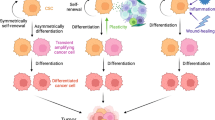
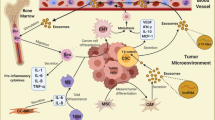
Abstract
Tumorigenic Cancer Stem Cells (CSCs), often called tumor-initiating cells (TICs), represent a unique subset of cells within the tumor milieu. They stand apart from the bulk of tumor cells due to their exceptional self-renewal, metastatic, and differentiation capabilities. Despite significant progress in classifying CSCs, these cells remain notably resilient to conventional radiotherapy and chemotherapy, contributing to cancer recurrence. In this review, our objective is to explore novel avenues of research that delve into the distinctive characteristics of CSCs within their surrounding tumor microenvironment (TME). We will start with an overview of the defining features of CSCs and then delve into their intricate interactions with cells from the lymphoid lineage, namely T cells, B cells, and natural killer (NK) cells. Furthermore, we will discuss their dynamic interplay with myeloid lineage cells, including macrophages, neutrophils, and myeloid-derived suppressor cells (MDSCs). Moreover, we will illuminate the crosstalk between CSCs and cells of mesenchymal origin, specifically fibroblasts, adipocytes, and endothelial cells. Subsequently, we will underscore the pivotal role of CSCs within the context of the tumor-associated extracellular matrix (ECM). Finally, we will highlight pre-clinical and clinical studies that target CSCs within the intricate landscape of the TME, including CAR-T therapy, oncolytic viruses, and CSC-vaccines, with the ultimate goal of uncovering novel avenues for CSC-based cancer immunotherapy.
Graphical abstract




Similar content being viewed by others
Data Availability
Not Applicable.
Code Availability
Not Applicable.
References
Siegel, R. L., et al. (2023). Cancer statistics, 2023. CA: A Cancer Journal for Clinicians, 73(1), 17–48.
Ma, Z. Q., & Richardson, L. C. (2022). Cancer Screening Prevalence and Associated Factors Among US Adults. Preventing Chronic Disease, 19, E22.
Sung, H., et al. (2021). Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 71(3), 209–249.
Dzobo, K., et al. (2020). Advances in therapeutic targeting of cancer stem cells within the tumor microenvironment: An updated review. Cells, 9(8), 1896.
O’Flaherty, J. D., et al. (2012). The cancer stem-cell hypothesis: Its emerging role in lung cancer biology and its relevance for future therapy. Journal of Thoracic Oncology, 7(12), 1880–1890.
Li, Y., et al. (2021). Drug resistance and Cancer stem cells. Cell Communication and Signaling: CCS, 19(1), 19.
Kovacic, B., et al. (2012). Diverging fates of cells of origin in acute and chronic leukaemia. EMBO Molecular Medicine, 4(4), 283–297.
Eun, K., Ham, S. W., & Kim, H. (2017). Cancer stem cell heterogeneity: Origin and new perspectives on CSC targeting. BMB Reports, 50(3), 117–125.
Zheng, X., Yu, C., & Xu, M. (2021). Linking Tumor Microenvironment to Plasticity of Cancer Stem Cells: Mechanisms and Application in Cancer Therapy. Frontiers in Oncology, 11, 678333.
Nimmakayala, R. K., Batra, S. K., & Ponnusamy, M. P. (2019). Unraveling the journey of cancer stem cells from origin to metastasis. Biochimica et Biophysica Acta - Reviews on Cancer, 1871(1), 50–63.
Arneth, B. (2019). Tumor microenvironment. Medicina (Kaunas), 56(1), 15.
Pattabiraman, D. R., & Weinberg, R. A. (2014). Tackling the cancer stem cells - what challenges do they pose? Nature Reviews. Drug Discovery, 13(7), 497–512.
Zhang, S., et al. (2018). Interplay between inflammatory tumor microenvironment and cancer stem cells. Oncology letters, 16(1), 679–686.
Langley, R. R., & Fidler, I. J. (2011). The seed and soil hypothesis revisited–the role of tumor-stroma interactions in metastasis to different organs. International Journal of Cancer, 128(11), 2527–2535.
Zeineddine, D., et al. (2014). The Oct4 protein: More than a magic stemness marker. Am J Stem Cells, 3(2), 74–82.
Wu, G., et al. (2015). Oct4 is a reliable marker of liver tumor propagating cells in hepatocellular carcinoma. Discovery Medicine, 20(110), 219–229.
Hatefi, N., et al. (2012). Evaluating the expression of oct4 as a prognostic tumor marker in bladder cancer. Iranian Journal of Basic Medical Sciences, 15(6), 1154–1161.
Leis, O., et al. (2012). Sox2 expression in breast tumours and activation in breast cancer stem cells. Oncogene, 31(11), 1354–1365.
Bareiss, P. M., et al. (2013). SOX2 expression associates with stem cell state in human ovarian carcinoma. Cancer Research, 73(17), 5544–5555.
Thirusangu, P., et al. (2022). PFKFB3 regulates cancer stemness through the hippo pathway in small cell lung carcinoma. Oncogene, 41(33), 4003–4017.
Lee, Y., et al. (2021). TMPRSS4 promotes cancer stem-like properties in prostate cancer cells through upregulation of SOX2 by SLUG and TWIST1. Journal of Experimental & Clinical Cancer Research, 40(1), 372.
Chiou, S. H., et al. (2010). Coexpression of Oct4 and Nanog enhances malignancy in lung adenocarcinoma by inducing cancer stem cell-like properties and epithelial-mesenchymal transdifferentiation. Cancer Research, 70(24), 10433–10444.
Amsterdam, A., et al. (2013). Localization of the stem cell markers LGR5 and Nanog in the normal and the cancerous human ovary and their inter-relationship. Acta Histochemica, 115(4), 330–338.
Bourguignon, L. Y., et al. (2008). Hyaluronan-CD44 interaction activates stem cell marker Nanog, Stat-3-mediated MDR1 gene expression, and ankyrin-regulated multidrug efflux in breast and ovarian tumor cells. Journal of Biological Chemistry, 283(25), 17635–17651.
Zbinden, M., et al. (2010). NANOG regulates glioma stem cells and is essential in vivo acting in a cross-functional network with GLI1 and p53. EMBO Journal, 29(15), 2659–2674.
Jeter, C. R., et al. (2009). Functional evidence that the self-renewal gene NANOG regulates human tumor development. Stem Cells, 27(5), 993–1005.
Singh, S., et al. (2013). Aldehyde dehydrogenases in cellular responses to oxidative/electrophilic stress. Free Radical Biology & Medicine, 56, 89–101.
Modok, S., Mellor, H. R., & Callaghan, R. (2006). Modulation of multidrug resistance efflux pump activity to overcome chemoresistance in cancer. Current Opinion in Pharmacology, 6(4), 350–354.
