Abstract
Background
Helical TomoTherapy® is widely used for total body irradiation as a component of conditioning regimens before allogeneic bone-marrow transplantation. However, this technique limits the maximum length of a planning target volume to 135 cm. Therefore, patients taller than 135 cm require two planning computed tomography scans and treatment plans. The junctional target between these two treatment plans is thus a critical region for treatment planning and delivery. Here, we compare radiation coverage of the junctional target between helical and static approaches to treatment planning and delivery to determine which approach allows high quality irradiation planning and provides more robustness against patient movement.
Methods
We retrospectively analyzed 10 patients who underwent total body irradiation using a static four-field box planning approach and nine patients who underwent total body irradiation using a helical planning approach. All patients were taller than 135 cm. The junctional target volume was divided into 10 slices of 1 cm thickness (JT1–JT10) for analysis. Dosimetric parameters and dose-volume histograms were compared to assess the quality of coverage of the junctional target between the helical and static planning approaches.
Results
The D50 for the total junctional target was slightly higher than the prescribed dose for both helical and static approaches, with a mean of 108.12% for the helical group and 107.81% for the static group. The mean D95 was 98.44% ± 4.19% for the helical group and 96.20% ± 4.59% for the static group. The mean homogeneity index covering the entire junctional target volume was 1.20 ± 0.04 for the helical group and 1.21 ± 0.05 for the static group. The mean homogeneity index ranged from 1.08 ± 0.01 in JT1 to 1.22 ± 0.06 in JT6 for the helical group and from 1.06 ± 0.02 in JT1 to 1.19 ± 0.05 in JT6 for the static group. There were no significant differences in parameters between helical and static groups. However, the static approach provided robustness against up to 30 mm of lateral movement of the patient.
Conclusions
As long as TBI using helical TomoTherapy® is limited to a maximum length of 135 cm, the junctional target must be addressed during treatment planning. Our analysis shows that the static four-field box approach is viable and offers higher robustness against lateral movement of the patient than the helical approach.
Similar content being viewed by others
Background
Total body irradiation (TBI) is an important component of allogeneic bone-marrow transplantation (BMT) conditioning regimens and is used as a myeloablative treatment [1, 2]. Several studies show that TBI is an outcome-improving tool for many diseases, such as acute lymphoblastic leukaemia (ALL), acute myeloblastic leukaemia (AML), and natural killer cell lymphoma [3,4,5]. One method of delivering TBI before BMT is helical tomotherapy. Not only does helical tomotherapy simplify the process of TBI, it also ensures minimal variance between planned and delivered doses and provides a homogeneous dose distribution [6, 7]. Indeed, several clinical studies provide examples of helical tomotherapy use and demonstrate its feasibility in BMT regimens [8,9,10,11].
However, TBI using helical tomotherapy is limited by a maximum treatment length of 135 cm. As a result, patients taller than 135 cm require two planning computed tomography (CT) scans to fully cover the body. These planning CT scans are performed in a cranial-to-caudal direction for the upper body and caudal-to-cranial direction for the lower body, which creates an overlapping junctional volume around the upper thigh region. This overlap presents a challenge to dose planning and delivery in terms of potential over- or underdosage. Furthermore, there is a risk of dose deviation in the junction area, as the position of patients—particularly their legs—changes during the rotation between upper treatment plan delivery and lower treatment plan delivery despite patient fixation. This can result in reduced compatibility between planned and delivered doses, resulting in reduced homogeneity in the junctional area and subtherapeutic doses.
One way to plan junctional target (JT) volumes for TBI for patients taller than 135 cm is by standardized treatment planning using a helical approach, in which irradiation is delivered with gantry rotation and couch translation into the bore, while the multileaf collimator is adjusted as planned throughout treatment if needed. Indeed, previous studies utilizing a helical approach have sought to determine the CT parameters that achieve optimal dose distribution in the JT target when two overlapping scans are required [12,13,14,15,16]. However, an alternative way to plan JT volumes for patients taller than 135 cm is via standardized treatment planning using a static approach with a four-field box. In this manner, no helical movement of the gantry needed, and a fixed jaw setting of the multileaf collimator may be used. This static approach could potentially allow a substantial increase in dose homogeneity against lateral movement during treatment and rotation compared with the helical approach.
