Abstract
Background
The use of total body irradiation (TBI) with linac-based volumetric modulated arc therapy (VMAT) has been steadily increasing. Helical tomotherapy has been applied in TBI and total marrow irradiation to reduce the dose to critical organs, especially the lungs. However, the methodology of TBI with Halcyon™ linac remains unclear. This study aimed to evaluate whether VMAT with Halcyon™ linac can be clinically used for TBI.
Methods
VMAT planning with Halcyon™ linac was conducted using a whole-body computed tomography data set. The planning target volume (PTV) included the body cropped 3 mm from the source. A dose of 12 Gy in six fractions was prescribed for 50% of the PTV. The organs at risk (OARs) included the lens, lungs, kidneys, and testes.
Results
The PTV D98%, D95%, D50%, and D2% were 8.9 (74.2%), 10.1 (84.2%), 12.6 (105%), and 14.2 Gy (118%), respectively. The homogeneity index was 0.42. For OARs, the Dmean of the lungs, kidneys, lens, and testes were 9.6, 8.5, 8.9, and 4.4 Gy, respectively. The V12Gy of the lungs and kidneys were 4.5% and 0%, respectively. The Dmax of the testes was 5.8 Gy. Contouring took 1–2 h. Dose calculation and optimization was performed for 3–4 h. Quality assurance (QA) took 2–3 h. The treatment duration was 23 min.
Conclusions
A planning study of TBI with Halcyon™ to set up VMAT-TBI, dosimetric evaluation, and pretreatment QA, was established.
Similar content being viewed by others
Background
Total body irradiation (TBI) is an important conditioning regimen for allogeneic hematopoietic stem cell transplantation. TBI with mega-voltage photon beams is an important treatment modality and is employed for treating several malignant diseases, including multiple myeloma, leukemia, lymphoma, and some solid tumors, and benign diseases, including severe aplastic and Fanconi anemia [1,2,3,4].
Most TBI procedures are based on techniques established on linear accelerators used for conventional radiation therapy. Large photon fields are generally achieved by treating a patient at an extended skin-surface distance (SSD) using standard linear accelerators. Equipment guidelines recommend the use of parallel-opposed pairs of high-energy photon beams that range from 4 to 18 MV for TBI [1]. However, conventional TBI has several limitations. Miral et al. reported that patients were required to stand for prolonged periods of time, often more than 40 min [5]. Additionally, conventional TBI has non-conformality of beam application with the inability to individually spare organs at risk (OARs), which results in acute and late toxicity such as pneumonitis or renal dysfunction [6, 7]. Moreover, irradiation of the gonads particularly increased the risk of reduced fertility after the successful completion of cancer treatment in adolescents and young adults (AYAs) [8]. Recovery of gonadal function occurred in only 10%–14% of women and in less than 20% of men [9, 10]. Therefore, fertility preservation in AYA patients treated with TBI should be considered.
The inverse optimization algorithm improved dose homogeneity as compared with conventional forward-planned techniques [11]. A previous study described the dose inhomogeneity of conventional techniques such as extended SSD [12]. In recent studies, helical tomotherapy was applied in TBI and total marrow irradiation (TMI) as an approach to reduce the dose delivered to critical organs, especially the lungs [13, 14]. Furthermore, several studies have reported the feasibility of TBI with linac-based volumetric modulated arc therapy (VMAT) [11, 15,16,17,18,19,20,21]. However, the full methodology of TBI with Halcyon™ linac is not well-established.
The present planning study aimed to evaluate whether VMAT with Halcyon™ linac can be clinically used for TBI.
Methods
Data sets and contouring
The pre-existing computed tomography (CT) dataset of an adult male patient with a malignant melanoma was used for planning purposes. He was imaged from the vertex of the skull up to the toe, with a total body length of 162 cm.
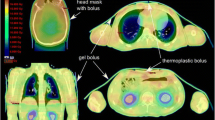
The planning target volume (PTV) included the entire body trimmed to 3 mm below the body. Furthermore, the PTV was divided into two structures, which are the PTV-BODY and the PTV-ARM (Fig. 1). These structures were 14 cm from the center in the left–right direction. The OARs included the lens, lungs, kidneys, and testes, which were excluded from the PTV.