Sterzyńska, K., et al. (2018). Mutual expression of ALDH1A1, LOX, and collagens in ovarian cancer cell lines as combined CSCs- and ECM-related models of drug resistance development. International Journal of Molecular Sciences, 20(1), 54.
Verma, A., Kapoor, R., & Mittal, R. D. (2017). Cluster of Differentiation 44 (CD44) Gene Variants: A Putative Cancer Stem Cell Marker in Risk Prediction of Bladder Cancer in North Indian Population. Indian Journal of Clinical Biochemistry, 32(1), 74–83.
Li, J., et al. (2022). Integrin β1 in Pancreatic Cancer: Expressions, Functions, and Clinical Implications. Cancers (Basel), 14(14), 3377.
Condello, S., et al. (2018). Tissue Tranglutaminase Regulates Interactions between Ovarian Cancer Stem Cells and the Tumor Niche. Cancer Research, 78(11), 2990–3001.
Gardelli, C., et al. (2021). Differential glycosylation of collagen modulates lung cancer stem cell subsets through β1 integrin-mediated interactions. Cancer Science, 112(1), 217–230.
Vadhan, A., et al. (2022). CD44 promotes breast cancer metastasis through AKT-mediated downregulation of nuclear FOXA2. Biomedicines, 10(10), 2488.
Zhang, Q., Cai, D. J., & Li, B. (2015). Ovarian cancer stem-like cells elicit the polarization of M2 macrophages. Molecular Medicine Reports, 11(6), 4685–4693.
Zöller, M. (2015). CD44, Hyaluronan, the Hematopoietic Stem Cell, and Leukemia-Initiating Cells. Frontiers in Immunology, 6, 235.
Connor, E. V., et al. (2019). Thy-1 predicts poor prognosis and is associated with self-renewal in ovarian cancer. Journal of Ovarian Research, 12(1), 112.
Luo, J., et al. (2016). The Notch pathway promotes the cancer stem cell characteristics of CD90+ cells in hepatocellular carcinoma. Oncotarget, 7(8), 9525–9537.
Zhu, J., et al. (2014). Overexpression of CD90 (Thy-1) in pancreatic adenocarcinoma present in the tumor microenvironment. Plos One, 9(12), e115507.
Schliekelman, M. J., et al. (2017). Thy-1(+) Cancer-associated Fibroblasts Adversely Impact Lung Cancer Prognosis. Science and Reports, 7(1), 6478.
Lobba, A. R. M., et al. (2018). High CD90 (THY-1) expression positively correlates with cell transformation and worse prognosis in basal-like breast cancer tumors. PLoS ONE, 13(6), e0199254.
Mazzoldi, E. L., et al. (2019). A juxtacrine/paracrine loop between C-Kit and stem cell factor promotes cancer stem cell survival in epithelial ovarian cancer. Cell Death & Disease, 10(6), 412.
Harris, K. S., et al. (2021). CD117/c-kit defines a prostate CSC-like subpopulation driving progression and TKI resistance. Science and Reports, 11(1), 1465.
Park, J., et al. (2021). Role of CD133/NRF2 Axis in the Development of Colon Cancer Stem Cell-Like Properties. Frontiers in Oncology, 11, 808300.
Hu, Z., et al. (2017). Targeting tissue factor as a novel therapeutic oncotarget for eradication of cancer stem cells isolated from tumor cell lines, tumor xenografts and patients of breast, lung and ovarian cancer. Oncotarget, 8(1), 1481–1494.
Liu, F., & Qian, Y. (2021). The role of CD133 in hepatocellular carcinoma. Cancer Biology & Therapy, 22(4), 291–300.
Yin, W., et al. (2022). Inhibition of autophagy promotes the elimination of liver cancer stem cells by CD133 aptamer-targeted delivery of doxorubicin. Biomolecules, 12(11), 1623.
Zhao, Q., et al. (2016). Prognostic value of the expression of cancer stem cell-related markers CD133 and CD44 in hepatocellular carcinoma: From patients to patient-derived tumor xenograft models. Oncotarget, 7(30), 47431–47443.
Horst, D., et al. (2009). Prognostic significance of the cancer stem cell markers CD133, CD44, and CD166 in colorectal cancer. Cancer Investigation, 27(8), 844–850.
Kim, W. T., & Ryu, C. J. (2017). Cancer stem cell surface markers on normal stem cells. BMB Reports, 50(6), 285–298.
Liao, W. T., et al. (2014). Metastatic cancer stem cells: From the concept to therapeutics. American Journal of Stem Cells, 3(2), 46–62.
Dieter, S. M., et al. (2011). Distinct types of tumor-initiating cells form human colon cancer tumors and metastases. Cell Stem Cell, 9(4), 357–365.
Zhang, S. S., et al. (2012). CD133(+)CXCR4(+) colon cancer cells exhibit metastatic potential and predict poor prognosis of patients. BMC Medicine, 10, 85.
Roato, I. & Ferracini, R. (2018). Cancer stem cells, bone and tumor microenvironment: Key players in bone metastases. Cancers (Basel), 10(2), 56.
Wu, Y., & Wu, P. Y. (2009). CD133 as a marker for cancer stem cells: Progresses and concerns. Stem Cells and Development, 18(8), 1127–1134.
Frank, N. Y., Schatton, T., & Frank, M. H. (2010). The therapeutic promise of the cancer stem cell concept. The Journal of Clinical Investigation, 120(1), 41–50.
Ruivo, C. F., et al. (2022). Extracellular Vesicles from Pancreatic Cancer Stem Cells Lead an Intratumor Communication Network (EVNet) to fuel tumour progression. Gut, 71(10), 2043–2068.
Kakarala, M., & Wicha, M. S. (2007). Cancer stem cells: Implications for cancer treatment and prevention. Cancer Journal, 13(5), 271–275.
Gasch, C., et al. (2017). Catching moving targets: Cancer stem cell hierarchies, therapy-resistance & considerations for clinical intervention. Molecular Cancer, 16(1), 43.
Liu, A., Yu, X., & Liu, S. (2013). Pluripotency transcription factors and cancer stem cells: Small genes make a big difference. Chinese Journal of Cancer, 32(9), 483–487.
Matsui, W. H. (2016). Cancer stem cell signaling pathways. Medicine (Baltimore), 95(1 Suppl 1), S8-s19.
Rahman, M., et al. (2016). Stem cell and cancer stem cell: A Tale of Two Cells. Progress in Stem Cell, 3(2), 97–108.
Wang, H., et al. (2021). Colorectal Cancer Stem Cell States Uncovered by Simultaneous Single-Cell Analysis of Transcriptome and Telomeres. Adv Sci (Weinh), 8(8), 2004320.
Luo, M., et al. (2020). Stem cell quiescence and its clinical relevance. World Journal of Stem Cells, 12(11), 1307–1326.
Plaks, V., Kong, N., & Werb, Z. (2015). The cancer stem cell niche: How essential is the niche in regulating stemness of tumor cells? Cell Stem Cell, 16(3), 225–238.
