Abstract
Background
Identifying breast cancer risk factors is a critical component of preventative strategies for this disease. This study aims to identify modifiable and non-modifiable risk factors of breast cancer in Iranian women.
Methods
We used international databases (PubMed/Medline, Scopus, Web of Knowledge, and Embase) and national databases (SID, Magiran, and ISC) to retrieve relevant studies until November 13, 2022. The odds ratio (OR) with a 95% confidence interval using the random-effect model was used to estimate the pooled effect. The publication bias was assessed by the Egger and Begg test. A sensitivity analysis was conducted to evaluate the effect of each included study on the final measurement.
Results
Of the 30,351 retrieved articles, 24 matched case–control records were included with 12,460 participants (5675 newly diagnosed cases of breast cancer and 6785 control). This meta-analysis showed that of the known modifiable risk factors for breast cancer, obesity (vs normal weight) had the highest risk (OR = 2.17, 95% CI 1.47 to 3.21; I2 = 85.7) followed by age at marriage (25–29 vs < 18 years old) (OR = 2.00, 95% CI 1.53 to 2.61; I2 = 0), second-hand smoking (OR = 1.86, 95% CI 1.58 to 2.19; I2 = 0), smoking (OR = 1.83, 95% CI 1.41 to 2.38; I2 = 18.9), abortion history (OR = 1.44, 95% CI 1.02 to 2.05; I2 = 66.3), oral contraceptive use (OR = 1.35, 95% CI 1.11 to 1.63; I2 = 74.1), age at marriage (18–24 vs < 18 years old) (OR: 1.22, 95% CI 1.02 to 1.47; I2 = 0). Of non-modifiable risk factors, history of radiation exposure (OR = 3.48, 95% CI 2.17 to 5.59; I2 = 0), family history of breast cancer (OR = 2.47, 95% CI 1.83 to 3.33; I2 = 73), and age at menarche (12–13 vs ≥ 14 years old) (OR = 1.67, 95% CI 1.31–2.13; I2 = 25.4) significantly increased the risk of breast cancer.
Conclusions
Since most risk factors related to breast cancer incidence are modifiable, promoting healthy lifestyles can play an influential role in preventing breast cancer. In women with younger menarche age, a family history of breast cancer, or a history of radiation exposure, screening at short intervals is recommended.
Similar content being viewed by others
Introduction
Women's breast cancer is the leading cause of cancer incidence in 2020, with about 2.3 million new cases, accounting for 11.7% of all cancer cases (1 in 4 cancer cases). It is the fifth leading cause of cancer mortality worldwide, with 685,000 deaths (1 in 6 cancer deaths) [1]. In Iran, breast cancer is the most frequent cancer among females [2]. Not only is the incidence increasing [3, 4], but also people with the disease are on average ten years younger than their Western counterparts [5]. It has been introduced leading cause of cancer mortality in women (age-standardized rate = 10.8 per 100,000) [6].
Breast cancer risk factors are divided into modifiable or lifestyle risk factors, which can be prevented, and non-modifiable risk factors [7]. Identifying these risk factors plays a significant role in primordial, primary, and secondary prevention. Breast cancer incidence varies widely among different populations globally [1]. So, it seems that there are no similar risk factors for all countries, and each country must identify the risk factors based on its demographic characteristics.
To identify risk factors, cohort studies are the best type of study, but case–control studies can also be an excellent alternative choice when the disease of interest is rare [8]. In Iran, there is no prospective study about breast cancer, and many conducted studies to identify risk factors of breast cancer are case–control studies, but their results vary. So, studies should be pooled to achieve consensus.
In this regard, a meta-analysis study was conducted in 2020 [9], but this study had some methodological shortcomings, such as a lack of comprehensive search strategy, combining of studies with different designs (case–control and cross-sectional studies), pooling of incident or prevalent cases in case–control studies, unclassified matched and unmatched case–control studies. These methodological problems can increase the recall and information bias and prevalent cases are mostly a sample of long disease duration and survival, so cannot be representative of the general population status. Besides the combination of studies with adjusted and crude odds ratios and including studies with different or non-defined reference categories in pooled estimation were important limitations of the published meta-analysis study which need to be considered in the current research.
Considering mentioned limitations of using prevalent cases, no access to cohort studies in Iran, and more efficient matched case–control studies than unmatched ones [10], we aimed to conduct a systematic review and meta-analysis study of matched case–control studies to determine breast cancer risk factors in Iranian women.
Materials and methods
This systematic review and meta-analysis were carried out using the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guideline [11]. A protocol was not registered at the international prospective register of systematic reviews (PROSPERO).
Search strategy
We used PubMed/Medline, Scopus, Web of Knowledge, Embase, and the Iranian database (SID, Magiran, and ISC) to retrieve the observational studies on breast cancer risk factors in Iran until November 13, 2022. Two groups of keywords were used for defining the breast cancer risk factors: the most important breast cancer risk factors presented in research worldwide and compatible with the MeSH library, and the keywords with the meaning of association such as correlation, relationship, etc. To find additional related studies, references of included studies, conducted systematic reviews, and meta-analyses were used. The search strategy details are presented in Additional file 1: Appendix S1 (Table A–D).
Eligibility criteria
The PECOS statement (Population, Exposure, Comparison, Outcomes, and Study design) is a framework to formulate eligibility criteria in systematic reviews. The research question was conducted using the PECOS framework (Table 1).
To be included in the meta-analysis, a published study had to meet the following criteria: (1) being original article, (2) published in Persian or English language, (3) compliance with PECOS criteria. Exclusion criteria consisted of the control group selection from patients with benign breast disease, matching on numerous variables, matching on variables except for age. Not reporting the odds ratio (OR) or not being able to calculate OR and the 95% confidence interval.