To determine whether the static treatment planning approach is feasible, meets International Commission on Radiation Units and Measurements (ICRU) guidelines [2, 17], and achieves dose homogeneity in the JT volume comparable to that of the helical treatment planning approach, we directly compared static versus helical approaches by performing retrospective dosimetric evaluation of 19 patients taller than 135 cm who underwent helical or static planning for TBI.
Methods
This analysis was conducted for routine quality assurance in line with requirements of the German radiation protection law. Therefore, ethical approval was not required.
Patients
Data from all patients taller than 135 cm at the time of TBI delivery between 2012 and 2020 who underwent planning with a direct field connection between upper and lower CT scans were analyzed. Patients who underwent planning using a dose gradient in the JT volume were excluded.
Treatment planning
In preparation for TBI, two planning CT scans were required, because the TomoTherapy® Hi-ART II is limited to a maximal couch movement of 135 cm. These CT scans were performed using a fixation mask for the head and a vacuum cushion for the body to help stabilize the patient and prevent significant alterations in the patient’s position during CT, between CT and treatment, and during treatment. The first CT scan was performed in the cranio-to-caudal direction, and the second CT scan was performed in the caudo-to-cranial direction, both with a slice thickness of 5 mm. To correctly match these two scans and assist in treatment planning, a radio-opaque marker was placed on the patient’s upper thigh. The exact position of the marker depended on the patient’s height to ensure that neither CT scan (and thus treatment plan) exceeded the maximum length of 135 cm.
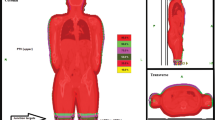
The matching of CT scans and all delineations and planning were performed using an Eclipse Treatment Planning unit (Varian Medical System, Palo Alto, CA, USA). To calculate the optimal dose for irradiating the junctional area, two treatment plans were fused and matched, with JT5 being the lowest part of the upper treatment plan and JT6 being the highest part of the lower treatment plan. The connecting area (JT4–7) was planned with 50% of the prescribed dose. Contouring of the whole body and organs at risk as well as generation of the planning target volume (PTV), sparing the lungs, were performed according to current institutional and international standards [18]. Nine patients underwent treatment planning and delivery using a helical approach (Fig. 1), and 10 patients underwent treatment planning and delivery using a static approach (Fig. 2). Both approaches used fixed jaws, a field width of 5 cm, a pitch of 0.4, and a constant feed rate fitting the pitch and prescribed dose. The modulation factors were 1.6 for the static approach and 2 for the helical approach. With the static approach, the dose was delivered from four angles, all covering the entire PTV. Treatment and planning times were equivalent between the two approaches.
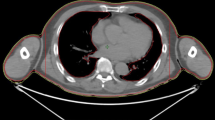
Considering the inverse square law, which states that the dose is inversely related to the square of the distance from the radiation source, lateral movement of the patient can be compensated due to an increase in field width, which can be addressed by opening five additional leaves of the multileaf collimator. As these five additionally opened leaves do not target the PTV directly, their dose is calculated using the mean opening time of the three outermost leaves that target the PTV directly and using that calculated amount as the dose for the additionally opened leaves. This is not possible when using a helical treatment plan. Because of these technical limitations when planning TBI with the TomoTherapy Hi-ART II, the static approach offers 30 mm safety before possible subtherapeutic doses, when the position of the patient changes laterally. Because a patient must be precisely positioned during irradiation to ensure an optimal treatment outcome, a simulation demonstrates the impact of lateral movement of the patients’ legs in the static approach as compared with the helical approach (Fig. 3). In this stimulation, we virtually misplaced the patient laterally at different distances and compared the resulting changes in the DVH between the helical and static approaches.
