Abstract
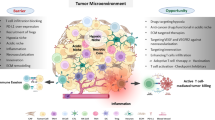
The tumor microenvironment is a result of dynamic interaction between different cellular and non-cellular components. In its essence it is not a solo performer, but an ensemble of performers that includes cancer cells, fibroblasts, myo-fibroblasts, endothelial cells and immune cells. The short review highlights important immune infiltrates within the tumor microenvironment that shape cytotoxic t lymphocyte (CTL)-rich immune hot and CTL-deficient immune cold tumors and novel strategies that have potential role in enhancing our immune responses in both immune hot and immune cold tumors.
Similar content being viewed by others
Introduction
Tumors are identified by their cellular features that include cytoplasmic appearance, nuclear differentiation and atypical makeup. Thoroughly described by Hanahan et al., cancer cells are programmed to resist cell death, proliferate, evade growth suppressors, metastasize, induce angiogenesis, attain replicative immortality and reprogram metabolism. They have the potenital to avoid immune destruction, undergo non-mutational epigenetic programming and possess phenotypic plasticity [1]. The dynamics between these oncogenetic processes and intrinsic host response molds the environment around it. This tumor microenvironment (TME) consists of various cellular and non-cellular components which is effectively the result of intrinsic host response such as cytokines, chemokines, and inflammation responding to disrupting cancer behavior, tumor surface antigens and mechanisms of cell defense/ growth [2]. The entire avenue of disease prognosis and treatment response is affected by the understanding of these various components of the tumor microenvironment.
Tumor immune microenvironment and immune infiltrates
One of the most significant components of the TME is the immune component. Immune infiltration and its role in cancer treatment were first reported more than a century back [3]. Immune infiltrates are the result of cancerous cells which are supported by two theories. First, cancer is antigenic and induces an active immune response [4] and second immune surveillance is vital for cancer progression and regression [5].
More recently, the concept of tumor immune microenvironment (TIME) has been well elaborated and their role in prognostication and treatment response has been established by various studies. A variety of immune cells including lymphocytic cells (T, B, natural killer cells), myeloid cells (neutrophils, macrophages, dendritic cells). Along with other effector and regulatory cells in adaptive immunity such as Type 1T helper cells (Th1), Type 2T helper cells (Th2), T helper 17 cells (Th17), T regulatory cells (Treg) have been identified within the tumor microenvironment. Amongst these cells infiltrating lymphocytes, dendritic cells, t-follicular helper have favorable prognostic role whereas m2 macrophages, polymorph nuclear cells identified as non-favorable cellular types [6].
Immune hot and immune cold tumors
Based on the characteristics of these immune infiltrates tumors can be classified as either immune hot or immune cold tumors. Immune hot tumors or t inflamed tumors usually express a higher number of programmed death ligand-1 (PDL-1) expressing tumors and infiltration of PD-1 expressing CTLs [7]. The infiltrating lymphoid and myeloid cells in the immune hot tumors have some unique functions such as, monocytes polarized into M1 types secrete lysosomes and destroy tumor cells. NK cells are capable of lysing cells with decreased expression of MHC proteins, e.g., virus infected cells, virus affected tumorous cells, mutating cancer cells [8]. Increased NK cell expression in tumor microenvironment (TME) milieu has shown better prognosis. Cytotoxic CD4 T cells (Th1, Th2 cells) not only provide immunologic help by enhancing anti-tumor cytotoxicity of CD8 T cells and macrophage activity, but also have direct action on cancer cells much like the CTLs which are both contact and granule dependent [9]. Most importantly, CTLs play a major role in our ability to recognize and destroy cancer cells, they do so via their ability to recognize tumor antigens presented on MHC I molecules and mount an effector function destroying malignant or pre malignant cells. Presence of CTL in tumor margins have proven good prognostic values in various cancers (e.g., colon cancer, lung cancer, melanoma [10,11,12] and its anti-tumor potential has been harnessed to develop effective immunotherapeutics drugs such as immune check point inhibitors and chimeric antigen receptor t cells (CARTs).
