-shen (Salvia miltiorrhiza). Tetrahedron Lett 29:4603–4606
González AG, Aguiar ZE, Grillo TA, Luis JG (1992) Diterpenes and diterpene quinones from the roots of Salvia apiana. Phytochemistry 31(5):1691–1695
Hanson RL, Lardy HA, Kupchan SM (1970) Inhibition of phosphofructokinase by quinone methide and α-methylene lactone tumor inhibitors. Science 168(3929):378–380
Ikeshiro Y, Hashimoto I, Iwamoto Y, Mase I, Tomita Y (1991) Diterpenoids from Salvia miltiorrhiza. Phytochemistry 30(8):2791–2792
Jamzad Z, Assadi M, Maassoumi A, Mozaffarian V (2012) Lamiaceae. In: Flora of Iran, vol. 76. Research Institute of Forest and Rangelands, Tehran
Jassbi AR, Mehrdad M, Eghtesadi F, Ebrahimi SN, Baldwin IT (2006) Novel rearranged abietane diterpenoids from the roots of Salvia sahendica. Chem Biodivers 3:916–922
Kintzios SE (2000) Sage: the genus salvia. Harwood Academic Publishers, Amsterdam, pp 55–68
Kolak U, Kabouche A, Öztürk M, Kabouche Z, Topçu G, Ulubelen A (2009) Antioxidant diterpenoids from the roots of Salvia barrelieri. Phytochem Anal 20(4):320–327
Kupchan SM, Karim A, Marcks C (1969) Tumor inhibitors. XLVIII. Taxodione and taxodone, two novel diterpenoid quinone methide tumor inhibitors from Taxodium distichum. J Org Chem 34(12):3912–3918
Kusumoto N, Ashitani T, Hayasaka Y, Murayama T, Ogiyama K, Takahashi K (2009) Antitermitic activities of abietane-type diterpenes from Taxodium distichum cones. J Chem Ecol 35(6):635–642
Machumi F, Samoylenko V, Yenesew A, Derese S, Midiwo JO, Wiggers FT, Jacob MR, Tekwani BL, Khan SI, Walker LA, Muhammad I (2010) Antimicrobial and antiparasitic abietane diterpenoids from the roots of Clerodendrum eriophyllum. Nat Prod Commun 5(6):853
Michavila A, Fernández-Gadea F, Rodríguez B (1985) Abietane diterpenoids from the root of Salvia lavandulaefolia. Phytochemistry 25(1):266–268
Michavila A, María C, Rodríguez B (1986) 20-Nor-abietane and rearranged abietane diterpenoids from the root of Salvia argentea. Phytochemistry 25(8):1935–1937
Moridi Farimani M, Mazarei Z (2014) Sesterterpenoids and other constituents from Salvia lachnocalyx Hedge. Fitoterapia 98:234–240
Moridi Farimani M, Miran M (2014) Labdane diterpenoids from Salvia reuterana. Phytochemistry 108:264–269
Moridi Farimani M, Taheri S, Matloubi Moghaddam F, Amin G (2008) Chemical constituents from Salvia macrosiphon Boiss. Chem Nat Comp 44:518–519
Moridi Farimani M, Matloubi Moghaddam F, Esmaeili MA, Amin GA (2012) Lupane triterpenoid and other constituents of Salvia eremophila. Nat Prod Res 26(21):2045–2049
Moridi Farimani M, Ebrahimi SN, Salehi P, Bahadori MB, Sonboli A, Khavasi HR, Zimmermann S, Kaiser M, Hamburger M (2013) Antitrypanosomal triterpenoid with an ε-lactone E-ring from Salvia urmiensis. J Nat Prod 76:1806–1809
Moujir L, Gutiérrez-Navarro AM, San Andrés L, Luis JG (1996) Bioactive diterpenoids isolated from Salvia mellifera. Phytother Res 10(2):172–174
Rechinger KH (1987) Flora Iranica. Akademische Druck- und Verlagsanstalt, Graz, pp 403–476
Saijo H, Kofujita H, Takahashi K, Ashitani T (2015) Antioxidant activity and mechanism of the abietane-type diterpene ferruginol. Nat Prod Res 29(18):1739–1743