Dose prescription and treatment planning
The prescribed dose was 12 Gy in six fractions, which was normalized as 12 Gy to 50% of the PTV-BODY. Our goals for dose-volume histograms (DVHs) are presented in Table 1. These goals were based on previous studies [11, 14, 18, 22].
VMAT plans were created using the Eclipse™ treatment planning system (TPS) ver. 15.6 (Varian Medical Systems Inc., Palo Alto, CA). We employed 6 MV flattening filter-free (FFF) photon beams. The VMAT plans were calculated using the Acuros® XB (AXB). We utilized the “base dose plan” function incorporated in the Eclipse™. An O-ring gantry linac with a single 6 MV FFF beam called Halcyon™ (Varian Medical Systems Inc., Palo Alto, CA) was recently introduced. The Halcyon™ linac has unique stacked and staggered dual-layer proximal and distal multileaf collimators (MLC) composed of 10.0-mm-wide leaves that produce an effective 5.0-mm resolution MLC. The maximum field size (28 × 28 cm2) at the isocenter is defined by a dual-layer MLC system without physical jaws or light field.
Splitting of the CT images into the cranial and caudal parts required a dosimetric alignment of these two body parts. The cranial part was created using the head-first position from the vertex of the skull to the upper thigh, while the caudal part was created using the feet-first position from the toes to the lower thigh because the PTV length exceeded the couch travel capability of the Halcyon™ linac. The overall PTV was split into seven segments with subsequent multi-isocentric planning. Each segment was divided at 13 cm from the center in the cephalad direction because of the capacity of the collimators. There were thirteen isocenters. Figure 2 shows the seven segments of the PTV with thirteen isocenters. Two to four full arcs of VMAT were applied (gantry angle, 181–179° clockwise and 179–181° counterclockwise; collimator angles, 270°, 280°, and 359°) for each isocenter (see Additional file 1: Table S1 for beam arrangement of VMAT-TBI with Halcyon™). The control point spacing was 2° from the angular separation. The beam arrangement was set to meet our goal for PTV coverage of D95% > 80% [23]. The collimator angle was fixed to maximize the field size in each segment. The maximum dose rate was 600 monitor units (MU) per minute. The dose rate was established using the MU calibration geometry setting (isocentric, 90 cm SSD) of the linac and could not be changed by the user.
Splitting the planning CT images into a cranial and a caudal part necessitates a dosimetric alignment of these two body parts. The cranial part is created using the head-first position from the vertex to upper thigh, and the caudal part is created using the feet-first position from the toes to lower thigh because the PTV length exceeds the couch travel capability of the Halcyon™ linac. The overall PTV is split into seven segments with a subsequent multi-isocentric planning. There are thirteen isocenters. Each segment is divided at 13 cm from the center in the caudal-cranial direction because of capacity of collimators
DVH analysis and patient-specific quality assurance (QA)
DVH parameters were evaluated in terms of the D98%, D95%, D50%, and D2% of the PTV, where D98%, D95%, D50%, and D2% were the doses received by 98%, 95%, 50%, and 2% of the PTV, respectively. The PTV indicated the overall target volume, including the PTV-BODY and PTV-ARM. In this study, the DVH parameters of the lens, testes, lungs, and kidneys were evaluated, wherein the Dmax was the maximum dose received by the testes; the Dmean was the mean dose received by the lungs, kidneys, lens, and testes; the V12Gy was the total volume of the lungs and kidneys that received 12 Gy; and the V5Gy was the total volume of the lungs that received 5 Gy. Moreover, we evaluated the homogeneity index (HI; defined as [D2% − D98%]/D50%).
Patient-specific QA was performed to ensure safe delivery of TBI. The difference between the calculated and measured dose distributions was evaluated using the γ pass rate. The ArcCHECK (SunNuclear, Melbourne, FL) and the electric portal imaging device (EPID) of the Halcyon™ linac were employed to evaluate the γ pass rate in each segment. The γ pass rate at each junction between the upper and lower segments, except for the junction between the segments of the head-first and foot-first positions, was evaluated using the ArcCHECK [24]. Furthermore, the absorbed doses at the low-dose gradient point in ArcCHECK in each segment and junction region were measured with a pinpoint ionization chamber (CC01, IBA, Schwarzenbruck, Germany) [25]. The tolerances in terms of dose difference and distance to agreement were 1%/1 mm and 2%/2 mm with a threshold of 10% for the portal dosimetry using the EPID, while they were 2%/2 mm, 3%/2 mm, and 3%/3 mm with a threshold of 10% for the ArcCHECK.