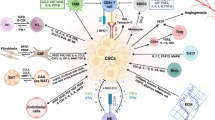
Immune cold tumors are t uninflamed tumors, they lack PDL-1 expressing tumor cells and PD-1 expressing CTL.A variety of reasons make some tumors immune cold, e.g., decreased antigenic expression [13] effective immune evasion methods [14], T cells repelling vasculature and high expression of immune suppressing immune infiltrates [15]. They have a characteristically increased expression of myeloid derived stromal cells (MDSC), M2 macrophages, PMNs, Tregs and Th17 cells [16, 17]. Tumor recruited MDSCs plays a central role in controlling and maintaining an immunosuppressive TME. It does so via immunologic and non-immunologic mechanism. MDSC scan remodel tissues with stromal components that are pro tumor, promote angiogenesis for tumor growth along with inhibition T cells proliferation, cytokine production and cytotoxic function [18]. In addition M2 polarized macrophages and T Regs support angiogenesis by secreting adrenomedullin and vascular epithelial growth factors (VEGFs) and express immunosuppressive molecules such as IL-10, programmed death-ligand 1 (PD-L1), and TGFβ, favoring tumor growth [19].
Immunotherapeutic agents in immune hot and immune cold tumors
Immune hot tumors have an endogenously activated T cell population. These are, however, suppressed by tumors expressing T cell inhibitory signals. Widely studied in clinical setting CTLA-4+/CD86, PD-1/PDL-1, MHC/LAG3 inhibitory pathway blockers have shown clinical benefit and is approved for treatment in a variety of advanced solid and hematologic malignancies [20, 21]. However, efficacy and durability of anti-tumor response with immune checkpoint inhibitors have a large space for improvement [22]. One of the key components for an effective and sustained immune response is APC activity. T cells need peptide-mhc complexes and co-stimulatory signals from APC to mount an effector response. These signals can be compounded with the use of CD40 agonists [23]. CD40 ligation on APCs induces increased surface expression of co-stimulatory and MHC molecules, production of pro-inflammatory cytokines, and enhanced T-cell triggering [24]. Hence CD40 agonists moAb can play an important role in enhancing APC function for a meaningful and sustained anti-tumor T cell function. Recently, immune check point refractory metastatic melanoma have demonstrated treatment benefit from combination use of CD40 agonistic antibody and nivolumab therapy [25]. Similarly, CD 40 moab (sotigalimab) had manageable toxicity and an early efficacy signal in a phase Ib pancreatic cancer trial [26].
Immune cold tumors are another side of the story it possesses a very unique and challenging clinical scenario. Besides being intratumorally CTL deficient [27], immune cold tumors contain effector T cells that have defective priming and homing signals. A deficient TAA, low MHC presentation of antigenic material, T cell/ DC interactions abnormal vasculature and immune suppressive TME, all contribute to defective priming/homing mechanisms in these types of tumor [28]. Thus treatment methods which could turn these unfavorable conditions to favorable ones are being explored. Among a few active agents CD137 agonist compounds in the form of monoclonal antibodies, CART cells and adoptive cell transfer have shown promising results. CD137 expressing T cells have been known to be highly cytotoxic. Moreover, in non-immunogenic tumors they can overcome effects of PDL1 inhibition and T reg associated suppression of microenvironment to mount an effective anti-tumor T cell activity [29]. In clinical setting CD137 agonist molecules (MCLA-145) in combination with PD-1 blocker are expected to enhance naïve T cell priming and promote long-term T cell immunity.
One of the major areas of interest in immune cold tumors is TAMS or M2 macrophages. They secrete IL-6, IL-8 promoting cancer cell growth and activate Th2 effector cells, and promote tumor angiogenesis via activation of VEGF and TGF beta. Furthermore they release matrix metalloproteinases promoting metastasis. Besides modulating the microenvironment TAMS directly inhibit CD8T cell activity inducing the expression of PD-L1 in monocytes and secretion of numerous immunosuppressive cytokines and factors, including IL-10, TGF-β and ROS, leading to CD8+ TIL exhaustion and dysfunction. TAMs can also directly inhibit CD8+ T cells cytotoxicity through the depletion of the amino acids, such as l-arginine and tryptophan.
Therapies targeting TAMS are either cytotoxic to TAMS (trabectedin, bisphosphonates), prevent its migration and recruitment by tumors (anti-CSF-1/CSF-1R [30] CCL2/CCR2, or the CXCL12/CXCR4 axis [31] or reprogram them to anti-tumor M1 subtypes [32], or via anti-CD47 mabs [33]). Clinically, selective TAM inhibitor bemcentinib has shown not only improved PFS when used in combination with pembrolizumab, but also induced epithelial differentiation and returned the tumor microenvironment to an immune stimulatory phenotype [34].