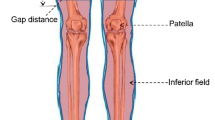
To evaluate the performance of the static approach using a four-field box method compared with the usual helical approach and in consideration of TBI guidelines, we divided the JT volume between the upper and lower CT scans into ten 1-cm-thick volumes (JT1-JT10) covering the entire PTV, spanning from 5 cm above to 5 cm below the marker on the patient’s thigh (Fig. 4). This additional contouring was performed after the completion of treatment for all patients. The dose-volume histogram and the D5, D50, D95, D98, and Dmean as well as the homogeneity index (HI) of each JT and all ten JT volumes combined (JTtotal) were calculated. The HI was calculated using the formula proposed by Kataria et al. (HI = D5/D95) [19].
Two-sided t tests were used to compare groups using SPSS v26.0 (IBM, Armonk, New York, USA).
A total of 19 patients were included; 10 patients underwent a static approach to planning and treatment, and 9 patients underwent a helical approach to planning and treatment. All patients underwent TBI in preparation for BMT. The most common disease for patients in the helical group was AML, and the most common disease for patients in the static group was ALL (Table 1). In addition, two patients in the static group were treated for diffuse large B cell lymphoma and mixed phenotype acute leukaemia, respectively. The delivered dose ranged from a single 2 Gy fraction to 2 × 2 Gy, 4 × 2 Gy, or 6 × 2 Gy, resulting in a total dose of 2, 4, 8, or 12 Gy, respectively. Most patients in the helical group received 4 Gy, and most patients in the static group received 8 Gy (Table 2).
Results
Several dosimetric parameters were evaluated to assess the quality of radiation therapy delivered using helical versus static approaches. D5 of JTtotal was calculated to assess the maximum dose absorbed by the PTV, D95 and D98 were calculated to assess the minimum dose, and D50 was calculated to assess the median dose as recommended by the ICRU [17] (Table 3).
For more in-depth evaluation, dosimetric parameters for each smaller fraction of the JTtotal were also evaluated. The standard deviation (SD) of D95 ranged from 1.45% (JT1) to 7.32% (JT6) for the helical group and from 1.86% (JT1) to 9.81% (JT5) for the static group (Table 4). The SD of D98 ranged from 3.18% (JT3) to 7.89% (JT7) for the helical group and from 2.02% (JT1) to 8.37% (JT5) for the static group (Table 5). For the helical group, the highest mean D95 and D98 were in JT9 and JT8, respectively, and the lowest mean D95 and D98 were both in JT6. For the static group, the highest mean D95 and D98 were both in JT2, and the lowest mean D95 and D98 were both in JT7.
Mean D50 ranged from 106.86% in JT1 to 111.40% in JT7 for the helical group and from 104.00% in JT10 to 111.63% in JT3 for the static group. Overall, these mean doses were slightly higher than the prescribed doses (Table 6).
The mean HI ranged from 1.08 in JT1 to 1.22 in JT6 for the helical group and from 1.06 in JT1 to 1.19 in JT6 for the static group (Table 7). The lowest and highest HI ranges were 0.04 in JT1 and 0.27 in JT4 for the helical group and 0.06 in JT1 and 0.20 in JT7 for the static group, respectively.
No significant differences in any dosimetric parameter were found between helical and static groups (Table 8).
Discussion
TBI is commonly used in allogeneic BMT conditioning regimens [20]. Patients often receive parallel chemotherapy to eradicate any malignant cells from the patient’s body, especially in the blood and haematopoietic tissue, such as bone marrow. In addition, in preparing for BMT, the patient’s own immune system is targeted to later implement the allogeneic transplant [21]. These systemic treatments result in high levels of toxicity. However, TBI is a feasible treatment option that can reduce total levels of toxicity [22]. In haematological malignancies, it is important to maximize treatment success while minimizing side effects. Thus, in this study, we aimed to maximize dose homogeneity by optimizing JT treatment and creating a robust plan for patients taller than 135 cm, who require two treatment plans when using the TomoTherapy® Hi-ART II.