To find additional studies, we used the reference of the included studies and the systematic reviews and meta-analyses studies. If there were several publications from a dataset, the article presenting the most risk factors was selected. If there were different risk factors in those publications, all of them were included in the study.
Data extraction
The search results of all databases were combined using EndNote, and duplicates were deleted. Two researchers (MKH and NGH) who were blinded to authors and journal names, reviewed the publications to identify those meeting the eligibility criteria being. A third author (SHH) addressed the possible lack of consensus between the two authors. There was 95% inter-author reliability by kappa statistics.
If the full text was not accessible or the type of selection cases (incident/prevalent cases) or controls were ambiguous, the corresponding authors were contacted by email for further data. After selecting the final records, two authors (MKH and NGH) started data extraction. Data included titles, first author's name, study design, sample size, publication year, patient recruitment period, city, study setting, risk factors, case and control description, number of cases and controls, number of exposed cases with risk factors, and number of exposed controls with risk factors, matching factors, crude and adjusted OR with 95% confidence interval (CI).
Association measurement
The measure of association between exposure and occurrence of disease in case–control studies is the odds ratio (OR). If there was no OR in a study, we calculated OR from the data of the article. OR refers to the odds of exposure to a specific risk factor in women with breast cancer compared to control group. Selected controls were matched on the various variables and age was common in all of them. A list of matched variables is reported in Table 2.
Risk of bias assessment
The risk of bias assessment was scored by the Newcastle–Ottawa Scale (NOS) from 0 to 9 stars [12]. It was divided into three groups of 0–3 (fair), 4–6 (moderate), and 7–9 (good).
Statistical analysis
Pooled measures were calculated based on a random-effect model [13]. The heterogeneity was assessed by statistical testing: Cochran's Q (χ2) test and I2. Quantitative assessment of heterogeneity was performed on the I2 and Higgins classifications. The heterogeneity < 50% was defined as low, between 50 and 74% as moderate, and ≥ 75% as high [14, 15]. The possibility of publication bias was explored by the Egger [16] and Begg [17] tests. If there was publication bias, the Trim and Fill method was employed [18]. A sensitivity analysis was conducted to evaluate the effect of each included study on the final measurement. A significant level was considered for heterogeneity (χ2) 0.1 and publication bias and pooled effects 0.05. The data were analyzed using Stata version 14.2 (StataCorp., College Station, TX, USA).
Results
Study selection
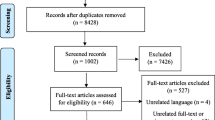
A total of 40,310 studies were retrieved, of which 33,630 were in English and 6680 were in Persian. Also, 18,681 articles were excluded due to duplication. After reading the title and abstract of 21,629 articles, the full text of 125 case–control studies was reviewed. In evaluating the full texts, there were two nested case–control studies which we assumed to be valuable for including in the study. But they had studied various and uncommon risk factors different from other included articles, so it was not possible to pool them in our analysis. In this step, 101 records were excluded because of unmatched case–control and unrelated nested case–control studies, the selection of cases and controls not meeting our inclusion criteria, the impossibility of calculating the association measurement, and multiple publications from one dataset. Finally, 24 matched case–control records (22 studies) were investigated in the meta-analysis. Figure 1 shows the steps for screening and selecting articles.
Study characteristics
A total number of 24 matched case–control records, involving 22 studies were included in this meta-analysis. The included records were conducted in nine cities as Tehran (10), Shiraz (3), Tabriz (2), Tehran/Tabriz (1), Kermanshah (1), Sabzevar (1), Babol (3), Isfahan (1), Arak (1), and Yazd (1) with the published date between 2008 and 2020. In the included studies, 12,460 participants (5675 newly diagnosed cases of breast cancer and 6785 control) were assessed, with the mean age ranging from 32.2 to 65 years in cases and 32.9–61 years in control groups. Control groups in 17 research had been selected from hospitals and clinics, and three research were from the general population. Also, in the four records, the place of control group selection was selected were not clear. Table 2 presents the information on the selected records in detail.
Risk of bias assessment
All the records were evaluated as moderate and high quality, with scores ranging from 4 to 8. Overall, the risk of bias score of 19 records was moderate and others were good (Table 3).
Modifiable risk factors
Occupation
According to nine studies, the overall effect measure showed that employee versus housewife was associated with increased odds of breast cancer by %37 [OR = 1.37 (95% CI 0.98 to 1.91)]; however, this association was not statistically significant (Fig. 2A). The results of the sensitivity analysis showed that excluding each study would change the overall estimate between 1.18 and 1.49 (Table 4).
The association between breast cancer and different risk factors. A: Occupation (employee vs housewife); B: education (lower than university vs university); C: marital status (single, divorced, widow vs married); D: place of residence (rural vs urban); E: age at marriage (≥ 18 vs < 18); F: BMI (BMI level vs normal range)
Education
In seven evaluated studies, there was no association between illiteracy and the odds of breast cancer [OR = 1.00 (95% CI 0.59 to 1.69)]. The overall estimate changed to 0.84 and 1.32 excluding the studies of Vahid [19] and Ghiasvand [20], respectively. Also, in included articles, no significant association was found between lower than diploma education and the odds of breast cancer [OR = 1.01 (95% CI, 0.73 to 1.39)]. The overall estimate changed to 0.90 and 1.14 excluding the studies of Dianatinasab [21] and Ghiasvand [20], respectively (Fig. 2B, Table 4).
Marital status
The overall effect measure of 14 studies showed that single, divorced, and widow versus married was associated with increased odds of breast cancer by %18 [OR = 1.18 (95% CI 0.85 to 1.64)]; however, this association was not statistically significant (Fig. 2C). In sensitivity analysis, the overall estimation changed between 1.09 and 1.34 by excluding each study. Egger test revealed publication bias (p = 0.09) (Table 4), but Trim and Fill analysis estimated no censored studies, and OR did not change.