Malta, T. M., et al. (2018). Machine Learning Identifies Stemness Features Associated with Oncogenic Dedifferentiation. Cell, 173(2), 338-354.e15.
Borlongan, M. C., & Wang, H. (2023). Profiling and targeting cancer stem cell signaling pathways for cancer therapeutics. Frontiers in Cell and Developmental Biology, 11, 1125174.
Bocci, F., et al. (2019). Toward understanding cancer stem cell heterogeneity in the tumor microenvironment. Proceedings of the National Academy of Sciences of the United States of America, 116(1), 148–157.
Shibue, T., & Weinberg, R. A. (2017). EMT, CSCs, and drug resistance: The mechanistic link and clinical implications. Nature Reviews Clinical Oncology, 14(10), 611–629.
Lu, W., & Kang, Y. (2019). Epithelial-Mesenchymal Plasticity in Cancer Progression and Metastasis. Developmental Cell, 49(3), 361–374.
Constant, S. L., & Bottomly, K. (1997). Induction of Th1 and Th2 CD4+ T cell responses: The alternative approaches. Annual Review of Immunology, 15, 297–322.
Philip, M., & Schietinger, A. (2022). CD8+ T cell differentiation and dysfunction in cancer. Nature Reviews Immunology, 22(4), 209–223.
Choo, S. Y. (2007). The HLA system: Genetics, immunology, clinical testing, and clinical implications. Yonsei Medical Journal, 48(1), 11–23.
Müller, L., et al. (2020). Bidirectional Crosstalk Between Cancer Stem Cells and Immune Cell Subsets. Frontiers in Immunology, 11, 140–140.
Di Tomaso, T., et al. (2010). Immunobiological characterization of cancer stem cells isolated from glioblastoma patients. Clinical Cancer Research, 16(3), 800–813.
Hirohashi, Y., et al. (2016). Immune responses to human cancer stem-like cells/cancer-initiating cells. Cancer Science, 107(1), 12–17.
Tsuchiya, H., & Shiota, G. (2021). Immune evasion by cancer stem cells. Regenerative Therapy, 17, 20–33.
Aramini, B., et al. (2021). Cancer stem cells and macrophages: Molecular connections and future perspectives against cancer. Oncotarget, 12(3), 230–250.
Kondĕlková, K., et al. (2010). Regulatory T cells (TREG) and their roles in immune system with respect to immunopathological disorders. Acta Medica (Hradec Kralove), 53(2), 73–77.
Chen, P., et al. (2021). Cancer Stemness Meets Immunity: From Mechanism to Therapy. Cell Reports, 34(1), 108597.
Yu, X., Li, H., & Ren, X. (2012). Interaction between regulatory T cells and cancer stem cells. International Journal of Cancer, 131(7), 1491–1498.
Munn, D. H. (2011). Indoleamine 2,3-dioxygenase, Tregs and cancer. Current Medicinal Chemistry, 18(15), 2240–2246.
Tesmer, L. A., et al. (2008). Th17 cells in human disease. Immunological Reviews, 223, 87–113.
Cancro, M. P., & Tomayko, M. M. (2021). Memory B cells and plasma cells: The differentiative continuum of humoral immunity. Immunological Reviews, 303(1), 72–82.
Shahaf, G., et al. (2016). B cell development in the bone marrow is regulated by homeostatic feedback exerted by mature B cells. Frontiers in Immunology, 7, 77.
de la Morena, M. (2008). CHAPTER 10 - Immunologic Development and Susceptibility to Infection. In S. S. Long (Ed.), Principles and Practice of Pediatric Infectious Disease (Third Edition) (pp. 86–94). W.B. Saunders.
Kurosaki, T., Kometani, K., & Ise, W. (2015). Memory B cells. Nature Reviews Immunology, 15(3), 149–159.
Rosser, E. C., & Mauri, C. (2015). Regulatory B cells: Origin, phenotype, and function. Immunity, 42(4), 607–612.
Franchina, D. G., Grusdat, M., & Brenner, D. (2018). B-Cell Metabolic Remodeling and Cancer. Trends Cancer, 4(2), 138–150.
Walcher, L., et al. (2020). Cancer Stem Cells-Origins and Biomarkers: Perspectives for Targeted Personalized Therapies. Frontiers in Immunology, 11, 1280.
Bruce, W. R., & Van Der Gaag, H. (1963). A quantitative assay for the number of murine lymphoma cells capable of proliferation in vivo. Nature, 199, 79–80.
Gross, E., et al. (2011). Cancer stem cells of differentiated B-cell malignancies: Models and consequences. Cancers (Basel), 3(2), 1566–1579.
Fearon, D. T., Manders, P., & Wagner, S. D. (2001). Arrested differentiation, the self-renewing memory lymphocyte, and vaccination. Science, 293(5528), 248–250.
Yang, H., & Green M. R. (2019). Epigenetic programing of B-cell lymphoma by BCL6 and its genetic deregulation. Frontiers in Cell and Developmental Biology, 7, 272.
Jamieson, C. H., et al. (2004). Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. New England Journal of Medicine, 351(7), 657–667.
Song, S., et al. (2020). Cancer Stem Cells of Diffuse Large B Cell Lymphoma Are Not Enriched in the CD45(+)CD19(-) cells but in the ALDH(high) Cells. Journal of Cancer, 11(1), 142–152.
Suárez-Sánchez, F.J., et al. (2021) Tumor-Infiltrating CD20(+) B Lymphocytes: Significance and Prognostic Implications in Oral Cancer Microenvironment. Cancers (Basel), 13(3)
DiLillo, D. J., Yanaba, K., & Tedder, T. F. (2010). B cells are required for optimal CD4+ and CD8+ T cell tumor immunity: Therapeutic B cell depletion enhances B16 melanoma growth in mice. The Journal of Immunology, 184(7), 4006–4016.
Luckey, C. J., et al. (2006). Memory T and memory B cells share a transcriptional program of self-renewal with long-term hematopoietic stem cells. Proceedings of the National Academy of Sciences of the United States of America, 103(9), 3304–3309.
Bhattacharya, R., et al. (2015). Bmi-1: At the crossroads of physiological and pathological biology. Genes & Diseases, 2(3), 225–239.
Chang, K.-C., et al. (2020). Stem cell characteristics promote aggressiveness of diffuse large B-cell lymphoma. Scientific Reports, 10(1), 21342.
Downs-Canner, S. M., et al. (2022). B Cell Function in the Tumor Microenvironment. Annual Review of Immunology, 40(1), 169–193.
PereraMolligodaArachchige, A. S. (2021). Human NK cells: From development to effector functions. The Innate and Adaptive Immune Systems, 27(3), 212–229.
Vivier, E., et al. (2011). Innate or adaptive immunity? The example of natural killer cells. Science, 331(6013), 44–49.