Regarding D50 values, we observed slightly higher treatment doses than prescribed doses for both the helical and static groups. Although this may be acceptable, because there are no organs at risk in the junctional area that might experience more toxicity from an increased radiation dose [23], this discrepancy is still important and should be addressed during treatment planning.
Dosimetric parameters for JTtotal showed only a narrow margin between median and mean values, indicating the existence of few outliers and a symmetric distribution suggestive of good dose distribution. Upon further analysis of 1-cm-wide volumes, the highest SD in D95 and D98 were observed in JT5, JT6, and JT7. These junctional volumes represent the last region of the upper treatment plan (J5) and the first two regions of the lower treatment plan (JT6 and JT7). Although overall coverage was acceptable, even in these specific junctional volumes, additional research may be needed to achieve ideal dosimetric parameters for this 3-cm region when planning TBI.
On the other hand, the mean value of D95 for JT1–JT10 ranged from 96.89% to 103.22% for helical group and 95.10–106.80% for the static group, while the mean value of D98 was only slightly lower, ranging from 92.56% to 100.89% for the helical group and 90.50% and 104.80% for the static group. Thus, the minimum dose did not fall rapidly between the dose delivered to 95% of the PTV and the dose delivered to 98% of the PTV, suggesting good overall dose coverage and dose-volume histograms for both helical and static groups.
The HI is commonly used for quality assessment in TBI [17, 19], with the Radiation Therapy Oncology Group (RTOG) recommending a maximum HI < 2 [17]. The 19 patients included in this study received treatment with a mean HI for the JTtotal of 1.20 in the helical group and 1.21 in the static group. Considering individual HI values for JT1–JT10, mean HI ranged from 1.08 to 1.22 in the helical group and 1.06 to 1.19 in the static group. These values are well under the maximum HI recommend by the RTOG. Even the maximum HI, found in JT6–7 with values of 1.35 in the helical group and 1.32 in the static group were still well below the limit of a minor violation, defined as a value between 2 and 2.5. Therefore, both helical and static approaches to delivering treatment showed acceptable homogeneity. The conformity index was not calculated, because the PTV was equal to the targeted volume [24].
Furthermore, statistical analysis showed no significant differences between helical and static groups considering any dosimetric parameter, indicating comparable performance between both treatment planning and delivery approaches.
Importantly, however, using a four-field box to irradiate the JT volume in the static approach provides 30 mm additional safety in case the patient changes their position. Technical limitations of the TomoTherapy® Hi-ART II do not allow the same possibility when using a helical approach. Therefore, considering the otherwise equivalent performance between static and helical approaches, using a four-field box for patients taller than 135 cm undergoing TBI may be considered and preferably chosen over the helical approach. Previous studies show that a virtual bolus can be used to reduce HT setup error when using a helical treatment regimen for the legs and JT [24, 25]. However, use of a virtual bolus can also lead to underdosage, especially in the smaller parts of the legs, as well as overdosage. In this regard, the inverse square law gives the static approach an advantage, as it prevents underdosage and overdosage as long as the setup error does not exceed the additional safety given by opening five additional leaves [26].
Conclusions
We compared the performance of helical versus static approaches to planning and delivering radiation to the JT volume in patients taller than 135 cm, who required two planning CT scans before undergoing TBI in allogeneic BMT conditioning regimens. The static approach utilized a four-field box to deliver the prescribed dose. Based on our institution’s experience, both helical and static treatment plans meet the ICRU and RTOG guidelines, and the static planning method is not inferior to the commonly used helical planning method. Furthermore, simulations and the physical law suggest that additional safety can be provided when using the static approach to treatment planning, which avoids the challenges of using a virtual bolus, making the four-field box a feasible method for TBI planning and delivery.
Availability of data and materials
All data relevant to this publication have been included into the manuscript’s body.