Place of residence
The association between residential place and the odds of breast cancer was assessed in 4 studies. No significant association was found between living in urban and the odds of breast cancer [OR = 1.46 (95% CI 0.96 to 2.21)] (Fig. 2D). Sensitivity analysis showed that the overall estimation changed between 1.13 and 1.69 by excluding each study (Table 4).
Age at marriage
According to three studies, the age at marriage of 18–24 vs < 18 years was associated with increased odds of breast cancer by %22 [OR = 1.22 (95% CI, 1.02 to 1.47)]. Also, the age group of 25–29 vs < 18 years was significantly associated with odds of two times for developing breast cancer [OR = 2 (95% CI 1.53 to 2.61). This effect was greater in the age group of ≥ 30 vs < 18 years [OR = 2.02 (95% CI, 0.68 to 5.98)] (Fig. 2E, Table 4).
Body mass index (BMI)
The overall effect measure in three studies showed that underweight vs normal weight was associated with decreased odds of breast cancer by 30% [OR = 0.70 (95% CI 0.34 to 1.45)]; however, this association was not statistically significant. According to sensitivity analysis, the overall estimate changed to 0.56 and 1.05 excluding the studies of Fararouei [22] and Dianatinasab [21], respectively. Nine studies showed that overweight vs normal weight was significantly associated with increased odds of breast cancer by 30% [OR = 1.30 (95% CI 1.00 to 1.70)]. Also, obesity vs normal weight was significantly associated with odds of 2.17 times for developing breast cancer [OR = 2.17 (95% CI 1.47 to 3.21)]. The tests revealed publication bias, but Trim and Fill analysis estimated no missing studies, and OR did not change (Fig. 2F, Table 4).
Physical activity
The overall effect of six evaluated studies showed that physical activity of occasionally and never versus actively was associated with increased odds of breast cancer by 37% [OR = 1.37 (95% CI 82 to 2.30)] and 54% [OR = 1.54 (95% CI 0.93 to 2.54)], respectively. However, these associations were not significant (Fig. 3A, Table 4).
Smoking
Results of nine studies showed that the overall effect of smoking was significantly associated with increased odds of breast cancer up to 83% [OR = 1.83 (95% CI 1.41 to 2.38)] (Fig. 3B). According to sensitivity analysis, the obtained OR for this variable was robust (range of OR changes: between 1.75 and 1.88) (Table 4).
Second-hand smoking
The three studies included in this group showed that second-hand smoking was significantly associated with increased odds of breast cancer by 86% [OR = 1.86 (95% CI 1.58 to 2.19)] (Fig. 3C). Sensitivity analysis showed that the obtained OR for this variable had good robustness (range of OR changes: between 1.83 and 1.90) (Table 4).
Alcohol use
In two evaluated studies, there was no association between alcohol use and the odds of breast cancer [OR = 0.59 (95% CI 0.15 to 2.29)] (Fig. 3D). According to sensitivity analysis, the overall estimate changed between 0.40 and 2.01 excluding each study (Table 4).
Supplement intake
Based on five studies, the overall effect measure showed that supplement intake was associated with decreased odds of breast cancer by 39% [OR = 0.61 (95% CI 0.35 to 1.07)]. However, this association was not significant (Fig. 3E). In sensitivity analysis, overall estimation changed between 0.46 and 0.73 excluding each study. The Begg and Egger test revealed publication bias, but the Trim and Fill analysis estimated no missing studies (Table 4).
Parity
According to five evaluated studies, the overall effect measure showed no association between parity and odds of breast cancer [OR = 0.94 (95% CI 0.71 to 1.24)] (Fig. 3F). The sensitivity analysis showed that the overall estimate changes between 0.88 and 1.06 excluding the studies of Tehranian [23] and Maleki [24], respectively (Table 4).
Abortion history
In five studies, the overall effect measure showed that abortion was significantly associated with increased odds of breast cancer by 44% [OR = 1.44 (95% CI 1.02 to 2.05)] (Fig. 4A). According to sensitivity analysis, the overall estimate changed between 1.21 and 1.63 excluding each study (Table 4).
Oral contraceptive (OCP) use
According to 13 studies, the overall effect measure indicated that OCP use was significantly associated with increased odds of breast cancer by 35% [OR = 1.35 (95% CI 1.11 to 1.63)] (Fig. 4B). According to sensitivity analysis, the obtained OR for this variable was robust (range of OR changes: between 1.26 and 1.41) (Table 4).
Hormone replacement therapy (HRT)
In five evaluated studies, the overall effect measure showed that HRT history was associated with increased odds of breast cancer by 3% [OR = 1.03 (95% CI 0.50 to 2.14)]. However, this association was not significant (Fig. 4C). The sensitivity analysis showed that the overall estimate changes between 0.83 and 1.31 excluding the studies of Sheikhi, Vahid [19, 25], and Sasanfar [26] (Table 4).
Breastfeeding history
The overall effect of eight studies indicated that breastfeeding was associated with decreased odds of breast cancer by 8% [OR = 0.92 (95% CI, 0.50 to 2.14)]. However, this association was not significant (Fig. 4D). According to sensitivity analysis, the obtained OR for this variable was robust (range of OR changes: between 0.83 and 0.93). The Begg and Egger test revealed publication bias (Table 4), but missing studies were not found with Trim and Fill analysis and OR did not change.
Breastfeeding duration
According to eight studies, the overall effect measure showed that breastfeeding duration < 24 versus ≥ 24 months was associated with increased odds of breast cancer by 47% [OR = 1.47 (95% CI 0.74 to 2.92)]. However, this association was not significant (Fig. 4E). The results of sensitivity analysis showed that the overall estimate changed between 1.09 and 2.14 excluding each study (Table 4).
