Abstract
Background
Previous studies on the link between macronutrients and breast cancer have mostly focused on individual macronutrients rather than their combination. This study investigates the association between adherence to a low carbohydrate diet and odds of breast cancer among women.
Methods
This hospital-based case-control study was carried out on 412 women with pathologically confirmed breast cancer within the past year and 456 apparently healthy controls that were matched in terms of age and residential place. Dietary data was collected using a 168-item validated FFQ. Participants were classified in terms of quintiles of percentages of energy intake from carbohydrates, proteins, and fats. Then, individuals in the highest quintile of fat and protein intake were given a score of 5 and those in the lowest quintile of these macronutrients were given a score of 1. Participants in the other quintiles of these macronutrients were given the corresponding score. In terms of carbohydrate intake, those in the highest quintile received a score of 1 and those in the lowest quintile received 5. The scores were then summed up to calculate the total low carbohydrate diet (LCD) score, which varied from 3 to 15. A higher score meant greater adherence to a low carbohydrate diet.
Results
The mean age of study participants was 45.2 y and mean BMI was 28.4 kg/m2. Mean LCD score of participants was 8.9 ± 2.5 (8.9 ± 2.6 in cases and 9.0 ± 2.5 in controls). Although no significant association was observed between adherence to the LCD score and odds of breast cancer in the study population, a trend toward significant positive association was seen between consumption of LCD and odds of breast cancer in postmenopausal women; after controlling for several potential confounders, individuals in the third quartile of LCD score were 1.94 times more likely to have breast cancer than those in the lowest quartile (95% CI: 1.00, 3.76). This association strengthened after controlling for dietary variables (2.50; 1.18–5.32). Even after further adjustment for BMI, this association remained significant (2.64, 1.23–5.67). No significant relationship was observed in premenopausal women, either before or after controlling for confounders.
Conclusion
Adherence to LCD may be associated with increased odds of breast cancer in postmenopausal women. Prospective cohort studies are needed to confirm these findings.
Similar content being viewed by others
Introduction
Low- carbohydrate diets (LCD) are popular diets, and studies have reported beneficial effects on several chronic diseases, including obesity [1], diabetes [2], epilepsy [3, 4], cardiovascular diseases [5] and some cancers [6, 7]. High dietary intake of carbohydrates is associated with low levels of total- and LDL cholesterol, as well as low concentrations of HDL-cholesterol and high levels of triglycerides [8]. On the other hand, consumption of low carbohydrate diets has been linked with elevated concentrations of inflammatory biomarkers [9,10,11], which could be underlying factors for cancer [12].
An inverse association was reported between adherence to LCD and risk of mortality from all causes, and also from cancer and cardiovascular disease [13,14,15,16,17]. Despite huge evidence on the protective role of low-carbohydrate diets in several cancers, some investigations have linked such diets with restricted tumor growth [16, 18]. Findings from other studies have provided null results with regards to the relation of LCD and incidence of cancers [19]. Data on the association between adherence to LCD and risk of breast cancer are scarce. In a published study from the Nurses’ Health Study, consumption of a low carbohydrate diet was associated with reduced risk of breast cancer in postmenopausal women who were estrogen receptor-negative [17].
The dietary intake of people in the Middle-East, due to its heterogeneity as well as other general characteristics, provides a unique opportunity to examine the relation between diet and disease. Most people in this region consume refined grains (refined bread and white rice) as their staple food [20]. In addition, due to the nutritional transition that is occurring in these countries, dietary fat intake, and in particular, detrimental fats, are increasing [21]. Such dietary characteristics make it reasonable to examine the contribution of different macronutrients to human health. Although the role of individual macronutrients in different cancers has been examined before [22, 23], no study is available linking breast cancer and a combination of macronutrients. Assessment of LCD in relation to chronic conditions is of great importance in Middle-Eastern countries since carbohydrates are the main source of energy in these countries and protein is expensive [24]. Dietary fat intake in this region is consumed at the level of DRI, however, type of dietary fat is a problem [25]. Given the aforementioned points, this study was done to assess the association between adherence to LCD and the odds of breast cancer in a case-control study in Iran.