Abbreviations
- ALL:
-
Acute lymphoblastic leukaemia
- AML:
-
Acute myeloblastic leukaemia
- BMT:
-
Bone-marrow transplantation
- CT:
-
Computed tomography
- HI:
-
Homogeneity index
- ICRU:
-
International Commission on Radiation Units and Measurements
- JT:
-
Junctional target
- PTV:
-
Planning target volume
- RTOG:
-
Radiation Therapy Oncology Group
- TBI:
-
Total body irradiation
References
Sabloff M, Tisseverasinghe S, Babadagli ME, Samant R. Total body irradiation for hematopoietic stem cell transplantation: what can we agree on? Curr Oncol. 2021;28(1):903–17. https://doi.org/10.3390/curroncol28010089.
Wong JYC, Filippi AR, Dabaja BS, Yahalom J, Specht L. Total body irradiation: guidelines from the international lymphoma radiation oncology group (ILROG). Int J Radiat Oncol Biol Phys. 2018;101(3):521–9. https://doi.org/10.1016/j.ijrobp.2018.04.071.
Marnitz S, Zich A, Martus P, et al. Long-term results of total body irradiation in adults with acute lymphoblastic leukemia. Strahlenther Onkol. 2014;190(5):453–8. https://doi.org/10.1007/s00066-014-0607-3.
Ringdén O, Labopin M, Ehninger G, et al. Reduced intensity conditioning compared with myeloablative conditioning using unrelated donor transplants in patients with acute myeloid leukemia. J Clin Oncol. 2009;27(27):4570–7. https://doi.org/10.1200/JCO.2008.20.9692.
Maeng CH, Ko YH, Lim DH, et al. Comparison of Total Body Irradiation (TBI) conditioning with non-TBI for autologous stem cell transplantation in newly diagnosed or relapsed mature T- and NK-cell non-hodgkin lymphoma. Cancer Res Treat. 2017;49(1):92–103. https://doi.org/10.4143/crt.2015.476.
Peñagarícano JA, Chao M, Van Rhee F, Moros EG, Corry PM, Ratanatharathorn V. Clinical feasibility of TBI with helical tomotherapy. Bone Marrow Transplant. 2011;46(7):929–35. https://doi.org/10.1038/bmt.2010.237.
Hui SK, Kapatoes J, Fowler J, et al. Feasibility study of helical tomotherapy for total body or total marrow irradiation. Med Phys. 2005;32(10):3214–24. https://doi.org/10.1118/1.2044428.
Schultheiss TE, Wong J, Liu A, Olivera G, Somlo G. Image-guided total marrow and total lymphatic irradiation using helical tomotherapy. Int J Radiat Oncol Biol Phys. 2007;67(4):1259–67. https://doi.org/10.1016/j.ijrobp.2006.10.047.
Shueng PW, Lin SC, Chong NS, et al. Total marrow irradiation with helical tomotherapy for bone marrow transplantation of multiple myeloma: first experience in Asia. Technol Cancer Res Treat. 2009;8(1):29–38. https://doi.org/10.1177/153303460900800105.
Wong JY, Rosenthal J, Liu A, Schultheiss T, Forman S, Somlo G. Image-guided total-marrow irradiation using helical tomotherapy in patients with multiple myeloma and acute leukemia undergoing hematopoietic cell transplantation. Int J Radiat Oncol Biol Phys. 2009;73(1):273–9. https://doi.org/10.1016/j.ijrobp.2008.04.071.
Wilhelm-Buchstab T, Leitzen C, Schmeel LC, et al. Total body irradiation: significant dose sparing of lung tissue achievable by helical tomotherapy. Z Med Phys. 2020;30(1):17–23. https://doi.org/10.1016/j.zemedi.2019.05.002.
Wang H, Liu J, Pi Y, et al. Technical note: factors affecting dose distribution in the overlap region of two-segment total body irradiation by helical tomotherapy. Radiat Oncol. 2020;15(1):257. https://doi.org/10.1186/s13014-020-01698-x.
Zeverino M, Agostinelli S, Taccini G, et al. Advances in the implementation of helical tomotherapy-based total marrow irradiation with a novel field junction technique. Med Dosim. 2012;37(3):314–20. https://doi.org/10.1016/j.meddos.2011.12.001.