Results
The DVH parameters are summarized in Table 2 and Fig. 3. Figure 4 shows the dose distributions. The Dmax of the whole body was 15.7 Gy (130%). The PTV D98%, D95%, D50%, and D2% were 8.9 Gy (74.2%), 10.1 Gy (84.2%), 12.6 Gy (105%), and 14.2 Gy (118%), respectively. The HI was 0.42. In terms of OARs, the Dmean of the lungs, kidneys, lens, and testes were 9.6 Gy, 8.5 Gy, 8.9 Gy, and 4.4 Gy, respectively. The Dmax of the testes was 5.8 Gy. The V12Gy of the lungs and kidneys were 4.5% and 0%, respectively. The V5Gy of the lungs was 100%. The DVH parameters met our goal for the target and almost all OARs, except for the V12Gy of the lungs (Table 2). Additionally, the total MU was 8996.
Table 3 shows the γ pass rates and point dose differences of each segment. The γ pass rates were > 96%, with a criterion of 1%/1 mm using the portal dosimetry with EPID. The γ pass rates were > 90%, with a criterion of 3%/2 mm using the ArcCHECK. All discrepancies in the point doses between the planned and measured doses at each segment were within 3%. Table 4 lists the γ pass rates at each junction. The γ pass rates were also > 90%, with a criterion of 3%/2 mm. In junction 4, which was between the head-first and feet-first positions, the discrepancy in the point dose was 1.91%. All discrepancies in the point doses at each junction were within 5%.
Treatment planning and QA measurements took longer than typical workflows. Contouring took 1–2 h, dose calculation and optimization took 3–4 h, and QA took 2–3 h. The beam-on time lasted 23 min.
Discussion
In the present study, a VMAT plan with Halcyon™ linac for TBI was evaluated. This study revealed that this method can be clinically used for TBI. Van et al. and Yao et al. reported that conventional TBI techniques required large treatment fields with lung blocks to irradiate the patient’s entire body, while the patient is in a standing or lying-on-the-side position at an extended SSD (for instance, 5 m), which required costly, large, linear accelerator vaults [26, 27]. In contrast to conventional TBI, the VMAT method with Halcyon™ linac for TBI can be applied in any treatment room that is large enough to fit the Halcyon™ linac. In addition, Gruen et al. described that in helical tomotherapy, the average beam-on time was 34.2 min, with a range of 28.7–39.6 min, for split plans for patients with body lengths over 145 cm [14]. Our beam-on time was 23 min. Thus, the in-room time could be shorter for patients with a body length of 162 cm, even if kilovoltage cone beam CT image guidance was performed for each segment. The standing or lying-on-the-side position at an extended SSD was exhausting for immunocompromised patients undergoing chemotherapy [28]. A shortened period of treatment contributes to patient compliance.
Springer et al. demonstrated that contouring, dose calculation, optimization, and QA took 5–6, 25–30, and 6–8 h, respectively [16]. These parameters were calculated using the RapidArc™ software of the Eclipse™ TPS, version 10.0 (Varian Medical Systems, Palo Alto, CA), in which the body lengths of several patients were over 160 cm [16]. These were much longer than those observed in our study. However, the dose calculation and optimization times in our study were still long because of repeated re-optimization.
In terms of PTV coverage, the D50% and Dmean were both 12.6 Gy (105%) in this study. The result was comparable with that of TBI with helical tomotherapy [14] and linac based VMAT [11], respectively. The D95% of the PTV in a previous study [14] was higher (11.7 Gy [97.5%]) than that in this study (10.1 Gy [84.2%]). However, Hirata et al. mentioned that helical tomotherapy increased the absorption dose at the exterior of the irradiation field [29]. Similarly, Mutic et al. demonstrated that the whole-body dose was greater with tomotherapy treatment modalities than with conventional treatments owing to scattered and leakage doses [30]. However, Tamura et al. described that the dose delivery of the Halcyon™ linac was accurate because of the minimal leakage dose and penumbra size of the dual-layer MLC [31].