Shaheen UY, Hussain MH, Ammar HA (2011) Cytotoxicity and antioxidant activity of new biologically active constituents from Salvia Lanigra and Salvia Splendens. Pharmacogn J 3(21):36–48
Tan N, Kaloga M, Radtke OA, Kiderlen AF, Öksüz S, Ulubelen A, Kolodziej H (2002) Abietane diterpenoids and triterpenoic acids from Salvia cilicica and their antileishmanial activities. Phytochemistry 61(8):881–884
Topçu G, Gören AC (2007) Biological activity of diterpenoids isolated from Anatolian Lamiaceae plants. Rec Nat Prod 1(1):1–16
Topcu G, Eriş C, Ulubelen A (1996) Rearranged abietane diterpenes from Salvia limbata. Phytochemistry 41(4):1143–1147
Topcu G, Kolak U, Ozturk M, Boga M, Hatipoglu SD, Bahadori F, Culhaoglu B, Dirmenci T (2013) Investigation of anticholinesterase activity of a series of Salvia extracts and the constituents of Salvia staminea. Nat Prod J 3(1):3–9
Ulubelen A (2003) Cardioactive and antibacterial terpenoids from some Salvia species. Phytochemistry 64(2):395–399
Ulubelen A, Topcu G, Tan N, Lin LJ, Cordell GA (1992) Microstegiol, a rearranged diterpene from Salvia microstegia. Phytochemistry 31(7):2419–2421
Ulubelen A, Topcu G, Eri C, Sönmez U, Kartal M, Kurucu S, Bozok-Johansson C (1994) Terpenoids from Salvia sclarea. Phytochemistry 36(4):971–974
Ulubelen A, Öksüz S, Kolak U, Tan N, Bozok-Johansson C, Çelik C, Kohlbau HJ, Voelter W (1999a) Diterpenoids from the roots of Salvia bracteata. Phytochemistry 52(8):1455–1459
Ulubelen A, Topçu G, Chai HB, Pezzuto JM (1999b) Cytotoxic activity of diterpenoids isolated from Salvia hypargeia. Pharm Boil 37(2):148–151
Ulubelen A, Oksüz S, Kolak U, Birman H, Voelter W (2000) Cardioactive terpenoids and a new rearranged diterpene from Salvia syriaca. Planta Med 66:627–629
Wu YB, Ni ZY, Shi QW, Dong M, Kiyota H, Gu YC, Cong B (2012) Constituents from Salvia species and their biological activities. Chem Rev 112:5967–6026
Yang Z, Kitano Y, Chiba K, Shibata N, Kurokawa H, Doi Y, Arakawa Y, Tada M (2001) Synthesis of variously oxidized abietane diterpenes and their antibacterial activities against MRSA and VRE. Bioorg Med Chem 9(2):347–356
Yong ZJH (1995) Two new diterpenoids, prtoketolactone and neoprionitone, from Salvia prionitis. Nat Prod Res Develop 4:1
Zaghloul AM, Gohar AA, Naiem ZAA, Bar FMA (2008) Taxodione, a DNA-binding compound from Taxodium distichum L. (Rich.). Z Naturforsch C 63(5–6):355–360
Authors’ contributions
Analysis and interpretation of data: by FE, MM and NH. All authors read and approved the final manuscript.
Acknowledgements
Financial support by the University of Sistan & Baluchestan Research Council is gratefully acknowledged. All spectra were performed at the Department of Pharmaceutical Sciences, Division of Pharmaceutical Biology, University of Basel. The kind assistance of Prof. M. Hamburger, Dr. S.N. Ebrahimi, and all other staff is gratefully appreciated.
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Eghtesadi, F., Moridi Farimani, M., Hazeri, N. et al. Abietane and nor-abitane diterpenoids from the roots of Salvia rhytidea . SpringerPlus 5, 1068 (2016). https://doi.org/10.1186/s40064-016-2652-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40064-016-2652-0