Lei, M. M. L., & Lee, T. K. W. (2021). Cancer Stem Cells: Emerging Key Players in Immune Evasion of Cancers. Front Cell Dev Biol, 9, 692940.
Luna, J. I., et al. (2017). Targeting Cancer Stem Cells with Natural Killer Cell Immunotherapy. Expert Opinion on Biological Therapy, 17(3), 313–324.
Hazini, A., Fisher K., & Seymour, L. (2021). Deregulation of HLA-I in cancer and its central importance for immunotherapy. The Journal for ImmunoTherapy of Cancer, 9(8), e002899.
Wang, B., et al. (2014). Metastatic consequences of immune escape from NK cell cytotoxicity by human breast cancer stem cells. Cancer Research, 74(20), 5746–5757.
Galland, S., et al. (2017). Tumor-Derived Mesenchymal Stem Cells Use Distinct Mechanisms to Block the Activity of Natural Killer Cell Subsets. Cell Reports, 20(12), 2891–2905.
Malaer, J. D., & Mathew, P. A. (2020). Role of LLT1 and PCNA as Natural Killer Cell Immune Evasion Strategies of HCT 116 Cells. Anticancer Research, 40(12), 6613.
Beano, A., et al. (2008). Correlation between NK function and response to trastuzumab in metastatic breast cancer patients. Journal of translational medicine, 6, 25–25.
Huergo-Zapico, L., et al. (2018). NK-cell Editing Mediates Epithelial-to-Mesenchymal Transition via Phenotypic and Proteomic Changes in Melanoma Cell Lines. Cancer Research, 78(14), 3913–3925.
Zaidi, M. R. (2019). The Interferon-Gamma Paradox in Cancer. Journal of Interferon and Cytokine Research, 39(1), 30–38.
Cruceriu, D., et al. (2020). The dual role of tumor necrosis factor-alpha (TNF-α) in breast cancer: Molecular insights and therapeutic approaches. Cellular Oncology (Dordrecht), 43(1), 1–18.
Liu, W., et al. (2020). TNF-α increases breast cancer stem-like cells through up-regulating TAZ expression via the non-canonical NF-κB pathway. Science and Reports, 10(1), 1804.
Hirayama, D., Iida, T., & Nakase, H. (2017). The phagocytic function of macrophage-enforcing innate immunity and tissue homeostasis. International Journal of Molecular Sciences, 19(1), 92.
Lau, E. Y., Ho, N. P., & Lee, T. K. (2017). Cancer Stem Cells and Their Microenvironment: Biology and Therapeutic Implications. Stem Cells International, 2017, 3714190.
Italiani, P., & Boraschi, D. (2014). From Monocytes to M1/M2 Macrophages: Phenotypical vs Functional Differentiation. Frontiers in Immunology, 5, 514.
Lu, H., et al. (2014). A breast cancer stem cell niche supported by juxtacrine signalling from monocytes and macrophages. Nature Cell Biology, 16(11), 1105–1117.
Vacchelli, E., et al. (2016). Trial Watch: Immunotherapy plus radiation therapy for oncological indications. Oncoimmunology, 5(9), e1214790.
Zhan, T., Rindtorff, N., & Boutros, M. (2017). Wnt signaling in cancer. Oncogene, 36(11), 1461–1473.
Wu, A., et al. (2010). Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro-Oncology, 12(11), 1113–1125.
Yang, J., et al. (2013). Tumor-associated macrophages regulate murine breast cancer stem cells through a novel paracrine EGFR/Stat3/Sox-2 signaling pathway. Stem Cells, 31(2), 248–258.
Wan, S., et al. (2014). Tumor-Associated Macrophages Produce Interleukin 6 and Signal via STAT3 to Promote Expansion of Human Hepatocellular Carcinoma Stem Cells. Gastroenterology, 147(6), 1393–1404.
Korkaya, H., Liu, S., & Wicha, M. S. (2011). Breast cancer stem cells, cytokine networks, and the tumor microenvironment. The Journal of Clinical Investigation, 121(10), 3804–3809.
Wu, K., et al. (2012). Hepatic transforming growth factor beta gives rise to tumor-initiating cells and promotes liver cancer development. Hepatology, 56(6), 2255–2267.
Malech, H. L., Deleo, F. R., & Quinn, M. T. (2014). The role of neutrophils in the immune system: An overview. Methods in Molecular Biology, 1124, 3–10.
Sharma, B., et al. (2013). Targeting CXCR2 enhances chemotherapeutic response, inhibits mammary tumor growth, angiogenesis, and lung metastasis. Molecular Cancer Therapeutics, 12(5), 799–808.
Chao, T., Furth, E. E., & Vonderheide, R. H. (2016). CXCR2-Dependent Accumulation of Tumor-Associated Neutrophils Regulates T-cell Immunity in Pancreatic Ductal Adenocarcinoma. Cancer Immunology Research, 4(11), 968–982.
Fridlender, Z. G., et al. (2009). Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell, 16(3), 183–194.
Ohms, M., Möller, S., & Laskay, T. (2020). An Attempt to Polarize Human Neutrophils Toward N1 and N2 Phenotypes in vitro. Frontiers in Immunology, 11, 532.
Casbon, A. J., et al. (2015). Invasive breast cancer reprograms early myeloid differentiation in the bone marrow to generate immunosuppressive neutrophils. Proceedings of the National Academy of Sciences of the United States of America, 112(6), E566–E575.
Andzinski, L., et al. (2016). Type I IFNs induce anti-tumor polarization of tumor associated neutrophils in mice and human. International Journal of Cancer, 138(8), 1982–1993.
Hwang, W.-L., et al. (2019). Tumor stem-like cell-derived exosomal RNAs prime neutrophils for facilitating tumorigenesis of colon cancer. Journal of Hematology & Oncology, 12(1), 10.
Anselmi, M., et al. (2022). Melanoma stem cells educate neutrophils to support cancer progression. Cancers (Basel), 14(14), 3391.
He, X., et al. (2021). Tumor-initiating stem cell shapes its microenvironment into an immunosuppressive barrier and pro-tumorigenic niche. Cell Reports, 36(10), 109674.
Zarbock, A., & Stadtmann, A. (2012). CXCR2: From bench to bedside. Frontiers in Immunology, 3, 263.
Wei, B., et al. (2016). The neutrophil lymphocyte ratio is associated with breast cancer prognosis: An updated systematic review and meta-analysis. Oncotargets and Therapy, 9, 5567–5575.
Gershkovitz, M., et al. (2018). TRPM2 Mediates Neutrophil Killing of Disseminated Tumor Cells. Cancer Research, 78(10), 2680–2690.
Mensurado, S., et al. (2018). Tumor-associated neutrophils suppress pro-tumoral IL-17+ γδ T cells through induction of oxidative stress. PLoS Biology, 16(5), e2004990.
Martin, A., et al. (2018). Tumor-derived granzyme B-expressing neutrophils acquire antitumor potential after lipid A treatment. Oncotarget, 9(47), 28364–28378.