A further advantage of VMAT with Halcyon™ linac for TBI was the dose reduction in the lens, testes, lungs, and kidneys, as observed in this study. Most centers use lung shielding to maintain the mean lung dose (MLD) of 8–10 Gy, which led to a reduction in the incidence of pneumonitis [32]. The MLD was reduced to 9.6 Gy in this study. The results were comparable to those of other studies for TBI with helical tomotherapy and linac based VMAT [11, 14, 16]. However, in this study, only the V12Gy of the lungs did not meet our goal. Fog et al. reported that their TBI method of step and shoot intensity-modulated radiation therapy with delivering extended SSD achieved a V12Gy of the lungs that was under 2.0% [18]. In our study, the ribs were regarded as the target, such as TMI [33]. The VMAT method ensured full dose coverage of the ribs. Therefore, our results in 4.5% V12Gy of the lungs were considered acceptable. Moreover, the Dmean of the kidneys and lens were reduced in this method, and the results were comparable to those of previous studies for TBI with helical tomotherapy and linac based VMAT [11, 14]. These results contributed to reducing the risk of renal dysfunction [34] and cataracts [35]. The most valuable advantage of this method was the reduced dose delivered to the testes. De Felice et al. reported that the threshold dose for permanent sterility in adults was 3–6 Gy [22]. In this study, the Dmax and Dmean of the testes were 5.8 and 4.4 Gy, respectively; therefore, the risk of permanent infertility could be reduced. Hence, the demand for fertility preservation in patients undergoing TBI has increased. However, the frequency of testicular relapse in acute lymphocytic leukemia was very high [36]. Therefore, the decision regarding the VMAT method with testes sparing should be carefully made. The TBI method used in the present study was also used for benign diseases, such as severe aplastic anemia or Fanconi anemia. In contrast, our method of VMAT with Halcyon™ linac could spare any OAR, including the testes.
In the patient-specific QA, the dosimetric accuracy in each segment was within the tolerance limit recommended by AAPM TG218 (γ pass rate of > 90% with a criterion of 3%/2 mm and point dose discrepancy between the planned and measured doses of < 3%) [37, 38]. The γ pass rates in each junction were also > 90% with a criterion of 3%/2 mm using the ArcCHECK. The point dose discrepancies were within 5%, which was within the action limit recommended by AAPM TG218. The reduced accuracy in each junction likely resulted from the detector shift and sagging variation between the upper and lower segments and using the doses in steep gradient areas of the FFF beam for each segment. Thus, this plan was considered acceptable for clinical use.
The present study had several limitations, including small sample size and only one case planning study. However, Chakraborty S et al. described that TBI with VMAT was feasible in one case planning study, the same as in our study [17]. Furthermore, the auto feathering algorithm was not applied for junctions because of the extension of the optimization time (over five hours for each segment). Maddalo et al. described the auto feathering algorithm for the cranio-spinal radiation treatment with the VMAT technique [39]. Therefore, the use of the auto feathering algorithm should be considered for TBI with VMAT. Alternatively, we utilized the “base dose plan” function, which could be achieving optimal plan sum by making up for inadequacies (hot and cold spots) [40].
Conclusions
A planning study of TBI with Halcyon™ to set up VMAT-TBI, dosimetric evaluation, and pretreatment QA, was established. This method is technically and clinically feasible.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- TBI:
-
Total body irradiation
- RT:
-
Radiation therapy
- SSD:
-
Skin-surface distance
- OARs:
-
Organs at risk
- AYA:
-
Adolescents and young adults
- TMI:
-
Total marrow irradiation
- VMAT:
-
Volumetric modulated arc therapy
- CT:
-
Computed tomography
- PTV:
-
Planning target volume
- DVH:
-
Dose-volume histogram
- TPS:
-
Treatment planning system
- FFF:
-
Flattening filter-free
- AXB:
-
Acuros® XB
- MU:
-
Monitor units
- QA:
-
Quality assurance
- D98%:
-
Doses received by 98% of the PTV
- D95%:
-
Doses received by 95% of the PTV
- D50%:
-
Doses received by 50% of the PTV
- D2%:
-
Doses received by 2% of the PTV
- Dmax:
-
Maximum dose
- Dmean:
-
Mean dose
- V12Gy:
-
Total volume receiving 12 Gy
- V5Gy:
-
Total volume receiving 5 Gy
- HI:
-
Homogeneity index
- EPID:
-
Electric portal imaging device
- DD:
-
Dose difference
- DTA:
-
Distance to agreement
- kV:
-
Kilo-voltage
- CBCT:
-
Cone beam CT
- MLD:
-
Mean lung doses
References
Levitt SH, Purdy JA, Perez CA, Poortmans P. Technical basis of radiation therapy: practical clinical applications. Berlin: Springer; 2012.