Non-modifiable risk factors
Age at menarche
Age at menarche was examined in four studies, and the results showed that age at menarche of 12–13 vs ≥ 14 years was significantly associated with increased odds of breast cancer by 67% [OR = 1.67 (95% CI, 1.31 to 2.13)]. Also, the age group < 12 vs ≥ 14 years was associated with odds of 2.72 times for developing breast cancer [OR = 2.72 (95% CI 0.93 to 7.99)] (Fig. 5A, Table 3).
The association between breast cancer and different risk factors. A: Age at menarche (< 14 vs ≥ 14); B: age at menopause (< 49 vs ≥ 49); C: menopausal status (yes vs no); D: family history of breast cancer (yes vs no); E: family history of cancer (yes vs no); F: history of radiation exposure (yes vs no)
Age at menopause
Age at menopause was investigated in three studies and the result showed that age at menopause of < 49 versus ≥ 49 years was associated with odds of 2.03 times for developing breast cancer [OR = 2.03 (95% CI, 0.77 to 5.34)]. However, this association was not significant (Fig. 5B). The Egger test revealed publication bias (p = 0.02) (Table 4), but missing studies were not found with Trim and Fill analysis.
Menopause status
In 12 evaluated studies, the overall effect measure showed that- menopause was associated with increased odds of breast cancer by 18% [OR = 1.18 (95% CI 0.90 to 1.55)]. However, this association was not significant (Fig. 5C). The results of the sensitivity analysis showed that excluding each study changed the overall estimate between 1.10 and 1.2 (Table 4).
Family history of breast cancer
Based on 11 studies, the overall effect measure showed that family history of breast cancer in the first-degree and second-degree relatives significantly was associated with odds of 2.47 times for developing breast cancer [OR = 2.47 (95% CI, 1.83 to 3.33)] (Fig. 5D). Sensitivity analysis showed that the obtained OR for this variable was robust (range of OR changes: between 2.28 and 2.65) (Table 4).
Family history of cancer
The overall effect of 11 studies showed that a family history of cancer was associated with odds of 2.57 times for developing breast cancer [OR = 2.57 (95% CI 0.84 to 7.85)]. However, this association was not significant (Fig. 5E). The results of the sensitivity analysis showed that excluding each study changed the overall estimate between 1.45 and 3.58 (Table 4).
History of radiation exposure
In two studies, the overall effect measure showed the history of radiation exposure was significantly associated with odds of 3.48 times for developing breast cancer [OR = 3.48 (95% CI 2.17 to 5.59)] (Fig. 5F). The results of the sensitivity analysis showed that excluding each study changed the overall estimate between 3.46 and 3.49 (Table 4).
Discussion
In this study, extracted breast cancer risk factors were categorized as modifiable and non-modifiable factors. Among the modifiable risk factors, obesity, age at marriage, second-hand smoking, smoking, abortion history, and OCP use were associated with an increased risk of breast cancer. Among the non-modifiable risk factors, history of radiation exposure, family history of breast cancer, and age at menarche increased the risk of developing breast cancer.
Modifiable risk factors
According to the results of this study, obesity vs normal weight significantly increased the risk of breast cancer. In a meta-analysis study, Liu et al. showed that every 5 units increase in BMI could lead to a 2% increased risk of breast cancer (P < 0.001). This dose–response study confirmed a significant linear relationship between BMI and breast cancer risk. In the analysis of premenopausal and postmenopausal subgroups, BMI in the premenopausal group played a protective role in developing breast cancer (P < 0.001, 95% CI 0.0–96.99, SRRFootnote 1: 0.98) while it was recognized as a significant risk factor in postmenopausal women (P = 0.001; 95% CI 1.1–02.07, SRR = 1.04) [27]. The relationship between increased BMI and the risk of breast cancer has been confirmed in most available sources [28, 29], and certain lifestyle modifications in women can be effective in modulating this risk factor.
Given that sexual relations are often formed in the context of marriage in Iranian culture, Iranian studies emphasize the age of marriage as a risk factor for breast cancer. In the present study, the age at marriage of 18–24 years vs < 18 years increased the risk of breast cancer by 22%, which was also observed in the age of 25–29 vs < 18 years and was significant in both groups. Reports from the Statistical Center of Iran show that the mean age at marriage increased from 25.6 to 27.4 years in men and from 22.4 to 23 years in women during 1996–2016 [30]. Other studies have shown a strong association between the age of the first marriage and the risk of breast cancer [31]. This emphasizes the need for a national policy to facilitate marriage.
The results of the present study confirmed that smoking and second-hand smoke were associated with increased odds of breast cancer by 83% and 86%, respectively. In particular, childhood exposure has been associated with an increased risk of premenopausal cancer [28]. Although smoking is less associated with breast cancer than second-hand smoking in this meta-analysis, this association is underestimated due to information bias. Because in Iran, smoking for women is not generally acceptable, so most women hide their smoking status due to this stigma while second-hand smoking is more presented by them. According to the American Cancer Society in 2019, women who smoked for more than 10 years before their first delivery were 18% more likely to develop breast cancer than non-smokers [28]. Also, a 2013 meta-analysis of 73,000 women showed that smoking before the first delivery significantly increased the risk of breast cancer (hazard ratio = 1.21, 95% CI = 1.14 to 1.28) [32]. Current smoking and past smoking increased the risk of breast cancer by 1.12 and 1.09, respectively [32]. According to US general surgeons’ consensus in 2004, the available evidence was insufficient to establish a causal effect between smoking and breast cancer [33]. Also, in 2009, the International Agency for Research on Cancer stated that there is insufficient evidence that cigarettes are carcinogenic [34]. Despite the significant relationship between smoking and increased risk of breast cancer in the present study, it is necessary to consider limitations such as the timing of smoking and the type of use (continuous or non-continuous). Given lifestyle changes in Iran, and various confounding factors, conducting more comprehensive research can be effective in health policymaking and control of non-communicable diseases.