Galdiero, M. R., et al. (2013). Tumor associated macrophages and neutrophils in cancer. Immunobiology, 218(11), 1402–1410.
Gabrilovich, D. I., & Nagaraj, S. (2009). Myeloid-derived suppressor cells as regulators of the immune system. Nature Reviews Immunology, 9(3), 162–174.
Safarzadeh, E., et al. (2019). Circulating myeloid-derived suppressor cells: An independent prognostic factor in patients with breast cancer. Journal of Cellular Physiology, 234(4), 3515–3525.
Allavena, P., & Mantovani, A. (2012). Immunology in the clinic review series; focus on cancer: Tumour-associated macrophages: Undisputed stars of the inflammatory tumour microenvironment. Clinical and Experimental Immunology, 167(2), 195–205.
Sawanobori, Y., et al. (2008). Chemokine-mediated rapid turnover of myeloid-derived suppressor cells in tumor-bearing mice. Blood, 111(12), 5457–5466.
Cui, Tracy X., et al. (2013). Myeloid-Derived Suppressor Cells Enhance Stemness of Cancer Cells by Inducing MicroRNA101 and Suppressing the Corepressor CtBP2. Immunity, 39(3), 611–621.
Panni, R. Z., et al. (2014). Tumor-induced STAT3 activation in monocytic myeloid-derived suppressor cells enhances stemness and mesenchymal properties in human pancreatic cancer. Cancer Immunology, Immunotherapy, 63(5), 513–528.
Welte, T., et al. (2016). Oncogenic mTOR signalling recruits myeloid-derived suppressor cells to promote tumour initiation. Nature Cell Biology, 18(6), 632–644.
Tracy, L. E., Minasian, R. A., & Caterson, E. J. (2016). Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound. Advances in Wound Care (New Rochelle), 5(3), 119–136.
Orimo, A., et al. (2005). Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell, 121(3), 335–348.
De Veirman, K., et al. (2014). Cancer Associated Fibroblasts and Tumor Growth: Focus on Multiple Myeloma. Cancers, 6, 1363–1381. https://doi.org/10.3390/cancers6031363
Strutz, F., et al. (2000). Basic fibroblast growth factor expression is increased in human renal fibrogenesis and may mediate autocrine fibroblast proliferation. Kidney International, 57(4), 1521–1538.
Furuhashi, M., et al. (2004). Platelet-derived growth factor production by B16 melanoma cells leads to increased pericyte abundance in tumors and an associated increase in tumor growth rate. Cancer Research, 64(8), 2725–2733.
Ham, I. H., Lee, D., & Hur, H. (2021). Cancer-associated fibroblast-induced eesistance to chemotherapy and radiotherapy in gastrointestinal cancers. Cancers (Basel), 13(5), 1172.
Vermeulen, L., et al. (2010). Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nature Cell Biology, 12(5), 468–476.
Xiong, S., et al. (2018). Cancer-associated fibroblasts promote stem cell-like properties of hepatocellular carcinoma cells through IL-6/STAT3/Notch signaling. American Journal of Cancer Research, 8(2), 302–316.
Tsuyada, A., et al. (2012). CCL2 mediates cross-talk between cancer cells and stromal fibroblasts that regulates breast cancer stem cells. Cancer Research, 72(11), 2768–2779.
Chen, W.-J., et al. (2014). Cancer-associated fibroblasts regulate the plasticity of lung cancer stemness via paracrine signalling. Nature Communications, 5(1), 3472.
Giannoni, E., et al. (2010). Reciprocal Activation of Prostate Cancer Cells and Cancer-Associated Fibroblasts Stimulates Epithelial-Mesenchymal Transition and Cancer Stemness. Cancer Research, 70(17), 6945–6956.
Soon, P. S., et al. (2013). Breast cancer-associated fibroblasts induce epithelial-to-mesenchymal transition in breast cancer cells. Endocrine-Related Cancer, 20(1), 1–12.
Yu, Y., et al. (2014). Cancer-associated fibroblasts induce epithelial-mesenchymal transition of breast cancer cells through paracrine TGF-β signalling. British Journal of Cancer, 110(3), 724–732.
Huang, T. X., Guan, X. Y., & Fu, L. (2019). Therapeutic targeting of the crosstalk between cancer-associated fibroblasts and cancer stem cells. American Journal of Cancer Research, 9(9), 1889–1904.
Marlatt, K. L., & Ravussin, E. (2017). Brown Adipose Tissue: An Update on Recent Findings. Current Obesity Reports, 6(4), 389–396.
Cypess, A. M., & Kahn, C. R. (2010). The role and importance of brown adipose tissue in energy homeostasis. Current Opinion in Pediatrics, 22(4), 478–484.
Seki, T., et al. (2022). Brown-fat-mediated tumour suppression by cold-altered global metabolism. Nature, 608(7922), 421–428.
De Pergola, G., & Silvestris, F. (2013). Obesity as a major risk factor for cancer. Journal of Obesity, 2013, 291546.
Maguire, O. A., et al. (2021). Creatine-mediated crosstalk between adipocytes and cancer cells regulates obesity-driven breast cancer. Cell Metabolism, 33(3), 499-512.e6.
Huh, J. Y., et al. (2014). Crosstalk between adipocytes and immune cells in adipose tissue inflammation and metabolic dysregulation in obesity. Molecules and Cells, 37(5), 365–371.
Dirat, B., et al. (2011). Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Research, 71(7), 2455–2465.
Wu, Q., et al. (2019). Cancer-associated adipocytes: Key players in breast cancer progression. Journal of Hematology & Oncology, 12(1), 95.
Rybinska, I., et al. (2021). Cancer-associated adipocytes in breast cancer: Causes and consequences. International Journal of Molecular Sciences, 22(7), 3775.
Zheng, Q., et al. (2011). Leptin deficiency suppresses MMTV-Wnt-1 mammary tumor growth in obese mice and abrogates tumor initiating cell survival. Endocrine-Related Cancer, 18(4), 491–503.
Martínez-Sánchez, N. (2020). There and Back Again: Leptin actions in white adipose tissue. International Journal of Molecular Sciences, 21(17), 6039.
Bowers, L. W., et al. (2018). Leptin Signaling Mediates Obesity-Associated CSC Enrichment and EMT in Preclinical TNBC Models. Molecular Cancer Research, 16(5), 869–879.
Choi, J., Cha, Y. J., & Koo, J. S. (2018). Adipocyte biology in breast cancer: From silent bystander to active facilitator. Progress in Lipid Research, 69, 11–20.
Han, G., et al. (2013). High expression of leptin receptor leads to temozolomide resistance with exhibiting stem/progenitor cell features in gliobalastoma. Cell Cycle, 12(24), 3833–3840.
Bao, Y., et al. (2013). A prospective study of plasma adiponectin and pancreatic cancer risk in five US cohorts. Journal of the National Cancer Institute, 105(2), 95–103.