Ishibashi N, Soejima T, Kawaguchi H, Akiba T, Hasegawa M, Isobe K, et al; Japanese Radiation Oncology Study Group. National survey of myeloablative total body irradiation prior to hematopoietic stem cell transplantation in Japan: survey of the Japanese Radiation Oncology Study Group (JROSG). J Radiat Res 2018;59:477–83. https://doi.org/10.1093/jrr/rry017.
Deeg HJ, Amylon ID, Harris RE, Collins R, Beatty PG, Feig S, et al. Marrow transplants from unrelated donors for patients with aplastic anemia: minimum effective dose of total body irradiation. Biol Blood Marrow Transplant. 2001;7:208–15. https://doi.org/10.1053/bbmt.2001.v7.pm11349807.
MacMillan ML, DeFor TE, Young JA, Dusenbery KE, Blazar BR, Slungaard A, et al. Alternative donor hematopoietic cell transplantation for Fanconi anemia. Blood. 2015;125:3798–804. https://doi.org/10.1182/blood-2015-02-626002.
Miralbell R, Rouzaud M, Grob E, Nouet P, Bieri S, Majno SB, et al. Can a total body irradiation technique be fast and reproducible? Int J Radiat Oncol Biol Phys. 1994;29:1167–73. https://doi.org/10.1016/0360-3016(94)90414-6.
Linsenmeier C, Thoennessen D, Negretti L, Bourquin JP, Streller T, Lütolf UM, et al. Total body irradiation (TBI) in pediatric patients: a single center experience after 30 years of low-dose rate irradiation. Strahlenther Onkol. 2010;186:614–20. https://doi.org/10.1007/s00066-010-2089-2.
Gerstein J, Meyer A, Sykora KW, Frühauf J, Karstens JH, Bremer M. Long-term renal toxicity in children following fractionated total body irradiation (TBI) before allogenic stem cell transplantation (SCT). Strahlenther Onkol. 2009;185:751–5. https://doi.org/10.1007/s00066-009-2022-8.
Furui T, Takai Y, Kimura F, Kitajima M, Nakatsuka M, Morishige KI, et al. Fertility preservation in adolescent and young adult cancer patients: from a part of a national survey on oncofertility in Japan. Reprod Med Biol. 2019;18:97–104. https://doi.org/10.1002/rmb2.12256.
Spinelli S, Chiodi S, Bacigalupo A, Brasca A, Menada MV, Petti AR, et al. Ovarian recovery after total body irradiation and allogeneic bone marrow transplantation: long-term follow up of 79 females. Bone Marrow Transplant. 1994;14:373–80.
Salooja N, Szydlo RM, Socie G, Rio B, Chatterjee R, Ljungman P, et al. Pregnancy outcomes after peripheral blood or bone marrow transplantation: a retrospective survey. Lancet. 2001;358:271–6. https://doi.org/10.1016/S0140-6736(01)05482-4.
Tas B, Durmus IF, Okumus A, Uzel OE, Gokce M, Goksoy HS, et al. Total-body irradiation using linac-based volumetric modulated arc therapy: its clinical accuracy, feasibility and reliability. Radiother Oncol. 2018;129:527–33. https://doi.org/10.1016/j.radonc.2018.08.005.
Hui SK, Das RK, Thomadsen B, Henderson D. CT-based analysis of dose homogeneity in total body irradiation using lateral beam. J Appl Clin Med Phys. 2004;5:71–9. https://doi.org/10.1120/jacmp.v5i4.1980.
Hui SK, Kapatoes J, Fowler J, Henderson D, Olivera G, Manon RR, et al. Feasibility study of helical tomotherapy for total body or total marrow irradiation. Med Phys. 2005;32:3214–24. https://doi.org/10.1118/1.2044428.