Subjects and methods
Participants
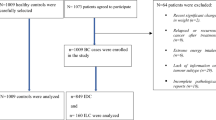
We conducted a hospital-based, case-control study between 2014 and 2016 among Iranian women aged 19–80 years old. Cases (n = 486) were breast cancer patients that referred to surgery, chemotherapy or radiotherapy departments of Iran Cancer Institute that is located at a major teaching and general hospital, Imam Khomeini complex in Tehran. All patients had pathologically confirmed breast cancer within the past year. They had no history of any other cancers and long term dietary restrictions. Controls were 523 apparently healthy subjects [frequency-matched to cases by age (±10 years) and place of residence] admitted to the same hospital as healthy visitors, relatives and friends of non-cancer patients. They had no long term dietary restrictions. Individuals with a total energy intake of > 4500 or < 800 kcal/d (n = 116) as well as those who had no response to more than 70 items of FFQ (n = 25) were excluded from the study. Eventually, 412 cases and 456 controls remained for the current analysis. The study was approved by the Bioethics Committee of Iran Cancer Institute, Tehran University of Medical Sciences, Tehran, Iran, and all participants gave written informed consent at the beginning of the study.
Assessment of dietary intake
Women with breast cancer were asked by trained interviewers to recall their usual diet over the preceding year using a validated semi-quantitative FFQ. It is clear that patients were likely to recall their dietary habits in the year prior to their diagnosis of breast cancer. The FFQ contained 168-food items with a standard serving size. Participants were asked to report their consumption on a daily, weekly or monthly basis. When the participants’ responses were not conformable with the given portion sizes, they were asked to report their own portion sizes for food items. Daily intake of reported food items was calculated and converted to grams per day using household measures. To compute energy and nutrient intakes, we used the USDA food composition database modified for Iranian foods [26, 27]. In a previous study, the validity of this FFQ was examined by comparing data from FFQ and the average of 12 dietary recalls [28]. The reliability was also examined through comparing data from two FFQs completed one year apart [29]. Findings from this validation study revealed that the FFQ provides reasonably valid and reliable data on long-term dietary intake [30].
Construction of the low carbohydrate diet (LCD) score
The contribution of macronutrients to total energy intake was used to construct the LCD score. Initially, the percentages of energy intake from fats, proteins, and carbohydrates were computed. Then, individuals were divided into quintiles based on the percentages of each macronutrient. Subsequently, individuals in the highest quintile of fat and protein were given a score of 5 and those in the lowest quintile of these macronutrients were given a score of 1. Participants in the other quintiles of these macronutrients were given a corresponding score. Reverse scoring was used for carbohydrates; those with the highest intake were given a score of 1 and those with the lowest intake were given a score of 5. The scores were then summed up to compute the total low carbohydrate diet (LCD) score. The score for each participant ranged from 3 to 15. A higher score meant greater adherence to a low carbohydrate diet.
Assessment of other variables
Weight was measured using digital scales to the nearest 100 g. Study participants were minimally clothed and without shoes during weighing. Height was measured while the women were standing and without shoes, using a tape meter. Body mass index (BMI) was calculated using measured weight and height. Data on physical activity was collected via a validated questionnaire, the Global Physical Activity Questionnaire (GPAQ) [31]. Patients were asked to recall their physical activity habits in the year preceding their cancer diagnosis. This questionnaire consists of 16 questions in 4 physical activity domains: job-related activities; transportation activities; recreation and sports activities; and sedentary behaviors. The measured data was processed according to the GPAQ Analysis Guide [32], and metabolic equivalent hours per week (MET-h/wk) values were calculated. Additional information on age, educational level, family history of breast cancer, alcohol and tobacco use, age at menarche, marital history, pregnancy history, parity, infertility treatment, age at menopause, postmenopausal hormone therapy, and contraceptive use were collected via questionnaire during a face-to-face interview.
