Abstract
Background
Epilepsy, a central neurological disorder, has a complex genetic architecture. There is some evidence suggesting that genetic factors play a role in both the occurrence of epilepsy and its treatment. However, the genetic determinants of epilepsy are largely unknown. This study aimed to identify potential therapeutic targets for epilepsy.
Methods
Differentially expressed genes (DEGs) were extracted from the expression profiles of GSE44031 and GSE1834. Gene co-expression analysis was used to confirm the regulatory relationship between newly discovered epilepsy candidate genes and known epilepsy genes. Expression quantitative trait loci analysis was conducted to determine if epilepsy risk single-nucleotide polymorphisms regulate DEGs’ expression in human brain tissue. Finally, protein–protein interaction analysis and drug–gene interaction analysis were performed to assess the role of DEGs in epilepsy treatment.
Results
The study found that the protein tyrosine phosphatase receptor-type O gene (PTPRO) and the growth arrest and DNA damage inducible alpha gene (GADD45A) were significantly upregulated in epileptic rats compared to controls in both datasets. Gene co-expression analysis revealed that PTPRO was co-expressed with RBP4, NDN, PAK3, FOXG1, IDS, and IDS, and GADD45A was co-expressed with LRRK2 in human brain tissue. Expression quantitative trait loci analysis suggested that epilepsy risk single-nucleotide polymorphisms could be responsible for the altered PTPRO and GADD45A expression in human brain tissue. Moreover, the protein encoded by GADD45A had a direct interaction with approved antiepileptic drug targets, and GADD45A interacts with genistein and cisplatin.
Conclusions
The results of this study highlight PTPRO and GADD45A as potential genes for the diagnosis and treatment of epilepsy.
Similar content being viewed by others
Background
Epilepsy, a central neurological disorder characterized by recurrent, spontaneous seizures, affects over 68 million people globally [1]. The primary causes of epilepsy are abnormal discharges in the hippocampus or cerebral cortex [2, 3]. While approximately 70% of seizures can be effectively managed with approved antiepileptic drugs [4], the remaining 30% are resistant to pharmacotherapy, resulting in significant psychological and physical burdens for patients [5].
The genetic determinants of epilepsy are largely unknown. There is evidence suggesting that genetic factors contribute to both generalized and focal epilepsies [6]. Mutations in certain genes have been associated with epilepsy [6, 7], but the role of common polymorphisms in epilepsy is still unclear [8, 9]. Several recent studies have identified new epilepsy loci [10,11,12,13,14,15], and further expanded analysis has revealed additional new loci for epilepsy [16]. Moreover, gene therapy has been reported as a potential treatment for refractory focal epilepsy [17].
Bioinformatics-based studies using microarray analysis are crucial for identifying disease-related gene expression patterns [18,19,20,21]. In this study, we analyzed gene expression between epileptic rat models and healthy samples to investigate their potential association with epilepsy. We further conducted integrative analyses with gene co-expression, expression quantitative trait loci (eQTL), protein–protein interaction (PPI) networks, and drug–gene interaction to clarify the potential role of newly discovered epilepsy-associated genes in the diagnosis and treatment of epilepsy.
Materials and methods
Gene Expression Omnibus datasets
In this study, we utilized two independent datasets from the Gene Expression Omnibus (GEO). These datasets, GSE44031 [22] and GSE1834 [23], were analyzed using the GPL9207 platform (Duke Operon Rat 27 k V3.0 printed oligonucleotide array) and the GPL85 platform ([RG_U34A] Affymetrix Rat Genome U34 Array), respectively. For detailed information on these datasets, please refer to the GEO database (http://www.ncbi.nlm.nih.gov/geo).