Haraldsson A, Engellau J, Lenhoff S, Engelholm S, Bäck S, Engström PE. Implementing safe and robust total marrow irradiation using helical tomotherapy—a practical guide. Phys Med. 2019;60:162–7. https://doi.org/10.1016/j.ejmp.2019.03.032.
Sresty NVNM, Gudipudi D, Krishnam Raju A, et al. Total body irradiation of bone marrow transplant using helical tomotherapy with a focus on the quality of dose contribution at junction target volumes. Strahlenther Onkol. 2021;197(8):722–9. https://doi.org/10.1007/s00066-021-01769-2.
Bao Z, Zhao H, Wang D, et al. Feasibility of a novel dose fractionation strategy in TMI/TMLI. Radiat Oncol. 2018;13(1):248. https://doi.org/10.1186/s13014-018-1201-0.
Hodapp N. The ICRU report 83: prescribing, recording and reporting photon-beam intensity-modulated radiation therapy (IMRT). Strahlenther Onkol. 2012;188(1):97–9. https://doi.org/10.1007/s00066-011-0015-x.
Quast U. Whole body radiotherapy: a TBI-guideline. J Med Phys. 2006;31(1):5–12. https://doi.org/10.4103/0971-6203.25664.
Kataria T, Sharma K, Subramani V, Karrthick KP, Bisht SS. Homogeneity index: an objective tool for assessment of conformal radiation treatments. J Med Phys. 2012;37(4):207–13. https://doi.org/10.4103/0971-6203.103606.
Cahu X, Labopin M, Giebel S, et al. Impact of conditioning with TBI in adult patients with T-cell all who receive a myeloablative allogeneic stem cell transplantation: a report from the acute leukemia working party of EBMT. Bone Marrow Transplant. 2016;51(3):351–7. https://doi.org/10.1038/bmt.2015.278.
Stein A, Forman SJ. Allogeneic transplantation for all in adults. Bone Marrow Transplant. 2008;41(5):439–46. https://doi.org/10.1038/bmt.2008.1.
DE Felice F, Grapulin L, Musio D, et al. Treatment complications and long-term outcomes of total body irradiation in patients with acute lymphoblastic leukemia: a single institute experience. Anticancer Res. 2016;36(9):4859–64. https://doi.org/10.21873/anticanres.11049.
Marks LB, Yorke ED, Jackson A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S10–9. https://doi.org/10.1016/j.ijrobp.2009.07.1754.
Feuvret L, Noël G, Mazeron JJ, Bey P. Conformity index: a review. Int J Radiat Oncol Biol Phys. 2006;64(2):333–42. https://doi.org/10.1016/j.ijrobp.2005.09.028.
Moliner G, Izar F, Ferrand R, Bardies M, Ken S, Simon L. Virtual bolus for total body irradiation treated with helical tomotherapy. J Appl Clin Med Phys. 2015;16(6):164–76.
Takenaka R, Haga A, Nawa K, et al. Improvement of the robustness to set up error by a virtual bolus in total scalp irradiation with Helical TomoTherapy. Radiol Phys Technol. 2019. https://doi.org/10.1007/s12194-019-00539-1.
Acknowledgements
Not applicable.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
MK formulated the research goals and aims, designed the study, and was responsible for research planning and execution, including providing mentorship external to the core team. JB performed the data collection. JB and FS performed the statistical analyses. MK, JB and FS analysed the data. MK and JB drafted the initial manuscript. All authors reviewed the drafted manuscript for critical content. All authors approved the final version of the manuscript and attest to the validity and legitimacy of the data as well as its interpretation.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
As this analysis was conducted for routine quality assurance in line with requirements of the German radiation protection law, ethical approval was not required.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Köksal, M., Baumert, J., Schoroth, F. et al. Helical versus static approaches to delivering tomotherapy to the junctional target for patients taller than 135 cm undergoing total body irradiation. Eur J Med Res 27, 265 (2022). https://doi.org/10.1186/s40001-022-00886-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-022-00886-7