Statistical methods
Analyses were done in the total study population as well as stratified by pre- and postmenopausal status. Individuals were categorized based on quartiles of LCD scores. We used one-way ANOVA and chi-square tests to compare continuous and categorical variables, respectively, across quartiles of LCD score. Multivariable logistic regression models were used to assess the association between adherence to LCD and risk of breast cancer. The analyses were first adjusted for age (continuous) and additionally for physical activity (continuous), family history of breast cancer (yes vs. no), educational level (categorical), parity (nulliparous, 1, 2–3, ≥4), oral contraceptive use (yes vs. no), menopausal hormone use (yes vs. no), tobacco use (yes vs. no), alcohol use (yes vs. no), infertility treatment (yes vs. no), marital status (married, unmarried). We further controlled for dietary intake of vitamin B6, iron, folic acid, vitamin A and vitamin E (all continuous). Finally, we made adjustments for body mass index (continuous). The trend of odds ratios across quartiles of LCD score was examined by considering the median value of LCD score in each category as a continuous variable. P values < 0.05 were considered statistically significant. The analysis was performed by STATA version 14 (State Corp., College Station, TX).
Results
Patients with BC were slightly older (46.3 νs. 44.2 years, P = 0.003), had lower BMI (28.1 νs. 28.8 kg/m2, P < 0.01), and were more likely to have a family history of breast cancer (10.1 νs. 1.54, P < 0.001) compared to controls (Table 1). They were less likely to be physically active (20.1 νs. 26.4 MET h/wk., P = 0.001), married (81.1 ν. 84.4%, P < 0.001), use oral contraceptives (52.8 vs. 61.3%, P = 0.02), use postmenopausal hormones (0.49 vs. 1.97%, P = 0.05) and drink alcohol (2.43 vs. 5.83%, P = 0.01) than controls (Table 1). Patients also had a lower intake of red meat and protein. The mean LCD score of participants was 8.9 ± 2.5 (8.9 ± 2.6 in cases and 9.0 ± 2.5 in controls).
Demographic, reproductive, and lifestyle characteristics of study participants (pre-and postmenopausal women) across quartile categories of LCD score are provided in Table 2. Mean (±SD) low-carbohydrate-diet score was 8.9 ± 2.5 for premenopausal and 9.0 ± 2.6 for postmenopausal women. Postmenopausal women in the top quartile of LCD score tended to be alcohol users, with a slightly lower age at menarche and lower family history of breast cancer compared to those in the bottom quartile.
Table 3 shows the mean daily dietary intake of food groups and nutrients across the quartiles of LCD score in pre-and postmenopausal women. Pre- and postmenopausal women in the top quartile of LCD score had a higher intake of red meat, full-fat dairy, protein, fat, saturated fat, and vitamin E and lower intake of whole grains, refined grains, fruits, and carbohydrate than those in the lowest quartile. In postmenopausal women, being in the top quartile of LCD score was associated with a lower intake of energy. Greater adherence to LCD in premenopausal women was associated with higher intake of vitamin A and lower intake of iron.
Multivariable-adjusted odds ratios and 95% CIs for breast cancer across categories of LCD score are provided in Table 4. Although no significant association was observed between adherence to LCD and odds of breast cancer in the whole study population, a trend toward significant positive association was seen between consumption of LCD and odds of breast cancer in postmenopausal women; after controlling for several potential confounders, individuals in the third quartile of LCD score were 1.94 times more likely to have breast cancer than those in the lowest quartile (95% CI: 1.00, 3.76). This association was strengthened after controlling for dietary variables (2.50; 1.18–5.32). Even after further adjustment for BMI, this association remained significant (2.64; 1.23–5.67). No such significant relationship was observed in premenopausal women, either before or after controlling for confounders.
Discussion
In this case-control study, we found that adherence to a low-carbohydrate diet was not significantly associated with increased odds of breast cancer in the study population, a trend toward significant positive association was seen between consumption of LCD and odds of breast cancer in postmenopausal women. To our knowledge, this is the first study examining the relationship between low-carbohydrate diet score and risk of breast cancer in a Middle- Eastern country.