Picon-Ruiz, M., et al. (2016). Interactions between Adipocytes and Breast Cancer Cells Stimulate Cytokine Production and Drive Src/Sox2/miR-302b–Mediated Malignant Progression. Cancer Research, 76(2), 491–504.
Tang, K. D., et al. (2016). Adipocytes promote prostate cancer stem cell self-renewal through amplification of the cholecystokinin autocrine loop. Oncotarget, 7(4), 4939–4948.
Kumar, S., et al. (2014). Functional modification of adipocytes by grape seed extract impairs their pro-tumorigenic signaling on colon cancer stem cells and the daughter cancer cells. Oncotarget, 5(20), 10151–10169.
Geevarghese, A., & Herman, I. M. (2014). Pericyte-endothelial crosstalk: Implications and opportunities for advanced cellular therapies. Translational Research, 163(4), 296–306.
Ribatti, D., Tamma, R., & Annese, T. (2021). The role of vascular niche and endothelial cells in organogenesis and regeneration. Experimental Cell Research, 398(1), 112398.
Ping, Y. F., Zhang, X., & Bian, X. W. (2016). Cancer stem cells and their vascular niche: Do they benefit from each other? Cancer Letters, 380(2), 561–567.
Carmeliet, P. (2005). VEGF as a key mediator of angiogenesis in cancer. Oncology, 69(Suppl 3), 4–10.
Levina, V., et al. (2008). Drug-selected human lung cancer stem cells: Cytokine network, tumorigenic and metastatic properties. Plos One, 3(8), e3077.
Lizárraga-Verdugo, E., et al. (2020). Cancer Stem Cells and Its Role in Angiogenesis and Vasculogenic Mimicry in Gastrointestinal Cancers. Frontiers in Oncology, 10, 413.
Zhang, Z., et al. (2014). Endothelial cell-secreted EGF induces epithelial to mesenchymal transition and endows head and neck cancer cells with stem-like phenotype. Cancer Research, 74(10), 2869–2881.
Peñarando, J., et al. (2018). A role for endothelial nitric oxide synthase in intestinal stem cell proliferation and mesenchymal colorectal cancer. BMC Biology, 16(1), 3.
Zhu, T. S., et al. (2011). Endothelial cells create a stem cell niche in glioblastoma by providing NOTCH ligands that nurture self-renewal of cancer stem-like cells. Cancer Research, 71(18), 6061–6072.
Lu, J., et al. (2013). Endothelial cells promote the colorectal cancer stem cell phenotype through a soluble form of Jagged-1. Cancer Cell, 23(2), 171–185.
Akil, A., et al. (2021). Notch Signaling in Vascular Endothelial Cells, Angiogenesis, and Tumor Progression: An Update and Prospective. Frontiers in Cell and Developmental Biology, 9, 642352.
Yan, G. N., et al. (2014). Endothelial cells promote stem-like phenotype of glioma cells through activating the Hedgehog pathway. The Journal of Pathology, 234(1), 11–22.
Wang, R., et al. (2019). Endothelial Cells Promote Colorectal Cancer Cell Survival by Activating the HER3-AKT Pathway in a Paracrine Fashion. Molecular Cancer Research, 17(1), 20–29.
Hsu, H. C., et al. (2019). Sequential cetuximab/bevacizumab therapy is associated with improved outcomes in patients with wild-type KRAS exon 2 metastatic colorectal cancer. Cancer Medicine, 8(7), 3437–3446.
Yue, B. (2014). Biology of the extracellular matrix: An overview. Journal of Glaucoma, 23(8 Suppl 1), S20–S23.
Tan, Y., et al. (2014). Matrix softness regulates plasticity of tumour-repopulating cells via H3K9 demethylation and Sox2 expression. Nature Communications, 5, 4619.
Pang, M. F., et al. (2016). Tissue Stiffness and Hypoxia Modulate the Integrin-Linked Kinase ILK to Control Breast Cancer Stem-like Cells. Cancer Research, 76(18), 5277–5287.
Motegi, H., et al. (2014). Type 1 collagen as a potential niche component for CD133-positive glioblastoma cells. Neuropathology, 34(4), 378–385.
Begum, A., et al. (2017). The extracellular matrix and focal adhesion kinase signaling regulate cancer stem cell function in pancreatic ductal adenocarcinoma. Plos One, 12(7), e0180181.
Nissen, N. I., Karsdal, M., & Willumsen, N. (2019). Collagens and Cancer associated fibroblasts in the reactive stroma and its relation to Cancer biology. Journal of Experimental & Clinical Cancer Research, 38(1), 115.
Wang, Z., et al. (2010). Type IIB procollagen NH(2)-propeptide induces death of tumor cells via interaction with integrins alpha(V)beta(3) and alpha(V)beta(5). Journal of Biological Chemistry, 285(27), 20806–20817.
Januchowski, R., et al. (2016). Increased Expression of Several Collagen Genes is Associated with Drug Resistance in Ovarian Cancer Cell Lines. Journal of Cancer, 7(10), 1295–1310.
Lim, Y. C., Oh, S. Y., & Kim, H. (2012). Cellular characteristics of head and neck cancer stem cells in type IV collagen-coated adherent cultures. Experimental Cell Research, 318(10), 1104–1111.
Rada, M., et al. (2018). Inhibitor of apoptosis proteins (IAPs) mediate collagen type XI alpha 1-driven cisplatin resistance in ovarian cancer. Oncogene, 37(35), 4809–4820.
Wu, Y. H., et al. (2017). Activation of TWIST1 by COL11A1 promotes chemoresistance and inhibits apoptosis in ovarian cancer cells by modulating NF-κB-mediated IKKβ expression. International Journal of Cancer, 141(11), 2305–2317.
Liu, C. C., et al. (2018). Collagen XVII/laminin-5 activates epithelial-to-mesenchymal transition and is associated with poor prognosis in lung cancer. Oncotarget, 9(2), 1656–1672.
Chang, C., et al. (2015). A laminin 511 matrix is regulated by TAZ and functions as the ligand for the α6Bβ1 integrin to sustain breast cancer stem cells. Genes & Development, 29(1), 1–6.
Govaere, O., et al. (2016). Laminin-332 sustains chemoresistance and quiescence as part of the human hepatic cancer stem cell niche. Journal of Hepatology, 64(3), 609–617.
Ou, J., et al. (2013). Fibronectin extra domain A (EDA) sustains CD133(+)/CD44(+) subpopulation of colorectal cancer cells. Stem Cell Res, 11(2), 820–833.
Sun, Y., et al. (2015). Targeted Therapy for Breast Cancer Stem Cells by Liposomal Delivery of siRNA against Fibronectin EDB. Advanced Healthcare Materials, 4(11), 1675–1680.
Ibrahim, S. A., et al. (2017). Syndecan-1 is a novel molecular marker for triple negative inflammatory breast cancer and modulates the cancer stem cell phenotype via the IL-6/STAT3, Notch and EGFR signaling pathways. Molecular Cancer, 16(1), 57.