Differentially expressed genes (DEGs) analysis
Differentially expressed genes (DEGs) were identified using R software. The gene expression levels were extracted from the GSE44031 dataset, which includes microarray data from eight hippocampal tissue samples from epileptic rats induced by kainic acid injection, and four samples from rats injected with phosphate buffer saline. These differentially expressed genes were further validated in the GSE1834 dataset, which contains data from 15 hippocampal tissue samples from epileptic rats induced by kainic acid injection, and 15 samples from rats injected with phosphate buffer saline. A statistical significance threshold was set at an adjusted p value ≤ 0.05 and |logFold change|≥ 1. The overlapping DEGs were selected for further analysis using a Venn map, which was created using the Venn online tool (http://bioinformatics.psb.ugent.be/webtools/Venn/).
Gene co-expression analysis
Gene co-expression analysis is a method used to discover the regulatory relationships between genes and subsequently identify potential disease candidate genes. Given that gene expression is tissue-specific, we investigated the co-expression relationships between DEGs and known epilepsy genes in human brain tissues. The gene co-expression database for brain tissue serves as an effective tool for analyzing the co-expression relationships of genes associated with brain diseases [24].
eQTL analysis
To determine the impact of epilepsy risk single-nucleotide polymorphisms (SNPs) on the expression of DEGs in human brain tissue, we performed eQTL analysis. This analysis was conducted using BRAINEAC (http://www.Braineac.org/) [25], a tool designed to investigate the association between SNPs and the expression of their target genes in human brain tissue.
Evaluation of DEGs in epilepsy treatment
To identify new antiepileptic drug targets and facilitate the translation of these findings into clinical therapy, we first identified approved antiepileptic drug therapeutic targets using DrugBank5.0 and the Therapeutic Target Database 2020 [26, 27]. We then conducted PPI analysis to explore the relationship between newly discovered epilepsy-related genes and approved antiepileptic drug target genes. This PPI analysis was performed using the STRING database (https://string-db.org/cgi/input.pl) [28], and a PPI network was constructed using Cytoscape software [29]. The findings from the PPI analysis were further validated by a drug–gene interaction analysis using the Drug–Gene Interaction Database [30].
Result
Analysis of DEGs in epilepsy rat models and controls
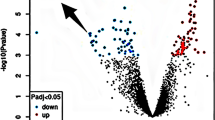
Based on the selection criteria for DEGs outlined in the “Methods” section, 91 DEGs were selected from the GSE44031 dataset (see Additional file 1: Table S1), and 425 DEGs were selected from the GSE1834 dataset (see Additional file 2: Table S2). As depicted in Fig. 1, five genes overlapped between the two datasets (see Additional file 3: Table S3). Specifically, among these five DEGs, the expression regulation of one gene was inconsistent across the two datasets, leading to its removal. The expression of the remaining four genes was upregulated in both datasets (Fig. 2), with two of them (GFAP and S100A4) previously reported to be associated with epilepsy [31, 32]. Consequently, we selected two newly discovered candidate genes, the protein tyrosine phosphatase receptor-type O gene (PTPRO) and the growth arrest and DNA damage inducible alpha gene (GADD45A), for further analysis. The Volcano map of DEGs was created using the online volcano plotting tool (http://sangerbox.com/AllTools?tool_id=9699135).
Gene co-expression analysis
As genetic research advances, an increasing number of epilepsy-related genes have been identified [33]. In this study, we utilized these reported epilepsy-related genes along with the newly discovered genes for gene co-expression analysis. The analysis revealed that PTPRO was co-expressed with RBP4, NDN, PAK3, FOXG1, and IDS in human brain tissue. Similarly, GADD45A was found to be co-expressed with LRRK2 in human brain tissue (Fig. 3).
eQTL analysis
In this study, we focused on SNPs that have been previously reported to be associated with epilepsy for eQTL analysis. Specifically, we explored whether the SNPs rs4794333, rs68082256, rs11943905, rs12185644, rs6432877, rs2212656, rs1402398, rs11890028, rs887696, rs1044352, rs13200150, and rs4671319, which have been associated with epilepsy in a multicenter study [16], regulate the expression levels of the newly discovered genes in human brain tissue. Interestingly, our findings suggest that these SNPs do indeed regulate the expression levels of PTPRO and GADD45A in human brain tissue (Table 1).