Earlier studies on the association between dietary carbohydrate intake and several outcomes have mostly examined total carbohydrate intake alone or in the context of low-carbohydrate, high-protein (LCHP) or low-carbohydrate, high-fat diets [2, 17, 23, 33,34,35]. The low-carbohydrate dietary pattern we focused on here, is usually characterized by low intake of carbohydrates and high intake of proteins and fats [36]. Therefore, this approach considers all macronutrients and can provide better insight into the link between macronutrient intake and risk of breast cancer compared to individual intake of these macronutrients. We found that adherence to a low carbohydrate diet was not significantly associated with increased risk of breast cancer in the study population. Two previous studies have reported association between adherence to LCD and risk of breast cancer [17, 33]. In a cohort study of Swedish women, adherence to LCHP was not associated with increased risk of respiratory tract cancer, even though a significant association was observed in men [33]. Another study was conducted among postmenopausal women of the Nurses’ Health Study and demonstrated an inverse association between consumption of a vegetable-based LCD and estrogen receptor-negative (ER-) breast cancer risk [17]. In addition to these observational studies, the effect of a low carbohydrate ketogenic diet on several cancers has been examined [37]. It has been shown that a low carbohydrate ketogenic diet had beneficial effects on cancer cells [38]. In addition to the carbohydrate content of the diet, other carbohydrate-related indices were also examined in relation to the risk of breast cancer. Farvid et al. within the framework of the Nurses’ Health Study population failed to find any significant association between dietary glycemic index (GI), glycemic load (GL) and insulin load during adolescence, and risk of breast cancer during adulthood [39]. Similarly, two prospective cohort studies on French and Canadian women, revealed no association between dietary GI, GL and carbohydrate intake with overall breast cancer risk [40, 41]. In contrast, in a case-control study on Mexican women, a positive association between carbohydrate intake and risk of breast cancer was found [42]. The quality and quantity of macronutrients in the diet might explain the null association we found. Low-carbohydrate diets have been reported to obtain less than 26% of their energy from carbohydrates [37], whereas the amount of energy from carbohydrates among those with the greatest adherence to the LCD in the current study was 41.5%. This might further help in understanding the difference in findings.
When we analyzed the results by menopausal status, a trend toward significant association was observed between adherence to LCD and risk of breast cancer among post-menopausal women. Despite lack of a significant association between dietary GI and risk of breast cancer in the population, Canadian researchers found a positive association between high GI diets and increased risk of breast cancer among postmenopausal women [41]. In a case-control study among premenopausal Mexican women [43], an increased risk of breast cancer with higher intake of carbohydrate was seen. The relationship between diet and risk of breast cancer has been different in pre- and postmenopausal women. It seems that the contribution of diet to the risk of breast cancer is strong in premenopausal women. However, we failed to find such an association in premenopausal women and further studies are needed to clarify the association between adherence to LCD and odds of breast cancer.
Although there are no clear mechanisms on the link between low carbohydrate dietary patterns and risk of breast cancer, some studies suggest some potential mechanisms. High carbohydrate intake was associated with elevated blood glucose and insulin levels, which can promote glucose intolerance, insulin resistance and hyperinsulinemia. Warburg et al. expressed that cancer cells depend on glucose as a fuel [44]. Insulin resistance results in decreased levels of insulin-like growth factor (IGF) binding proteins 1 and 2, therefore, the availability of IGF-Ι, which can in turn increase tumor cell proliferation, would increase [45, 46]. Elevated levels of insulin and IGF-Ι result in higher levels of free estrogen and androgen via inhibiting the hepatic synthesis of the sex hormone binding globulin [47, 48]. Therefore, adherence to a diet with a low carbohydrate content might suppress tumor cell proliferation and regulate apoptosis via cell signaling pathways, the PI3K/Akt/Mtor and RAS/RAF/MEK/ERK sequences, which are insulin or IGF-Ι-dependent [7, 46, 49]. The significant association between circulating levels of IGF-Ι and increased risk of breast cancer in premenopausal women has previously been shown [50].