Sun, B., et al. (2017). Significance of Glypican-3 (GPC3) Expression in Hepatocellular Cancer Diagnosis. Medical Science Monitor, 23, 850–855.
Cao, J., et al. (2018). Targeting glypican-4 overcomes 5-FU resistance and attenuates stem cell-like properties via suppression of Wnt/β-catenin pathway in pancreatic cancer cells. Journal of Cellular Biochemistry, 119(11), 9498–9512.
Pang, L., et al. (2016). Membrane type 1-matrix metalloproteinase induces epithelial-to-mesenchymal transition in esophageal squamous cell carcinoma: Observations from clinical and in vitro analyses. Scientific Reports, 6(1), 22179.
Raja, A. M., et al. (2015). Differential remodeling of extracellular matrices by breast cancer initiating cells. Journal of Biophotonics, 8(10), 804–815.
Bourguignon, L. Y. W. (2016). Matrix Hyaluronan Promotes Specific MicroRNA Upregulation Leading to Drug Resistance and Tumor Progression. International Journal of Molecular Sciences, 17(4), 517.
Shiina, M., & Bourguignon, L. Y. (2015). Selective Activation of Cancer Stem Cells by Size-Specific Hyaluronan in Head and Neck Cancer. International Journal of Cell Biology, 2015, 989070.
Cui, X., et al. (2021). CAR-T therapy: Prospects in targeting cancer stem cells. Journal of Cellular and Molecular Medicine, 25(21), 9891–9904.
Xu, J., Melenhorst, J. J., & Fraietta, J. A. (2018). Toward precision manufacturing of immunogene T-cell therapies. Cytotherapy, 20(5), 623–638.
Locke, F. L., et al. (2019). Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): A single-arm, multicentre, phase 1–2 trial. The Lancet Oncology, 20(1), 31–42.
Boyiadzis, M. M., et al. (2018). Chimeric antigen receptor (CAR) T therapies for the treatment of hematologic malignancies: Clinical perspective and significance. Journal for Immunotherapy of Cancer, 6(1), 137.
Chavez, J. C., Bachmeier, C., & Kharfan-Dabaja, M. A. (2019). CAR T-cell therapy for B-cell lymphomas: Clinical trial results of available products. Therapeutic Advances in Hematology, 10, 2040620719841581.
Subklewe, M., von Bergwelt-Baildon, M., & Humpe, A. (2019). Chimeric Antigen Receptor T Cells: A Race to Revolutionize Cancer Therapy. Transfusion Medicine and Hemotherapy, 46(1), 15–24.
Lanitis, E., et al. (2013). Chimeric antigen receptor T Cells with dissociated signaling domains exhibit focused antitumor activity with reduced potential for toxicity in vivo. Cancer Immunology Research, 1(1), 43–53.
Yan, L. E., et al. (2020). Targeting Two Antigens Associated with B-ALL with CD19-CD123 Compound Car T Cell Therapy. Stem Cell Reviews and Reports, 16(2), 385–396.
Duan, D., et al. (2021). The BCMA-Targeted Fourth-Generation CAR-T Cells Secreting IL-7 and CCL19 for Therapy of Refractory/Recurrent Multiple Myeloma. Frontiers in Immunology, 12, 609421.
Tschumi, B. O., et al. (2018). CART cells are prone to Fas- and DR5-mediated cell death. Journal for Immunotherapy of Cancer, 6(1), 71.
Liu, D., Zhao, J., & Song, Y. (2019). Engineering switchable and programmable universal CARs for CAR T therapy. Journal of Hematology & Oncology, 12(1), 69.
Mehrabadi, A. Z., et al. (2022). Therapeutic potential of CAR T cell in malignancies: A scoping review. Biomedicine & Pharmacotherapy, 146, 112512.
Volonté, A., et al. (2014). Cancer-initiating cells from colorectal cancer patients escape from T cell-mediated immunosurveillance in vitro through membrane-bound IL-4. The Journal of Immunology, 192(1), 523–532.
Zhu, X., et al. (2015). Patient-derived glioblastoma stem cells are killed by CD133-specific CAR T cells but induce the T cell aging marker CD57. Oncotarget, 6(1), 171–184.
Feng, K. C., et al. (2017). Cocktail treatment with EGFR-specific and CD133-specific chimeric antigen receptor-modified T cells in a patient with advanced cholangiocarcinoma. Journal of Hematology & Oncology, 10(1), 4.
Sun, S., et al. (2018). Immunotherapy with CAR-Modified T Cells: Toxicities and Overcoming Strategies. Journal of Immunology Research, 2018, 2386187.
McLellan, A. D., & S.M. Ali Hosseini Rad. (2019). Chimeric antigen receptor T cell persistence and memory cell formation. Immunology & Cell Biology, 97(7), 664–674.
Nallanthighal, S., Heiserman, J. P., & Cheon, D. J. (2019). The Role of the Extracellular Matrix in Cancer Stemness. Frontiers in Cell and Developmental Biology, 7, 86.
Bao, S., et al. (2006). Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature, 444(7120), 756–760.
Kooti, W., et al. (2021). Oncolytic Viruses and Cancer, Do You Know the Main Mechanism? Frontiers in Oncology, 11, 761015.
Friedman, G. K., et al. (2012). Targeting pediatric cancer stem cells with oncolytic virotherapy. Pediatric Research, 71(4 Pt 2), 500–510.
Zimmerman, O., et al. (2023). Entry receptors — the gateway to alphavirus infection. The Journal of Clinical Investigation, 133(2), e165307.
Liang, Z., et al. (2021). Cyr61 from adipose-derived stem cells promotes colorectal cancer metastasis and vasculogenic mimicry formation via integrin α(V) β(5). Molecular Oncology, 15(12), 3447–3467.
Saha, D., Wakimoto, H., & Rabkin, S. D. (2016). Oncolytic herpes simplex virus interactions with the host immune system. Current Opinion in Virology, 21, 26–34.
Lemos de Matos, A., Franco, L. S., & McFadden, G. (2020). Oncolytic Viruses and the Immune System: The Dynamic Duo. Molecular Therapy Methods & Clinical Development, 17, 349–358.
Saha, D., Martuza, R. L., & Rabkin, S. D. (2017). Macrophage Polarization Contributes to Glioblastoma Eradication by Combination Immunovirotherapy and Immune Checkpoint Blockade. Cancer Cell, 32(2), 253-267.e5.
Tong, Y., et al. (2013). Potent antitumor activity of oncolytic adenovirus expressing Beclin-1 via induction of autophagic cell death in leukemia. Oncotarget, 4(6), 860–874.
Wang, H., et al. (2012). Oncolytic vaccinia virus GLV-1h68 strain shows enhanced replication in human breast cancer stem-like cells in comparison to breast cancer cells. Journal of Translational Medicine, 10, 167.