The results of 5 studies showed that having a history of abortion increased the risk of breast cancer by 44%. A similar meta-analysis of 403,000 women showed an increased risk of developing breast cancer in women with ≥ 3 abortions (OR = 2.39; 95% CI: 1.78–3.21) [35]. Despite the limited number of studies in Iran, the odds ratio is almost equal compared to other studies. Since estrogen as a breast cancer risk factor increases in the first half of pregnancy, abortion exposes undifferentiated breast cells to high concentrations of estrogen during this period [36]. Attention to this pathophysiology emphasizes the importance of preventive measures and opportunistic screening for breast cancer in these individuals.
Thirteen examined studies have shown that the risk of developing breast cancer is significantly increased by 35% by taking oral contraceptive pills. The American Cancer Society states that the recent use of birth control pills is associated with about 20% higher risk of breast cancer, especially before the first pregnancy [28]. OCPs are prescribed as a method of contraception and as a method of treating hormonal disorders. On the other hand, there is no integrated database in Iran that records the duration, amount, and continuity of using these pills. Given the relatively proven role of hormonal compounds in the development of breast cancer, access to the above information can lead to a more accurate estimate of the contribution of hormonal pills in the development of breast cancer.
In nine articles, the risk of developing breast cancer in employed women was 37% higher than that in housewives, although it was not significant at an error level of 5%. The lack of uniform definition and job changes in different periods is an important limitation in examining the causal relationship between occupation and breast cancer. In one meta-analysis, increasing the number of years of jobs with night shift work increased the risk of developing breast cancer by about 1.1 times [37]. The lack of welfare standards in similar occupations is a confounding factor that complicates the study of this relationship. Therefore, multidisciplinary studies that can show the role of occupational factors on breast cancer appear necessary.
In this study, urbanization was associated with an increased risk of developing breast cancer (OR = 1.46; 95% CI 0.96–2.21). In a meta-analysis of 31 studies, Akinyemiju et al. showed a positive relationship between an increased risk of breast cancer and urbanization compared to rural life (Relative Risk = 1.09; 95% CI 1.1–1.19) [38]. The design of population cohort studies in several provinces with appropriate national distribution provides good evidence in this regard.
Physical activity plays a protective role in developing cancer with some hormonal regulation mechanisms such as lowering insulin levels. It is also effective in weight loss, which is indirectly associated with the reduction of breast cancer incidence by lowering insulin levels [39, 40]. Data from 6 studies showed that moderate/occasional physical activity increased the risk of breast cancer by 37% compared to regular exercise. A meta-analysis study of 139 articles, confirms the protective role of physical activity against breast cancer and that this effect size was similar in premenopausal and postmenopausal women [29]. Promoting a healthy lifestyle that includes regular physical activity and a proper diet should be considered a preventative factor in breast cancer.
Data from 47 epidemiological studies in 30 countries showed a 7% reduction in the relative risk of breast cancer with each delivery after adjusting for breastfeeding [41]. The present study also showed a 6% reduction in the risk of breast cancer by having children, which was not statistically significant. Considering the overall decrease in fertility from 2.07 in 2017 to 1.71 in 2020 in Iran [42], addressing this variable can improve the demographic characteristics of Iran and play an influential role in controlling the incidence of breast cancer.
In the present study, the risk of developing breast cancer with HRT after menopause increased by 3%. Other studies confirm this relationship, too [43]. Since the type of hormone consumed has not been reported separately in the studies in Iran, the analysis of this increased risk is worth considering. Certainly, performing breast cancer screening in HRT users increases the likelihood of being diagnosed with breast cancer. Therefore, it is necessary to examine the possibility of undifferentiated misclassification and overdiagnosis.
According to the present meta-analysis, breastfeeding reduced the risk of breast cancer by up to 8%. Also, breastfeeding for less than 2 years compared to 2 years and more increased the risk of breast cancer by 47%. None of these estimates were significant at an error level of 5%. In examining the relationship between breastfeeding and breast cancer, it is necessary to define this exposure more accurately in terms of duration, continuity of time, and quality of breastfeeding.
Non-modifiable risk factors
In this study, the earlier age of menarche increased the risk of breast cancer. Yi-Sheng et al. showed that the risk of breast cancer decreased by 5–10% for every 1-year delay in menarche [44]. A meta-analysis of 27 studies on Asian women found that age at menarche of 12 years and lower increased the risk of breast cancer by 1.26 times (95% CI 0.93–7.99) [35].
Results showed that the family history of breast cancer in first-degree and second-degree relatives increased the risk of developing breast cancer by 2.47 times, although this estimate did not consider distinguishing between family relationships and the number of people involved. A review study on 113000 women in the UK showed family history in one first-degree relative increased OR by 1.75 times and in the case of two or three people involved, this ratio increased to 2.5 times [45]. Accordingly, in countries with limited resources where population-based screening is not possible, measures for early detection of breast cancer in high-risk populations (positive family history of breast cancer) are recommended.
In two studies, a history of radiation exposure significantly increased the risk of breast cancer by 3.48 times, but this odds ratio is biased because of considering any kind of radiation exposure such as radiography as a risk factor. There was no clear definition for this variable without determining the dose and time of exposure. The results of a systematic review study also showed that a history of radiation exposure to the chest area linearly increased the risk of breast cancer in young women, with a standardized incidence ratio of 13.3 to 55.5 [46]. Our results were derived from only two studies may affect their generalizability and should be interpreted with caution. It seems more accurate for quantitative studies to introduce the attributable risk of radiation exposure in developing breast cancer are needed.