The strengths of the current study include considering several potential confounders, recruiting participants from a referral hospital, in which subjects are from all across the country. Stratified analysis by menopausal status is a strength of this study. However, several limitations need to be considered. First, due to the case-control design of the study with its inherent recall and selection bias, one cannot infer causality. Second, as with all epidemiologic studies that apply FFQ, misclassification of participants in terms of dietary intake cannot be excluded. Third, prominence in the third quartile may be due to chance as the sample size is limited in each quartile. Therefore further studies with larger sample sizes are needed to confirm this finding. Fourth, we did not gather information on the hormone receptor status of participants. This may affect our findings.
Conclusion
Based on the present findings, we found no evidence on association between consumption of a low carbohydrate diet (LCD), and odds of breast cancer risk in the study population; however, adherence to LCD might be associated with increased odds of breast cancer in postmenopausal women. Further research, especially cohort studies are needed to confirm these findings.
References
Volek JS, Sharman MJ, Gómez AL, Judelson DA, Rubin MR, et al. Comparison of energy-restricted very low-carbohydrate and low-fat diets on weight loss and body composition in overweight men and women. Nutr Metab. 2004;1:13.
Halton TL, Liu S, Manson JE, Hu FB. Low-carbohydrate-diet score and risk of type 2 diabetes in women. Am J Clin Nutr. 2008;87:339–46.
Lin A, Turner Z, Doerrer SC, Stanfield A, Kossoff EH. Complications during ketogenic diet initiation: prevalence, treatment, and influence on seizure outcomes. Pediatr Neurol. 2017;68:35–9.
Geyelin HR. Fasting as a method for treating epilepsy. Med Rec. 1921;99:1037–9.
Hu T, Yao L, Reynolds K, Whelton PK, Niu T, et al. The effects of a low-carbohydrate diet vs. a low-fat diet on novel cardiovascular risk factors: a randomized controlled trial. Nutrients. 2015;7:7978–94.
Allen BG, Bhatia SK, Anderson CM, Eichenberger-Gilmore JM, Sibenaller ZA, et al. Ketogenic diets as an adjuvant cancer therapy: history and potential mechanism. Redox Biol. 2014;2:963–70.
Klement RJ, Kämmerer U. Is there a role for carbohydrate restriction in the treatment and prevention of cancer? Nutr Metab. 2011;8:75.
Mente A, Dehghan M, Rangarajan S, McQueen M, Dagenais G, et al. Association of dietary nutrients with blood lipids and blood pressure in 18 countries: a cross-sectional analysis from the PURE study. Lancet Diabetes Endocrinol. 2017;5:774–87.
Ebbeling CB, Swain JF, Feldman HA, Wong WW, Hachey DL, et al. Effects of dietary composition on energy expenditure during weight-loss maintenance. Jama. 2012;307:2627–34.
Stimson RH, Johnstone AM, Homer NZ, Wake DJ, Morton NM, et al. Dietary macronutrient content alters cortisol metabolism independently of body weight changes in obese men. J Clin Endocrinol Metab. 2007;92:4480–4.
Rankin JW, Turpyn AD. Low carbohydrate, high fat diet increases C-reactive protein during weight loss. J Am Coll Nutr. 2007;26:163–9.
Tete S, Nicoletti M, Saggini A, Maccauro G, Rosati M, et al. Nutrition and cancer prevention. Int J Immunopathol Pharmacol. 2012;25:573–81.
Trichopoulou A, Psaltopoulou T, Orfanos P, Hsieh C, Trichopoulos D. Low-carbohydrate–high-protein diet and long-term survival in a general population cohort. Eur J Clin Nutr. 2007;61:575.
Lagiou P, Sandin S, Weiderpass E, Lagiou A, Mucci L, et al. Low carbohydrate–high protein diet and mortality in a cohort of Swedish women. J Intern Med. 2007;261:366–74.
Sjögren P, Becker W, Warensjö E, Olsson E, Byberg L, et al. Mediterranean and carbohydrate-restricted diets and mortality among elderly men: a cohort study in Sweden. Am J Clin Nutr. 2010;92:967–74.