Conclusion
Enhanced anti-tumor immunity in the form immune check point inhibitors has improved clinical outcomes, but a lot remains to be explored especially exploring novel methods to enhance anti-tumor functions and long-lived anti-tumor immune response. This review explores in brief a few novel ways of enhancing anti-tumor response by highlighting roles of some important immune cells in the tumor microenvironment and factors that can be considered in circumstances when current day immunologic armaments do not work. We are, however, unable to explore the world of long-lived tumor immunity and novel structures that could hold tumor specific clonal effector T cells. There is evidence suggesting naïve T cells are selectively in contact with mature DC in tumor-associated tertiary lymphoid structures (TLS) giving us some direction as TLS may be an active site for the priming and the proliferation but a lot remains to be explored. We conclude by highlighting the significant role of TME and its immune infiltrate in determining effective therapeutic strategies and opportunities to explore novel approach to cancer treatment in the coming future.
Availability of data and materials
Not applicable.
References
Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12(1):31–46.
Baghban R, Roshangar L, Jahanban-Esfahlan R, et al. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun Signal. 2020;18:59. https://doi.org/10.1186/s12964-020-0530-4.
McCarthy EF. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop J. 2006;26:154–8.
Lurquin C, Van Pel A, Mariamé B, De Plaen E, Szikora JP, Janssens C, Reddehase MJ, Lejeune J, Boon T. Structure of the gene of tum-transplantation antigen P91A: the mutated exon encodes a peptide recognized with Ld by cytolytic T cells. Cell. 1989;58(2):293–303. https://doi.org/10.1016/0092-8674(89)90844-1.
Foley EJ. Antigenic properties of methylcholanthrene-induced tumors in mice of the strain of origin. Cancer Res. 1953;13(12):835–7.
Gentles AJ, Newman AM, Liu CL, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21(8):938–45. https://doi.org/10.1038/nm.3909. (Epub 2015 July 20).
Binnewies M, Roberts EW, Kersten K, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24(5):541–50. https://doi.org/10.1038/s41591-018-0014-x. (Epub 2018 Apr 23).
Vivier E, Raulet DH, Moretta A, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331(6013):44–9. https://doi.org/10.1126/science.1198687.
Cachot A, Bilous M, Liu YC, et al. Tumor-specific cytolytic CD4 T cells mediate immunity against human cancer. Sci Adv. 2021;7(9): eabe3348. https://doi.org/10.1126/sciadv.abe3348.
Reissfelder C, Stamova S, Gossmann C, et al. Tumor-specific cytotoxic T lymphocyte activity determines colorectal cancer patient prognosis [published correction appears in J Clin Invest. 2015;125(3):1364]. J Clin Invest. 2015;125(2):739–51. https://doi.org/10.1172/JCI74894.
Tian C, Lu S, Fan Q, et al. Prognostic significance of tumor-infiltrating CD8+ or CD3+ T lymphocytes and interleukin-2 expression in radically resected non-small cell lung cancer. Chin Med J. 2015;128(1):105–10. https://doi.org/10.4103/0366-6999.147828.
Yuan Y, Zhu Z, Lan Y, et al. Development and validation of a CD8+ T cell infiltration-related signature for melanoma patients. Front Immunol. 2021;12: 659444. https://doi.org/10.3389/fimmu.2021.659444.
Coulie PG, Van den Eynde BJ, van der Bruggen P, Boon T. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat Rev Cancer. 2014;14(2):135–46. https://doi.org/10.1038/nrc3670.
Dhatchinamoorthy K, Colbert JD, Rock KL. Cancer immune evasion through loss of MHC class I antigen presentation. Front Immunol. 2021;12: 636568. https://doi.org/10.3389/fimmu.2021.636568.
Zarychta E, Ruszkowska-Ciastek B. Cooperation between angiogenesis, vasculogenesis, chemotaxis, and coagulation in breast cancer metastases development: pathophysiological point of view. Biomedicines. 2022;10(2):300. https://doi.org/10.3390/biomedicines10020300.
Holmgaard RB, Zamarin D, Li Y, Gasmi B, Munn DH, Allison JP, Merghoub T, Wolchok JD. Tumor-expressed IDO recruits and activates MDSCs in a Treg-dependent manner. Cell Rep. 2015;13(2):412–24. https://doi.org/10.1016/j.celrep.2015.08.077. (Epub 2015 Sep 24).
Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14(7):399–416. https://doi.org/10.1038/nrclinonc.2016.217. (Epub 2017 Jan 24).
Dysthe M, Parihar R. Myeloid-derived suppressor cells in the tumor microenvironment. Adv Exp Med Biol. 2020;1224:117–40. https://doi.org/10.1007/978-3-030-35723-8_8.
van Dalen FJ, van Stevendaal MHME, Fennemann FL, et al. Molecular repolarisation of tumour-associated macrophages. Molecules. 2018;24(1):9. https://doi.org/10.3390/molecules24010009.
Mok TSK, Wu YL, Kudaba I, KEYNOTE-042 Investigators, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–30. https://doi.org/10.1016/S0140-6736(18)32409-7. (Epub 2019 Apr 4).
Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381(16):1535–46. https://doi.org/10.1056/NEJMoa1910836. (Epub 2019 Sep 28).
O’Donnell JS, Teng MWL, Smyth MJ. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol. 2019;16:151–67.
Tutt AL, O’Brien L, Hussain A, et al. T cell immunity to lymphoma following treatment with anti-CD40 monoclonal antibody. J Immunol. 2002;168(6):2720–8. https://doi.org/10.4049/jimmunol.168.6.2720.
vanKooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol. 2000;67(1):2–17. https://doi.org/10.1002/jlb.67.1.2.
Weiss S, Sznol M, Shaheen M, et al. 389 Phase II of CD40 agonistic antibody sotigalimab (APX005M) in combination with nivolumab in subjects with metastatic melanoma with confirmed disease progression on anti-PD-1 therapy. J ImmunoTherapy Cancer. 2021. https://doi.org/10.1136/jitc-2021-SITC2021.389.
American Association for Cancer Research. A CD40 agonistic antibody shows signs of efficacy in pancreatic cancer. Cancer Discov. 2021;11(3):OF7. https://doi.org/10.1158/2159-8290.CD-RW2021-006.
Hegde PS, Chen DS. Top 10 challenges in cancer immunotherapy. Immunity. 2020;52(1):17–35. https://doi.org/10.1016/j.immuni.2019.12.011.
Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10.
Geuijen C, Tacken P, Wang LC, Klooster R, et al. A human CD137×PD-L1 bispecific antibody promotes anti-tumor immunity via context-dependent T cell costimulation and checkpoint blockade. Nat Commun. 2021;12(1):4445. https://doi.org/10.1038/s41467-021-24767-5.
Cannarile MA, Weisser M, Jacob W, et al. Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J Immunother Cancer. 2017;5(1):53. https://doi.org/10.1186/s40425-017-0257-y.
Song JS, Chang CC, Wu CH, et al. A highly selective and potent CXCR4 antagonist for hepatocellular carcinoma treatment. Proc Natl Acad Sci USA. 2021;118(13): e2015433118. https://doi.org/10.1073/pnas.2015433118.
Feng Y, Mu R, Wang Z, et al. A toll-like receptor agonist mimicking microbial signal to generate tumor-suppressive macrophages. Nat Commun. 2019;10(1):2272. https://doi.org/10.1038/s41467-019-10354-2.
Schürch CM, Roelli MA, Forster S, et al. Targeting CD47 in anaplastic thyroid carcinoma enhances tumor phagocytosis by macrophages and is a promising therapeutic strategy. Thyroid. 2019;29(7):979–92. https://doi.org/10.1089/thy.2018.0555. (Epub 2019 May 10).
Felip E, Brunsvig P, Vinolas N, et al. A phase II study of bemcentinib (BGB324), a first-in-class highly selective AXL inhibitor, with pembrolizumab in pts with advanced NSCLC: OS for stage I and preliminary stage II efficacy. J Clin Oncol. 2019;37(15_suppl):9098–9098.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
SR is the primary author involved in envisioning the core idea, concept, complete manuscript writing, reference review and publication process. HD and SS are co-authors involved with the review paper from its initiation, and contributed in reviewing the manuscript and ideas that ultimately led to the completion of the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Yes by author and co-authors.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rajbhandary, S., Dhakal, H. & Shrestha, S. Tumor immune microenvironment (TIME) to enhance antitumor immunity. Eur J Med Res 28, 169 (2023). https://doi.org/10.1186/s40001-023-01125-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-023-01125-3