Menopausal age less than 49 years increased the risk of breast cancer by 2.03 times compared to older ages, which was not statistically significant. This contradictory result, in addition to the limited number of studies, could be due to the induced menopause of young patients. Due to the young population composition of Iran, the age of breast cancer incidence is about a decade lower than that in other countries [47] and in most studies, physiological menopause has not been distinguished from induced menopause, which often occurs at a young age. Perhaps the earlier onset of menopause in the case group than that in the control group led to a misclassification of exposure and bias.
Having a family history of cancer increased the risk of breast cancer by 2.57 times. According to the American Cancer Society, a family history of ovarian, pancreatic, and prostate cancer is associated with an increased risk of breast cancer [28]. However, one of the limitations of the present study was the heterogeneity in recording different types of cancers, the number of people involved, and the family relationships of individuals, which makes it difficult to provide a definitive analysis of the risk of this variable in breast cancer.
Some of the variables mentioned could not be pooled because they were only in one of the articles. These variables included socioeconomic status, infertility treatment, night bra use, hair coloring, past life stress, prenatal age, hysterectomy, cosmetic use, day sleep duration, parity number, diabetes, irregular menstruation, and ovarian cyst that were not significantly associated with breast cancer, while variables such as day bra use, sunlight exposure, stress, high-fat diet, migration, history of > 20 kg weight gain after 18 years old were introduced as risk factors and regular bedtime, quality sleep, diet containing sufficient fruit and vegetables, were as protective factors for breast cancer.
Strengths
One of the issues which make distinguish this meta-analysis from the previous ones is the clarity of the reference group, use of incident cases of breast cancer, lack of combination of different study designs, and conducting sensitivity analysis. This is the first research that has tried to estimate more accurately the attributable risk factors of breast cancer in Iran by considering the methodological limitations of the published studies.
Limitation
In this study, there are several limitations. Despite the inclusion of studies with newly diagnosed patients, there is still a recall bias publication bias tests were not significant in a small sample size, so the absence of publication bias is not ruled out. In risk of bias assessment, most records had moderate quality due to not selecting the control group from the community and not mentioning ascertainment of exposure and non-response rate. Due to the small number of studies, it was impossible to do metaregression for finding the cause of heterogeneity. Although there were the same risk factors in many studies, due to the lack of similar reference groups, we cannot use all of these studies. There was no complete geographical distribution of breast cancer risk factors. Due to the absence of cohort studies, it is not possible to conclude with certainty the causal relationship of the obtained risk factors. The obtained odds ratios may be overestimated because of not achieving the conditional logistic regression in the primary studies.
Conclusion
In general, in the present study, age at marriage of 18–29 years, obesity, smoking, second-hand smoking, abortion history, OCP use, age at menarche of 12–13 years, family history of breast cancer, and history of radiation exposure were introduced as risk factors for breast cancer. Given that most of the above are modifiable risk factors, lifestyle changes can play an influential role in the primordial prevention of breast cancer. In women whose risk factors are non-modifiable (women with younger age at menarche, family history of breast cancer, or history of radiation exposure), screening at short intervals can play an effective role in the secondary prevention of breast cancer.
Availability of data and materials
The datasets generated and analyzed during the current study are available through contact with the corresponding authors: haghighat@acecr.ac.ir, sha_haghighat@yahoo.com.
Notes
Summary Relative Risk.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2021;71(3):209–49.
Nafissi N, Khayamzadeh M, Zeinali Z, Pazooki D, Hosseini M, Akbari ME. Epidemiology and histopathology of breast cancer in Iran versus other Middle Eastern countries. Middle East J Cancer. 2018;9(3):243–51.
Zahmatkesh B, Keramat A, Alavi N, Khosravi A, Kousha A, Motlagh AG, et al. Breast cancer trend in Iran from 2000 to 2009 and prediction till 2020 using a trend analysis method. Asian Pac J Cancer Prev. 2016;17(3):1493–8.
Rafiemanesh H, Salehiniya H, Lotfi Z. Breast cancer in Iranian woman: Incidence by age group, morphology and trends. Asian Pac J Cancer Prev. 2016;17(3):1393–7.
Harirchi I, Karbakhsh M, Kashefi A, Momtahen AJ. Breast cancer in Iran: results of a multi-center study. Asian Pac J Cancer Prev. 2004;5(1):24–7.
Tartof SY, Slezak JM, Fischer H, Hong V, Ackerson BK, Ranasinghe ON, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398(10309):1407–16.
Centers for Disease Control and Prevention. What are the risk factors for breast cancer? Centers for Disease Control and Prevention 2022. https://www.cdc.gov/cancer/breast/basic_info/risk_factors.htm.
Van Stralen KJ, Dekker FW, Zoccali C, Jager KJ. Case-control studies–an efficient observational study design. Nephron Clin Pract. 2010;114(1):c1–c4.
Shamshirian A, Heydari K, Shams Z, Aref AR, Shamshirian D, Tamtaji OR, et al. Breast cancer risk factors in Iran: a systematic review & meta-analysis. Horm Mol Biol Clin Invest. 2020. https://doi.org/10.1515/hmbci-2020-0021.
Thompson WD, Kelsey JL, Walter SD. Cost and efficiency in the choice of matched and unmatched case-control study designs. Am J Epidemiol. 1982;116(5):840–51.
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology a proposal for reporting. JAMA. 2000;283(15):2008–12.
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in. Meta-Anal. 2000. https://doi.org/10.1016/0197-2456(86)90046-2.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11(2):193.
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994. https://doi.org/10.2307/2533446.
Duval S, Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc. 2000;95(449):89–98.
Vahid F, Shivappa N, Hatami M, Sadeghi M, Ameri F, Naeini YJ, et al. Association between dietary inflammatory index (DII) and risk of breast cancer: a case-control study. APJCP. 2018;19(5):1215.