Wakimoto, H., et al. (2009). Human glioblastoma-derived cancer stem cells: Establishment of invasive glioma models and treatment with oncolytic herpes simplex virus vectors. Cancer Research, 69(8), 3472–3481.
Cheema, T. A., et al. (2013). Multifaceted oncolytic virus therapy for glioblastoma in an immunocompetent cancer stem cell model. Proceedings of the National Academy of Sciences of the United States of America, 110(29), 12006–12011.
Saha, D., et al. (2018). Combinatorial Effects of VEGFR Kinase Inhibitor Axitinib and Oncolytic Virotherapy in Mouse and Human Glioblastoma Stem-Like Cell Models. Clinical Cancer Research, 24(14), 3409–3422.
Hu, J. C., et al. (2006). A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clinical Cancer Research, 12(22), 6737–6747.
Yang, H., et al. (2016). Treatment of colon cancer with oncolytic herpes simplex virus in preclinical models. Gene Therapy, 23(5), 450–459.
Yoo, S. Y., et al. (2016). A cancer-favoring oncolytic vaccinia virus shows enhanced suppression of stem-cell like colon cancer. Oncotarget, 7(13), 16479–16489.
Bach, P., et al. (2013). Specific Elimination of CD133+ Tumor Cells with Targeted Oncolytic Measles Virus. Cancer Research, 73(2), 865–874.
Chaurasiya, S., Chen, N. G., & Warner, S. G. (2018). Oncolytic virotherapy versus cancer stem cells: A review of approaches and mechanism. Cancers (Basel), 10(4), 124.
Schirrmacher, V. (2020). Cancer vaccines and oncolytic viruses exert profoundly lower side effects in cancer patients than other systemic therapies: A comparative analysis. Biomedicines 8(3), 61.
Sobhani, N., et al. (2022). Therapeutic cancer vaccines: From biological mechanisms and engineering to ongoing clinical trials. Cancer Treatment Reviews, 109, 102429.
Stanley, M. (2017). Tumour virus vaccines: Hepatitis B virus and human papillomavirus. Philosophical Transactions of the Royal Society B, 372(1732), 20160268.
Anassi, E., & Ndefo, U. A. (2011). Sipuleucel-T (provenge) injection: The first immunotherapy agent (vaccine) for hormone-refractory prostate cancer. P t, 36(4), 197–202.
Guallar-Garrido, S., & Julián, E. (2020). Bacillus Calmette-Guérin (BCG) Therapy for Bladder Cancer: An Update. ImmunoTargets and Therapy, 9, 1–11.
Lee, A. (2023). Nadofaragene Firadenovec: First Approval. Drugs, 83(4), 353–357.
Ferrucci, P. F., et al. (2021). Talimogene Laherparepvec (T-VEC): An intralesional cancer immunotherapy for advanced melanoma. Cancers (Basel), 13(6), 1383.
Caudill, M. M., & Li, Z. (2001). HSPPC-96: A personalised cancer vaccine. Expert Opinion on Biological Therapy, 1(3), 539–547.
Ji, N., et al. (2018). Heat shock protein peptide complex-96 vaccination for newly diagnosed glioblastoma: a phase I, single-arm trial. JCI Insight, 3(10), e99145.
Testori, A., et al. (2008). Phase III comparison of vitespen, an autologous tumor-derived heat shock protein gp96 peptide complex vaccine, with physician’s choice of treatment for stage IV melanoma: The C-100-21 Study Group. Journal of Clinical Oncology, 26(6), 955–962.
Mazzaferro, V., et al. (2003). Vaccination with autologous tumor-derived heat-shock protein gp96 after liver resection for metastatic colorectal cancer. Clinical Cancer Research, 9(9), 3235–3245.
Wood, C. G., & Mulders, P. (2009). Vitespen: A preclinical and clinical review. Future Oncology, 5(6), 763–774.
Chu, N. R., et al. (2000). Immunotherapy of a human papillomavirus (HPV) type 16 E7-expressing tumour by administration of fusion protein comprising Mycobacterium bovis bacille Calmette-Guérin (BCG) hsp65 and HPV16 E7. Clinical and Experimental Immunology, 121(2), 216–225.
Ahrends, T., & Borst, J. (2018). The opposing roles of CD4(+) T cells in anti-tumour immunity. Immunology, 154(4), 582–592.
Guo, J., et al. (2022). Cancer vaccines from cryogenically silicified tumour cells functionalized with pathogen-associated molecular patterns. Nature Biomedical Engineering, 6(1), 19–31.
Santos, P. M., & Butterfield, L. H. (2018). Dendritic Cell-Based Cancer Vaccines. The Journal of Immunology, 200(2), 443–449.
Lin, M., et al. (2017). Development and application of cancer stem cell-targeted vaccine in cancer immunotherapy. Janssen Vaccine, 8(6), 371.
Zhou, L., et al. (2015). Promise of cancer stem cell vaccine. Human Vaccines & Immunotherapeutics, 11(12), 2796–2799.
Zheng, F., et al. (2018). Cancer Stem Cell Vaccination With PD-L1 and CTLA-4 Blockades Enhances the Eradication of Melanoma Stem Cells in a Mouse Tumor Model. Journal of Immunotherapy, 41(8), 361–368.
Rouzbahani, E., et al. (2022). Cancer stem cells in immunoregulation and bypassing anti-checkpoint therapy. Biomedicine & Pharmacotherapy, 156, 113906.
Lu, L., et al. (2015). Abstract 4796: Cancer stem cell vaccine inhibits metastases of primary tumors and induces humoral immune responses against cancer stem cells. OncoImmunology, 4, 00–00.
El-Ashmawy, N. E., et al. (2020). Dual-targeted therapeutic strategy combining CSC-DC-based vaccine and cisplatin overcomes chemo-resistance in experimental mice model. Clinical and Translational Oncology, 22(7), 1155–1165.
Wei, H. (2019). Interleukin 6 signaling maintains the stem-like properties of bladder cancer stem cells. Translational Cancer Research, 8(2), 557–566.
Kumari, N., et al. (2016). Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biology, 37(9), 11553–11572.
Funding
D.S. was supported in part by a fund from the DOD (W81XWH-20–1-0702).
Author information
Authors and Affiliations
Contributions
M.B. and H.W. contributed to the study of conception and design. M.B., D.S. and H.W. contributed literature search, data analysis, writing, and editing.
Corresponding authors
Ethics declarations
Conflicts of Interest/Competing Interests
Not Applicable
Ethics Approval
Not Applicable
Consent to Participate
Not Applicable.
Consent for Publication
All authors approved the version of the manuscript to be published.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Borlongan, M.C., Saha, D. & Wang, H. Tumor Microenvironment: A Niche for Cancer Stem Cell Immunotherapy. Stem Cell Rev and Rep 20, 3–24 (2024). https://doi.org/10.1007/s12015-023-10639-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-023-10639-6