Gruen A, Ebell W, Wlodarczyk W, Neumann O, Kuehl JS, Stromberger C, et al. Total body irradiation (TBI) using helical tomotherapy in children and young adults undergoing stem cell transplantation. Radiat Oncol. 2013;8:92. https://doi.org/10.1186/1748-717X-8-92.
Tas B, Durmus IF, Okumus A, Uzel OE. Dosimetric evaluation of total body irradiation (TBI) treatment by volumetric modulated arc therapy (VMAT) on the coach. J Biochem Biophys. 2017;1:103. https://doi.org/10.15744/2576-7623.1.103.
Springer A, Hammer J, Winkler E, Track C, Huppert R, Böhm A, et al. Total body irradiation with volumaetric modulated arc therapy: dosimetric data and first clinical experience. Radiat Oncol. 2016;11:46. https://doi.org/10.1186/s13014-016-0625-7.
Chakraborty S, Cheruliyil S, Bharthan R, Muttath G. Total body irradiation using VMAT (Rapidarc): a planning study of a novel treatment delivery method. Int J Cancer Ther Oncol. 2015;3:03028. https://doi.org/10.14319/ijcto.0302.8.
Fog LS, Hansen VN, Kjær-Kristoffersen F, Berlon TE, Petersen PM, Mandeville H, et al. A step and shoot intensity modulated technique for total body irradiation. Tech Innov Patient Support Radiat Oncol. 2019;10:1–7. https://doi.org/10.1016/j.tipsro.2019.05.002.
Teruel JR, Taneja S, Galavis PE, Osterman KS, McCarthy A, Malin M, et al. Automatic treatment planning for VMAT-based total body irradiation using Eclipse scripting. J Appl Clin Med Phys. 2021;22(3):119–30. https://doi.org/10.1002/acm2.13189.
Blomain ES, Kovalchuk N, Neilsen BK, Skinner L, Hoppe RT, Hiniker SM. A preliminary report of gonadal-sparing TBI using a VMAT technique. Pract Radiat Oncol. 2021;11(2):e134–8. https://doi.org/10.1016/j.prro.2020.07.006.
Symons K, Morrison C, Parry J, Woodings S, Zissiadis Y. Volumetric modulated arc therapy for total body irradiation: a feasibility study using Pinnacle3 treatment planning system and Elekta Agility™ linac. J Appl Clin Med Phys. 2018;19(2):103–10. https://doi.org/10.1002/acm2.12257.
De Felice F, Marchetti C, Marampon F, Cascialli G, Muzii L, Tombolini V. Radiation effects on male fertility. Andrology. 2019;7:2–7. https://doi.org/10.1186/s12958-018-0431-1.
Nalichowski A, Eagle DG, Burmeister J. Dosimetric evaluation of total marrow irradiation using 2 different planning systems. Med Dosim. 2016;41(3):230–5. https://doi.org/10.1016/j.meddos.2016.06.001.
Morrison CT, Symons KL, Woodings SJ, House MJ. Verification of junction dose between VMAT arcs of total body irradiation using a Sun Nuclear ArcCHECK phantom. J Appl Clin Med Phys. 2017;18:177–82. https://doi.org/10.1002/acm2.12208.
Aydogan B, Yeginer M, Kavak GO, Fan J, Radosevich JA, Gwe-Ya K. Total marrow irradiation with RapidArc volumetric arc therapy. Int J Radiat Oncol Biol Phys. 2011;81:592–9. https://doi.org/10.1016/j.ijrobp.2010.11.035.
Van Dyke J, Galvin JM, Glasgow GP, Podgorsak EB. The physical aspect of total and half body photon irradiation. AAPM Report No. 17. 1986. https://doi.org/10.37206/16.
Yao R, Bernard D, Turian J, Abrams RA, Sensakovic W, Fung HC, et al. A simplified technique for delivering total body irradiation (TBI) with improver dose homogeneity. Med Phys. 2012;39:2239–48. https://doi.org/10.1118/1.3697526.
Ouyang L, Folkerts M, Zhang Y, Hrycushko B, Lamphier R, Lee P, et al. Volumetric modulated arc therapy based total body irradiation: work flow and clinical experience with an indexed rotational immobilization system. phiRO. 2017;4:22–5. https://doi.org/10.1016/j.phro.2017.11.002.