Ghiasvand R, Bahmanyar S, Zendehdel K, Tahmasebi S, Talei A, Adami H-O, et al. Postmenopausal breast cancer in Iran; risk factors and their population attributable fractions. BMC Cancer. 2012;12(1):1–9.
Dianatinasab M, Fararouei M, Mohammadianpanah M, Zare-Bandamiri M, Rezaianzadeh A. Hair coloring, stress, and smoking increase the risk of breast cancer: a case-control study. Clin Breast Cancer. 2017;17(8):650–9.
Fararouei M, Iqbal A, Rezaian S, Gheibi Z, Dianatinasab A, Shakarami S, et al. Dietary habits and physical activity are associated with the risk of breast cancer among young Iranian women: a case-control study on 1010 premenopausal women. Clin Breast Cancer. 2019;19(1):e127–34.
Tehranian N, Shobeiri F, Pour FH, Hagizadeh E. Risk factors for breast cancer in Iranian women aged less than 40 years. Asian Pac J Cancer Prev. 2010;11(6):1723–5.
Maleki F, Fotouhi A, Ghiasvand R, Harirchi I, Talebi G, Rostami S, et al. Association of physical activity, body mass index and reproductive history with breast cancer by menopausal status in Iranian women. Cancer Epidemiol. 2020;67: 101738.
Mobarakeh ZS, Mirzaei K, Hatmi N, Ebrahimi M, Dabiran S, Sotoudeh G. Dietary habits contributing to breast cancer risk among Iranian women. Asian Pac J Cancer Prev. 2014;15(21):9543–7.
Sasanfar B, Toorang F, Esmaillzadeh A, Zendehdel K. Adherence to the low carbohydrate diet and the risk of breast cancer in Iran. Nutr J. 2019;18(1):86.
Liu K, Zhang W, Dai Z, Wang M, Tian T, Liu X, et al. Association between body mass index and breast cancer risk: evidence based on a dose-response meta-analysis. Cancer management and research. 2018;10:143–51.
American Cancer Society. Breast cancer facts and figures 2019–2020. Atlanta: American Cancer Society; 2019.
Hardefeldt PJ, Penninkilampi R, Edirimanne S, Eslick GD. Physical activity and weight loss reduce the risk of breast cancer: a meta-analysis of 139 prospective and retrospective studies. Clin Breast Cancer. 2018;18(4):e601–12.
Statistical Center of Iran. Data and statistical information: Statistical Center of Iran; 2022. Available from: https://amar.org.ir/.
Kinlen LJ, Gilham C, Ray R, Thomas DB, Peto J. Cohabitation, infection and breast cancer risk. Int J Cancer. 2021;148(6):1408–18.
Gaudet MM, Gapstur SM, Sun J, Diver WR, Hannan LM, Thun MJ. Active smoking and breast cancer risk: original cohort data and meta-analysis. J Natl Cancer Inst. 2013;105(8):515–25.
Office of the Surgeon General (US); Office on Smoking and Health (US). The health consequences of smoking: a report of the surgeon general. Atlanta, GA: Centers for Disease Control and Prevention (US); 2004.
Lauby-Secretan B, Straif K, Baan R, Grosse Y, Ghissassi F, Bouvard V, et al. A review of human carcinogens–Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009;10:1033–4.
Tao P, Hu Y, Huang Y, Li J. Risk factors of breast cancer in Asian women: a meta-analysis. Zhonghua liu xing bing xue za zhi Zhonghua liuxingbingxue zazhi. 2011;32(2):164–9.
Parkins T. Does abortion increase breast cancer risk? J Natl Cancer Inst. 1993;85(24):1987–8.
Lin X, Chen W, Wei F, Ying M, Wei W, Xie X. Night-shift work increases morbidity of breast cancer and all-cause mortality: a meta-analysis of 16 prospective cohort studies. Sleep Med. 2015;16(11):1381–7.
Akinyemiju TF, Genkinger JM, Farhat M, Wilson A, Gary-Webb TL, Tehranifar P. Residential environment and breast cancer incidence and mortality: a systematic review and meta-analysis. BMC Cancer. 2015;15(1):191.
Friedenreich CM, Neilson HK, Woolcott CG, McTiernan A, Wang Q, Ballard-Barbash R, et al. Changes in insulin resistance indicators, IGFs, and adipokines in a year-long trial of aerobic exercise in postmenopausal women. Endocr Relat Cancer. 2011;18(3):357–69.
Hirose K, Toyama T, Iwata H, Takezaki T, Hamajima N, Tajima K. Insulin, insulin-like growth factor-I and breast cancer risk in Japanese women. Asian Pac J Cancer Prev. 2003;4(3):239–46.
Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50 302 women with breast cancer and 96 973 women without the disease. The lancet. 2002;360(9328):187–95.
Fathi E. Fertility in Iran, from 2017 to 2020. Iran: Statistical Center of Iran; 2021.
Barbieri RL. Patient education: Menopausal hormone therapy (Beyond the Basics): UpToDate; 2021. Available from: https://www.uptodate.com/contents/menopausal-hormone-therapy-beyond-the-basics?search=Patient%20education:%20Menopausal%20hormone%20therapy%20(Beyond%20the%20Basics)&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1.
Sun Y-S, Zhao Z, Yang Z-N, Xu F, Lu H-J, Zhu Z-Y, et al. Risk factors and preventions of breast cancer. Int J Biol Sci. 2017;13(11):1387.
Brewer HR, Jones ME, Schoemaker MJ, Ashworth A, Swerdlow AJ. Family history and risk of breast cancer: an analysis accounting for family structure. Breast Cancer Res Treat. 2017;165(1):193–200.
Henderson TO, Amsterdam A, Bhatia S, Hudson MM, Meadows AT, Neglia JP, et al. Systematic review: surveillance for breast cancer in women treated with chest radiation for childhood, adolescent, or young adult cancer. Ann Int Med. 2010. https://doi.org/10.7326/0003-4819-152-7-201004060-00009.