Evaluation of PTPRO and GADD45A in epilepsy treatment
To explore the potential role of PTPRO and GADD45A in epilepsy treatment, we first conducted an interaction analysis between the proteins encoded by PTPRO and GADD45A and the targets of approved antiepileptic drugs (Table 2). We found that the protein encoded by GADD45A had direct interactions with the targets of approved antiepileptic drugs, including PPARA and PPARG (Fig. 4). Further drug–gene interaction analysis revealed that GADD45A interacts with genistein and cisplatin.
Discussion
In this comprehensive analysis of epilepsy, we identified two new potential genes, PTPRO and GADD45A, and highlighted their crucial roles in epilepsy. Our results suggest that GADD45A might serve as a potential therapeutic target for epilepsy. The Consortium on Complex Epilepsies has made significant strides in identifying several loci with genome-wide significance for epilepsy [15, 16]. Research aimed at understanding the role of genes in epilepsy will contribute to a deeper understanding of the genetic architecture of epilepsy. To the best of our knowledge, the association of PTPRO and GADD45A with epilepsy has not been evaluated previously.
In this study, we initially identified five DEGs that overlapped in the GSE44031 and GSE1834 gene expression profiles. Further analysis revealed that two of these DEGs, PTPRO and GADD45A, had not been previously reported to be associated with epilepsy. Gene co-expression analysis uncovered specific regulatory relationships between these two newly discovered epilepsy-associated genes and known epilepsy genes (Fig. 3). These findings suggest that these potential genes are functionally associated with the reported epilepsy genes, further underscoring the role of these newly discovered genes in epilepsy. This could facilitate the diagnosis and treatment of epilepsy and may provide a new direction in the understanding of the disease.
SNPs located in noncoding regions can influence disease risk by regulating the expression of their target genes [34, 35]. Our eQTL analysis revealed that several noncoding SNPs associated with epilepsy risk alter the expression of PTPRO and GADD45A in brain tissue (Table 1). Moreover, these SNPs have been reported to be associated with epilepsy [16]. Therefore, we hypothesize that these SNPs may contribute to epilepsy risk by regulating PTPRO and GADD45A expression in human brain tissue. These findings further elucidate the mechanisms of PTPRO and GADD45A in the pathogenesis of epilepsy.
PTPRO, located on chromosome 12, encodes the receptor-type tyrosine-protein phosphatase O. It is highly expressed in the brain and promotes the formation of excitatory synapses [36]. PTPRO also plays a role in regulating the development and function of the sensory nervous system [37]. A genome-wide study has shown that PTPRO is associated with learning and memory [38]. On the other hand, GADD45A has been found to influence cortical evolution and diversity depending upon its expression levels [39, 40]. Therefore, both PTPRO and GADD45A may play significant roles in the pathogenesis of brain diseases.
PPI networks play a crucial role in drug target discovery and present drug discovery process [41]. Our PPI analysis revealed that the protein encoded by GADD45A directly interacts with the targets of approved antiepileptic drugs, PPARA and PPARG (Fig. 4). By integrating data from DrugBank5.0 and the Therapeutic Target Database 2020, we found that PPARA and PPARG are targets of Valproate. Furthermore, we discovered that GADD45A interacts with genistein and cisplatin. Genistein has been implicated in antiepileptic effects [42, 43], while cisplatin has been reported to induce seizures [44, 45]. Therefore, the results of the PPI networks and drug–gene interaction further underscore the significant role of GADD45A in epilepsy therapy. While recent studies have discovered new epilepsy-related genes [46, 47], our study not only identified new epilepsy-related genes but also explored the potential mechanisms of these new genes in the pathogenesis and treatment of epilepsy, which could be more conducive to the transformation of clinical application.