Hirata M, Monzen H, Hanaoka K, Nishimura Y. Measurement of absorption dose outside irradiation field in IMRT. Radiat Prot Dosimetry. 2017;176:425–33. https://doi.org/10.1093/rpd/ncx027.
Mutic S, Low DA. Whole-body dose from tomotherapy delivery. Int J Radiat Oncol Biol Phys. 1998;42:229–32. https://doi.org/10.1016/s0360-3016(98)00199-0.
Tamura M, Matsumoto K, Otsuka M, Monzen H. Plan complexity quantification of dual-layer multi-leaf collimator for volumetric modulated arc therapy with Halcyon linac. Phys Eng Sci Med. 2020;43:947–57. https://doi.org/10.1007/s13246-020-00891-2.
Pinnix CC, Smith GL, Milgrom S, Osborne EM, Reddy JP, Akhtari M, et al. Predictors of radiation pneumonitis in patients receiving intensity modulated radiation therapy for Hodgkin and non-Hodgkin lymphoma. Int J Radiat Oncol Biol Phys. 2015;92:175–82. https://doi.org/10.1016/j.ijrobp.2015.02.010.
Fogliata A, Cozzi L, Clivio A, Ibatici A, Mancosu P, Navarria P, et al. Preclinical assessment of volumetric modulated arc therapy for total marrow irradiation. Int J Radiat Oncol Biol Phys. 2011;80:628–36. https://doi.org/10.1016/j.ijrobp.2010.11.028.
Watanabe Nemoto M, Isobe K, Togasaki G, Kanazawa A, Kurokawa M, Saito M, et al. Delayed renal dysfunction after total body irradiation in pediatric malignancies. J Radiat Res. 2014;55:996–1001. https://doi.org/10.1093/jrr/rru041.
Belkacemi Y, Labopin M, Vernant JP, Prentice HG, Tichelli A, Schattenberg A, et al. Cataracts after total body irradiation and bone marrow transplantation in patients with acute leukemia in complete remission: a study of the European Group for Blood and Marrow Transplantation. Int J Radiat Oncol Biol Phys. 1998;41:659–68. https://doi.org/10.1016/s0360-3016(98)00077-7.
Bowman WP, Aur RJ, Hustu HO, Rivera G. Isolated testicular relapse in acute lymphocytic leukemia of childhood: categories and influence on survival. J Clin Oncol. 1984;2:924–9. https://doi.org/10.1200/JCO.1984.2.8.924.
Miften M, Olch A, Mihailidis D, Moran J, Pawlicki T, Molineu A, et al. Tolerance limits and methodologies for IMRT measurement-based verification QA: recommendations of AAPM Task Group No. 218. Med Phys. 2018;45:e53-83. https://doi.org/10.1002/mp.12810.
Laugeman E, Heermann A, Hilliard J, Watts M, Roberson M, Morris R, et al. Comprehensive validation of halcyon 2.0 plans and the implementation of patient specific QA with multiple detector platforms. J Appl Clin Med Phys. 2020;21:39–48. https://doi.org/10.1002/acm2.12881.
Maddalo M, Benecchi G, Altabella L, Ghetti C, Dabbiero N. Automatic feathering algorithm for VMAT craniospinal irradiation: a comprehensive comparison with other VMAT planning strategies. Med Dosim. 2021;46(2):103–10. https://doi.org/10.1016/j.meddos.2020.09.003.
Lu JY, Lin Z, Zheng J, Lin PX, Cheung ML, Huang BT. Dosimetric evaluation of a simple planning method for improving intensity-modulated radiotherapy for stage III lung cancer. Sci Rep. 2016;24(6):23543. https://doi.org/10.1038/srep23543.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Study concept and design: TU, HM and MT. Treatment planning: TU, HM, MT, MI, MO, KI, HD and KM. Data analysis and interpretation: TU, HM, MT, MI, MO and KM. Manuscript preparation and editing: TU, HM, MT, MI, KI, HD and YN. Supervised project: YN. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This planning study was approved by our institutional review board (R02-278).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1
. Beam arrangement of VMAT-TBI with Halcyon™.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Uehara, T., Monzen, H., Tamura, M. et al. Feasibility study of volumetric modulated arc therapy with Halcyon™ linac for total body irradiation. Radiat Oncol 16, 236 (2021). https://doi.org/10.1186/s13014-021-01959-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13014-021-01959-3