Harirchi I, Karbakhsh M, Kashefi A, Momtahen AJ. Breast cancer in Iran: results of a multi-center study. APJCP. 2004;5(1):24–7.
Safabakhsh M, Imani H, Shab-Bidar S. Higher dietary total antioxidant capacity is not associated with risk of breast cancer in Iranian women. Breast Cancer. 2020. https://doi.org/10.1002/cnr2.1212.
Heidari Z, Jalali S, Sedaghat F, Ehteshami M, Rashidkhani B. Dietary patterns and breast cancer risk among Iranian women: a case-control study. Eur J Obstet Gynecol Reproduct Biol. 2018;230:73–8.
Pourzand A, Tajaddini A, Pirouzpanah S, Asghari-Jafarabadi M, Samadi N, Ostadrahimi A-R, et al. Associations between dietary allium vegetables and risk of breast cancer: a hospital-based matched case-control study. J Breast Cancer. 2016;19(3):292.
Montazeri V, Jafarpour Sadegh F, Hosseinpour S, Mirzaei H, Akbari E, Ehsanin M, et al. Reproductive risk factors of breast cancer among women in Tehran and Northwest of Iran: a case-control study. Iran J Epidemiol. 2016;12(1):1–9.
Salarabadi A, Bidgoli SA, Madani SH. Roles of Kermanshahi oil, animal fat, dietary and non-dietary vitamin D and other nutrients in increased risk of premenopausal breast cancer: a case control study in Kermanshah. Iran Asian Pacific Journal of Cancer Prevention. 2015;16(17):7473–8.
Bidgoli SA, Azarshab H. Role of vitamin D deficiency and lack of sun exposure in the incidence of premenopausal breast cancer: a case control study in Sabzevar. Iran Asian Pac J Cancer Prev. 2014;15(8):3391–6.
Hosseinzadeh M, Ziaei JE, Mahdavi N, Aghajari P, Vahidi M, Fateh A, et al. Risk factors for breast cancer in Iranian women: a hospital-based case-control study in Tabriz, Iran. J Breast Cancer. 2014;17(3):236.
Bahadoran Z, Karimi Z, Houshiar-rad A, Mirzayi H-R, Rashidkhani B. Is dairy intake associated to breast cancer? A case control study of Iranian women. Nutr Cancer. 2013;65(8):1164–70.
Hajian K, Gholizadehpasha A, Bozorgzadeh S. Association of obesity and central obesity with breast cancer risk in pre- and postmenopausal Women. J Babol Univ Med Sci. 2013;15(3):7–15.
Hajian-Tilaki K, Kaveh-Ahangar T, Hajian-Tilaki E. Is educational level associated with breast cancer risk in Iranian women? Breast Cancer. 2012;19(1):64–70.
Hajian-Tilaki KO, Kaveh-Ahangar T. Reproductive factors associated with breast cancer risk in northern Iran. Med Oncol. 2011;28(2):441–6.
Rezaeiian F, Rashidkhani B, Mirzaei H, Akbari E, Foroutan Ghaznavi M, Shadman Z, et al. Association between folate intake and breast cancer risk among Tehrani women a case control study. Iranian Journal of Nutri Sci Food Technol. 2012;6(4):21–31.
Bidgoli SA, Eftekhari T, Sadeghipour R. Role of xenoestrogens and endogenous sources of estrogens on the occurrence of premenopausal breast cancer in Iran. Asian Pac J Cancer Prev. 2011;12(9):2425–30.
Ghosn B, Benisi-Kohansal S, Ebrahimpour-Koujan S, Azadbakht L, Esmaillzadeh A. Association between healthy lifestyle score and breast cancer. Nutr J. 2020;19(1):1–11.
Saremi A, Mohammadi Bonchenari S. Physical activity and breast cancer prevalence: a case-control study in Arak, Iran (2017–2018). Iran Q J Breast Dis. 2019;12(1):29–38.
Lotfi M, Charkhati S, Shobeyri S. Breast cancer risk factors in an urban area of Yazd city. Yazd: ACTA MEDICA IRANIC; 2008.
Acknowledgements
The authors thank the research deputy of ACECR (Academic Center for Education and Research) and the manager of “The Iranian Health Network” for their financial support. Malihe Khoramdad collaborated on this project as part of her Epidemiology Ph.D. thesis at the Iran University of Medical Sciences and we appreciate the IUMS education deputy and their professors for this cooperation.
Funding
This project was supported financially by “Academic Center for Education and Research” and “The Iranian Health Network”.
Author information
Authors and Affiliations
Contributions
MK: conceptualization, methodology, analysis, interpreting of data, writing—original draft, writing—review and editing. MS-D: methodology, data curation, writing—original draft. AK: methodology, writing—original draft, supervision, writing—review and editing. NG: acquisition, data curation. ESH: writing—original draft, supervision, writing—review and editing. NF: writing—original draft, supervision, writing—review and editing. ZO: acquisition, interpreting of data, writing—original draft, writing—review and editing. MAM: writing—original draft, supervision, writing—review and editing. O: writing—original draft, supervision, writing—review and editing. HS: writing—original draft, supervision, writing—review and editing. SH: conceptualization, methodology, formal analysis, validation, data curation, writing—original draft, supervision, project administration, writing—review and editing. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Motamed Cancer Institute (IR.ACECR.IBCRC.REC.1399.010).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Appendix S1. The search strategy of breast cancer risk factors in Iran.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Khoramdad, M., Solaymani-Dodaran, M., Kabir, A. et al. Breast cancer risk factors in Iranian women: a systematic review and meta-analysis of matched case–control studies. Eur J Med Res 27, 311 (2022). https://doi.org/10.1186/s40001-022-00952-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-022-00952-0