While our study integrated data from gene expression, gene co-expression, eQTL, PPI network, and drug–gene interaction to uncover the role of PTPRO and GADD45A in epilepsy diagnosis and therapy, there are still some limitations. First, further validation of our findings in independent populations is necessary to strengthen our conclusions. Second, additional functional characterization would help to better understand the mechanisms of PTPRO and GADD45A in the pathogenesis and treatment of epilepsy.
Conclusion
In summary, our findings underscore the potential of PTPRO and GADD45A as promising targets for the diagnosis and treatment of epilepsy.
Availability of data and materials
The datasets generated during the current study are available in the GEO repository.
Abbreviations
- DEGs:
-
Differentially expressed genes
- eQTL:
-
Expression quantitative trait loci
- PPI:
-
Protein–protein interaction
- SNPs:
-
Single-nucleotide polymorphisms
- GFAP :
-
Glial Fibrillary Acidic Protein
- S100A4 :
-
S100 Calcium-Binding Protein A4
- PTPRO :
-
Protein Tyrosine Phosphatase Receptor Type O
- GADD45A :
-
Growth Arrest and DNA Damage Inducible Alpha
References
Ngugi AK, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Estimation of the burden of active and life-time epilepsy: a meta-analytic approach. Epilepsia. 2010;51(5):883–90.
Avanzini G, Franceschetti S. Cellular biology of epileptogenesis. Lancet Neurol. 2003;2(1):33–42.
Pitkänen A, Sutula TP. Is epilepsy a progressive disorder? Prospects for new therapeutic approaches in temporal-lobe epilepsy. Lancet Neurol. 2002;1(3):173–81.
Kwan P, Schachter SC, Brodie MJ. Drug-resistant epilepsy. N Engl J Med. 2011;365(10):919–26.
Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342(5):314–9.
Helbig I, Scheffer IE, Mulley JC, Berkovic SF. Navigating the channels and beyond: unravelling the genetics of the epilepsies. Lancet Neurol. 2008;7(3):231–45.
Poduri A, Lowenstein D. Epilepsy genetics—past, present, and future. Curr Opin Genet Dev. 2011;21(3):325–32.
Epi4K Consortium. Epi4K: gene discovery in 4,000 genomes. Epilepsia. 2012;53(8):1457–67.
Dibbens LM, Heron SE, Mulley JC. A polygenic heterogeneity model for common epilepsies with complex genetics. Genes Brain Behav. 2007;6(7):593–7.
International League Against Epilepsy Consortium on Complex Epilepsies. Genetic determinants of common epilepsies: a meta-analysis of genome-wide association studies. Lancet Neurol. 2014;13(9):893–903.
International League Against Epilepsy Consortium on Complex Epilepsies. GWAS meta-analysis of over 29,000 people with epilepsy identifies 26 risk loci and subtype-specific genetic architecture. Nat Genet. 2023;55(9):1471–82.
Song M, Liu J, Yang Y, Lv L, Li W, Luo XJ. Genome-wide meta-analysis identifies two novel risk loci for Epilepsy. Front Neurosci. 2021;15: 722592.
Mirza N, Appleton R, Burn S, du Plessis D, Duncan R, Farah JO, et al. Genetic regulation of gene expression in the epileptic human hippocampus. Hum Mol Genet. 2017;26(9):1759–69.
Lu M, Feng R, Zhang C, Xiao Y, Yin C. Identifying novel drug targets for Epilepsy through a Brain Transcriptome-Wide Association Study and Protein-Wide Association Study with chemical-gene-interaction analysis. Mol Neurobiol. 2023;60(9):5055–66.
Rawat C, Kushwaha S, Srivastava AK, Kukreti R. Peripheral blood gene expression signatures associated with epilepsy and its etiologic classification. Genomics. 2020;112(1):218–24.
International League Against Epilepsy Consortium on Complex Epilepsies. Genome-wide mega-analysis identifies 16 loci and highlights diverse biological mechanisms in the common epilepsies. Nat Commun. 2018;9(1):5269.