Fung TT, van Dam RM, Hankinson SE, Stampfer M, Willett WC, et al. Low-carbohydrate diets and all-cause and cause-specific mortality: two cohort studies. Ann Intern Med. 2010;153:289–98.
Fung TT, Hu FB, Hankinson SE, MD WWCH. Low-carbohydrate diets, dietary approaches to stop hypertension-style diets, and the risk of postmenopausal breast cancer. Am J Epidemiol. 2011;174:652–60.
Lv M, Zhu X, Wang H, Wang F, Guan W. Roles of caloric restriction, ketogenic diet and intermittent fasting during initiation, progression and metastasis of cancer in animal models: a systematic review and meta-analysis. PLoS One. 2014;9:e115147.
Kelemen LE, Kushi LH, Jacobs DR Jr, Cerhan JR. Associations of dietary protein with disease and mortality in a prospective study of postmenopausal women. Am J Epidemiol. 2005;161:239–49.
Barak F, Falahi E, Keshteli AH, Yazdannik A, Esmaillzadeh A. Adherence to the dietary approaches to stop hypertension (DASH) diet in relation to obesity among Iranian female nurses. Public Health Nutr. 2015;18:705–12.
Musaiger AO. Food consumption patterns in the Eastern Mediterranean Region. Manama: Arab Center for Nutrition; 2011, pp101.
Franceschi S, Favero A, Russo A, Decarli A. La vecchia C et al.:Intake of macronutrients and risk of breast cancer. Lancet. 1996;347:1351–6.
Key TJ, Balkwill A, Bradbury KE, Reeves GK, Kuan AS, Simpson RF, Green J, Beral V. Foods, macronutrients and breast cancer risk in postmenopausal women: a large UK cohort. Int J Epidemiol. 2018;48(2):489–500.
Esmaillzadeh A, Mirmiran P, Azizi F. Whole-grain consumption and the metabolic syndrome: a favorable association in Tehranian adults. Eur J Clin Nutr. 2005;59:353.
Esmaillzadeh A, Azadbakht L. Consumption of hydrogenated versus nonhydrogenated vegetable oils and risk of insulin resistance and the metabolic syndrome among Iranian adult women. Diabetes Care. 2008;31:223–6.
Azar M, Sarkisian E. Food composition table of Iran. Tehran: National Nutr Food Res Inst, Shaheed Beheshti University. 1980;65.
David H, Linda L, Pamela P. USDA National Nutrient Database for Standard Reference, Release 24. Beltsville: Nutrient Data Laboratory, USDA National Nutrient Database for Standard Reference; 2016.
Mirmiran P, Esfahani FH, Mehrabi Y, Hedayati M, Azizi F. Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. Public Health Nutr. 2010;13:654–62.
Esfahani FH, Asghari G, Mirmiran P, Azizi F. Reproducibility and relative validity of food group intake in a food frequency questionnaire developed for the Tehran lipid and glucose study. J Epidemiol. 2010;20:150–8.
Esmaillzadeh A, Mirmiran P, Azizi F. Whole-grain intake and the prevalence of hypertriglyceridemic waist phenotype in Tehranian adults. Am J Clin Nutr. 2005;81:55–63.
Armstrong T, Bull F. Development of the world health organization global physical activity questionnaire (GPAQ). J Public Health. 2006;14:66–70.
Organization WH (2012) Global physical activity questionnaire (GPAQ) analysis guide: Geneva.
Nilsson LM, Winkvist A, Johansson I, Lindahl B, Hallmans G, et al. Low-carbohydrate, high-protein diet score and risk of incident cancer; a prospective cohort study. Nutr J. 2013;12:58.
Hession M, Rolland C, Kulkarni U, Wise A Broom J:Systematic review of randomized controlled trials of low-carbohydrate vs. low-fat/low-calorie diets in the management of obesity and its comorbidities. Obes Rev 10, 36–50,2009.
Sacks FM, Katan M. Randomized clinical trials on the effects of dietary fat and carbohydrate on plasma lipoproteins and cardiovascular disease. Am J Med. 2002;113:13–24.