Kullmann DM, Schorge S, Walker MC, Wykes RC. Gene therapy in epilepsy-is it time for clinical trials? Nat Rev Neurol. 2014;10(5):300–4.
Deng JL, Xu YH, Wang G. Identification of potential crucial genes and key pathways in breast cancer using bioinformatic analysis. Front Genet. 2019;10:695.
Ceylan H. Identification of hub genes associated with obesity-induced hepatocellular carcinoma risk based on integrated bioinformatics analysis. Med Oncol. 2021;38(6):63.
Ma L, Lu H, Chen R, Wu M, Jin Y, Zhang J, et al. Identification of key genes and potential new biomarkers for ovarian aging: a study based on RNA-sequencing data. Front Genet. 2020;11: 590660.
Ceylan H. Integrated bioinformatics analysis to identify alternative therapeutic targets for Alzheimer’s disease: insights from a synaptic machinery perspective. J Mol Neurosci. 2022;72(2):273–86.
Friedman LK, Mancuso J, Patel A, Kudur V, Leheste JR, Iacobas S, et al. Transcriptome profiling of hippocampal CA1 after early-life seizure-induced preconditioning may elucidate new genetic therapies for epilepsy. Eur J Neurosci. 2013;38(1):2139–52.
Wilson DN, Chung H, Elliott RC, Bremer E, George D, Koh S. Microarray analysis of postictal transcriptional regulation of neuropeptides. J Mol Neurosci. 2005;25(3):285–98.
Freytag S, Burgess R, Oliver KL, Bahlo M. brain-coX: investigating and visualising gene co-expression in seven human brain transcriptomic datasets. Genome Med. 2017;9(1):55.
Ramasamy A, Trabzuni D, Guelfi S, Varghese V, Smith C, Walker R, et al. Genetic variability in the regulation of gene expression in ten regions of the human brain. Nat Neurosci. 2014;17:1418–28.
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018;46(D1):D1074–82.
Wang Y, Zhang S, Li F, Zhou Y, Zhang Y, Wang Z, et al. Therapeutic target database 2020: enriched resource for facilitating research and early development of targeted therapeutics. Nucleic Acids Res. 2020;48(D1):D1031–41.
von Mering C, Huynen M, Jaeggi D, Schmidt S, Bork P, Snel B. STRING: a database of predicted functional associations between proteins. Nucleic Acids Res. 2003;31:258–61.
Su G, Morris JH, Demchak B, Bader GD. Biological network exploration with Cytoscape 3. Curr Protoc Bioinformatics. 2014;47:1–24.
Cotto KC, Wagner AH, Feng YY, Kiwala S, Coffman AC, Spies G, et al. DGIdb 3.0: a redesign and expansion of the drug–gene interaction database. Nucleic Acids Res. 2018;46(D1):D1068–73.
Wang M, Yu J, Xiao X, Zhang B, Tang J. Changes of biochemical biomarkers in the serum of children with convulsion status epilepticus: a prospective study. BMC Neurol. 2022;22(1):196.
Lipponen A, Paananen J, Puhakka N, Pitkänen A. Analysis of post-traumatic brain injury gene expression signature reveals tubulins, Nfe2l2, Nfkb, Cd44, and S100a4 as treatment targets. Sci Rep. 2016;6:31570.
Wang J, Lin ZJ, Liu L, Xu HQ, Shi YW, Yi YH, et al. Epilepsy-associated genes. Seizure. 2017;44:11–20.
Wang S, Zhang X, Zhou L, Wu Q, Han Y. Analysis of GABRG2 C588T polymorphism in genetic epilepsy and evaluation of GABRG2 in drug treatment. Clin Transl Sci. 2021;14(5):1725–33.
Wang S, Zhou L, He C, Wang D, Cai X, Yu Y, et al. The association between STX1B polymorphisms and treatment response in patients with Epilepsy. Front Pharmacol. 2021;12: 701575.