Nilsson LM, Winkvist A, Eliasson M, Jansson J-H, Hallmans G, et al. Low-carbohydrate, high-protein score and mortality in a northern Swedish population-based cohort. Eur J Clin Nutr. 2012;66:694.
Fine EJ, Feinman RD. Insulin, carbohydrate restriction, metabolic syndrome and cancer. Expert Rev Endocrinol Metab. 2015;10:15–24.
Oliveira CL, Mattingly S, Schirrmacher R, Sawyer MB, Fine EJ, et al. A nutritional perspective of ketogenic diet in cancer: a narrative review. J Acad Nutr Diet. 2018;118:668–88.
Farvid MS, Eliassen AH, Cho E, Chen W, Willett WC. Adolescent and early adulthood dietary carbohydrate quantity and quality in relation to breast cancer risk. Cancer Epidemiol Biomarkers Prev, cebp. 2015;1401:2014.
Lajous M, Boutron-Ruault M-C, Fabre A, Clavel-Chapelon F, Romieu I. Carbohydrate intake, glycemic index, glycemic load, and risk of postmenopausal breast cancer in a prospective study of French women. Am J Clin Nutr. 2008;87:1384–91.
Silvera SAN, Jain M, Howe GR, Miller AB, Rohan TE. Dietary carbohydrates and breast cancer risk: a prospective study of the roles of overall glycemic index and glycemic load. Int J Cancer. 2005;114:653–8.
Romieu I, Lazcano-Ponce E, Sanchez-Zamorano LM, Willett W, Hernandez-Avila M. Carbohydrates and the risk of breast cancer among Mexican women. Cancer Epidemiol Biomark Prev. 2004;13:1283–9.
Amadou A, Degoul J, Hainaut P, Chajes V, Biessy C, et al. Dietary carbohydrate, glycemic index, glycemic load, and breast cancer risk among Mexican women. Epidemiology. 2015;26:917–24.
Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41:211–8.
Kaaks R. Plasma insulin, IGF-I and breast cancer. Gynecol Obstet Fertil. 2001;29:185–91.
Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer. 2012;12:159.
Lipworth L, Adami HO, Trichopoulos D, Carlström K, Mantzoros C. Serum steroid hormone levels, sex hormone-binding globulin, and body mass index in the etiology of postmenopausal breast cancer. Epidemiology. 1996;1:96-100.
THOMAS DB, EA NOONAN. Neoplasia WCSo Contreceptives S:breast cancer and prolonged lactation. Int J Epidemiol. 1993;22:619–26.
Spindler SR, Dhahbi JM. Conserved and tissue-specific genic and physiologic responses to caloric restriction and altered IGFI signaling in mitotic and postmitotic tissues. Annu Rev Nutr. 2007;27:193–217.
Chen W, Wang S, Tian T, Bai J, Hu Z, et al. Phenotypes and genotypes of insulin-like growth factor 1, IGF-binding protein-3 and cancer risk: evidence from 96 studies. Eur J Hum Genet. 2009;17:1668.
Acknowledgments
We would like to express our special thanks to Soraiya Ebrahimpour-Koujan for her kind help in data analysis.
Availability of supporting data
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding
This study was financially supported by the Cancer Research Center of Tehran University of Medical Sciences (no. 93–03–51-27113).
Author information
Authors and Affiliations
Contributions
BS and FT participated in designing the study, analysis and drafting the initial version. FT helped in data analysis. BS implemented the comments and suggestions of the co-authors. KZ and AE contributed in the conception, design and data analysis. All authors reviewed the final version of the manuscript. KZ and AE supervised the study.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the Tehran University of Medical Sciences. Written informed consent was obtained from all subjects/patients.
Consent for publication
There is no personal information regarding any patients in our article.
Competing interests
None of the authors declared any conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Sasanfar, B., Toorang, F., Esmaillzadeh, A. et al. Adherence to the low carbohydrate diet and the risk of breast Cancer in Iran. Nutr J 18, 86 (2019). https://doi.org/10.1186/s12937-019-0511-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12937-019-0511-x