Jiang W, Wei M, Liu M, Pan Y, Cao D, Yang X, et al. Identification of Protein Tyrosine Phosphatase Receptor Type O (PTPRO) as a synaptic adhesion molecule that promotes synapse formation. J Neurosci. 2017;37(41):9828–43.
Gonzalez-Brito MR, Bixby JL. Protein tyrosine phosphatase receptor type O regulates development and function of the sensory nervous system. Mol Cell Neurosci. 2009;42(4):458–65.
LeBlanc M, Kulle B, Sundet K, Agartz I, Melle I, Djurovic S, et al. Genome-wide study identifies PTPRO and WDR72 and FOXQ1-SUMO1P1 interaction associated with neurocognitive function. J Psychiatr Res. 2012;46(2):271–8.
Matsunaga E, Nambu S, Oka M, Iriki A. Comparative analysis of developmentally regulated expressions of Gadd45a, Gadd45b, and Gadd45g in the mouse and marmoset cerebral cortex. Neuroscience. 2015;284:566–80.
Sarkisian MR, Siebzehnrubl D. Abnormal levels of Gadd45alpha in developing neocortex impair neurite outgrowth. PLoS ONE. 2012;7(9): e44207.
Chakraborty C, Doss CGP, Chen L, Zhu H. Evaluating Protein-protein Interaction (PPI) networks for diseases pathway, target discovery, and drug-design using ‘In silico Pharmacology.’ Curr Protein Pept Sci. 2014;15(6):561–71.
Amiri Gheshlaghi S, Mohammad Jafari R, Algazo M, Rahimi N, Alshaib H, Dehpour AR. Genistein modulation of seizure: involvement of estrogen and serotonin receptors. J Nat Med. 2017;71(3):537–44.
Elsayed AA, Menze ET, Tadros MG, Ibrahim BMM, Sabri NA, Khalifa AE. Effects of genistein on pentylenetetrazole-induced behavioral and neurochemical deficits in ovariectomized rats. Naunyn Schmiedebergs Arch Pharmacol. 2018;391(1):27–36.
Cattaneo MT, Filipazzi V, Piazza E, Damiani E, Mancarella G. Transient blindness and seizure associated with cisplatin therapy. J Cancer Res Clin Oncol. 1988;114(5):528–30.
Dana R, Spartacus RK, Mutha S, Bhat P. Seizure following chemotherapy (paclitaxel and cisplatin) in a patient of carcinoma cervix. Indian J Pharmacol. 2016;48(6):736–8.
Riaz M, Abbasi MH, Sheikh N, Saleem T, Virk AO. GABRA1 and GABRA6 gene mutations in idiopathic generalized epilepsy patients. Seizure. 2021;93:88–94.
Kaminski VL, Kulmann-Leal B, Tyska-Nunes GL, Beltrame BP, Riesgo RDS, Schüler-Faccini L, et al. Association between NKG2/KLR gene variants and epilepsy in Autism Spectrum Disorder. J Neuroimmunol. 2023;381: 578132.
Acknowledgements
The authors would like to thank all the professionals and databases managers for helping us in this study.
Funding
This study was financially supported by Natural Science Foundation of Anhui Provincial Education Department (2022AH050756).
Author information
Authors and Affiliations
Contributions
SW and XD contributed to the conception and design of the project. ZX, ZL, and XD performed all the figures. HX, XM, MZ, and JL drafted the manuscript. JT and FR supervised the findings of this work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors consented for the publication.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
91 DEGs were identified in GSE44031 series.
Additional file 2: Table S2.
425 DEGs were identified in GSE1834 series.
Additional file 3: Table S3.
5 overlapping DEGs were identified in GSE1834 and GSE44031.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, S., Xie, Z., Jun, T. et al. Identification of potential crucial genes and therapeutic targets for epilepsy. Eur J Med Res 29, 43 (2024). https://doi.org/10.1186/s40001-024-01643-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-024-01643-8