Abstract
Background
Covid-19 has been one of the major concerns around the world in the last 2 years. One of the challenges of this disease has been to determine its prevalence. Conflicting results of the serology test in Covid explored the need for an updated meta-analysis on this issue. Thus, this systematic review aimed to estimate the prevalence of global SARS-CoV-2 serology in different populations and geographical areas.
Methods
To identify studies evaluating the seroprevalence of SARS-CoV-2, a comprehensive literature search was performed from international databases, including Medline (PubMed), Web of Sciences, Scopus, EMBASE, and CINHAL.
Results
In this meta-analysis, the results showed that SARS-CoV-2 seroprevalence is between 3 and 15% worldwide. In Eastern Mediterranean, the pooled estimate of seroprevalence SARS-CoV-2 was 15% (CI 95% 5–29%), and in Africa, the pooled estimate was 6% (CI 95% 1–13%). In America, the pooled estimate was 8% (CI 95% 6–11%), and in Europe, the pooled estimate was 5% (CI 95% 4–6%). Also the last region, Western Pacific, the pooled estimate was 3% (CI 95% 2–4%). Besides, we analyzed three of these areas separately. This analysis estimated the prevalence in subgroups such as study population, diagnostic methods, sampling methods, time, perspective, and type of the study.
Conclusion
The present meta-analysis showed that the seroprevalence of SARS-CoV-2 has been between 3 and 15% worldwide. Even considering the low estimate of this rate and the increasing vaccination in the world, many people are still susceptible to SARS-CoV-2.
Similar content being viewed by others
Background
Scientists first reported infection due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in Wuhan, China, in December 2019 [1], and due to its contagious nature, it rapidly spread throughout China and the world as the WHO declared a pandemic on March 11, 2020 [2, 3]. According to the World Health Organization (WHO), more than 220 million cases have been identified worldwide; more than 5 million have died [4]. The presented statistics show only a part of the total cases because the clinical manifestations of patients with SARS-CoV-2 vary from acute diseases with severe pneumonia, acute respiratory distress syndrome, or multiple organ failure up to asymptomatic infection. Asymptomatic carriers are essential sources of the infection spread during the incubation period and interfere with the prevention and control of the disease. So, this group of people is an important challenge in the current management of the pandemic [5,6,7].
The ideal method for detecting Covid-19 is a real-time reverse transcription-polymerase chain reaction (RT-PCR). Still, the disease may not be detectable for various reasons, including low viral concentrations in the upper respiratory tract, non-standard sampling methods, and reduced viral load one week after the onset of symptoms. False-negative results may be reported [3, 8]. However, because SARS-COV-2 infection can induce innate and acquired immunity, resulting in widespread inflammatory responses in the disease [9], and neutralizing antibodies (Nabs) made against spike glycoprotein or SARS-CoV-2 nucleocapsid protein are often lead to a long-term immune response in viral infections which in most patients with different titers can be detected within 14 to 21 days after the onset of symptoms and at least for several months thereafter [8, 10], the method of serological testing replaces and complements molecular testing by detecting virus-specific antibodies in blood samples such as IgM and IgG and through commercially available tests including lateral flow immunoassays (LFIAs), enzyme-linked immunoassays (ELISAs), fluorescence immunoassays (FIA), chemiluminescence assays (CLIAs), electro-chemiluminescent immunoassay (ECLIA), and pseudovirus neutralization assays (PsVN assay or VN), and it is used to estimate the serum prevalence in the population and thus the total number of previous infections to diagnose asymptomatic cases, post-clinical convalescence, post-vaccine responses and as a diagnostic aid method in false-negative cases reported by PCR [11,12,13].
To date, epidemiologists from many countries conducted seroprevalence studies on different populations. The results are significantly different between studies, and in many cases, the actual number of patients is higher than the recorded cases. Therefore, they cannot be the exact measure of serum prevalence in the general population and the true extent of pandemic dynamics. As a result, differences in the presented statistics can lead to inappropriate policies and harm to public health [7, 8, 10]. Because Covid-19 has become a global threat and its spread depends on social interactions, population density, education, health promotion, and other related factors, determining the prevalence of infection and collective immunity against SARS-CoV-2 and the use of these data are necessary for making decisions about control measures, management, and assessment of epidemic risks. Therefore, in this meta-analysis, we aimed to estimate the prevalence of global SARS-CoV-2 serology in different populations and geographical areas and investigate the factors affecting it.
Methods
This systematic review and meta-analysis were based on PRISMA guidelines which are specific to the systematic review and meta-analysis of observational studies [14, 15].
Search strategy
All original articles published from December 2019 to December 2021 were searched without language restrictions in international databases, including Medline (PubMed), Web of Sciences, Scopus, EMBASE, and CINHAL. The search strategy in this study was performed using the main study keywords, including serologic tests (with synonyms of serologic, serology, serology studies) SARS-CoV-2 (with synonyms of Covid-19).
Gray Literature was then searched to access unpublished articles and dissertations or international reports. In addition, after the final selection of articles, a manual search was performed by reviewing the references of related articles. Also, medrxiv and bioRxiv websites were used for findings preprint studies related to seroprevalence of SARS-CoV-2 from inception to December 2021.
Study selection and eligibility criteria
The search strategy in international databases was independently performed by the two researchers (MA and AM), and the disputes were resolved by the third person (YM).
Inclusion criteria
In this meta-analysis, studies were considered whose main purpose was to determine the prevalence of positive serological tests in different communities; that is, after performing tests at different times in other communities, the prevalence of the number of positive tests was examined. Therefore, cohort and cross-sectional studies were included in this meta-analysis. The statistical population studied in these initial articles were all individuals, whether with a specific disease or healthy. There were no particular restrictions on the method of serological diagnosis of Covid-19 in this study for inclusion of studies, and various serological tests such as ELISA, LFIA, VN, CLIA, and ECLIA were included in the research. The definition of Covid-19 disease in this study was based on its international definition affected by the transmission of the SARS-CoV-2 virus.
Exclusion criteria
Other studies, including case reports or case series, systematic reviews, and meta-analyses, as well as letters or editorials, were excluded from this study.
Data extraction
To extract information, first, a checklist including questions on the first author’s name, date of publication, country, WHO region, type of sampling (random or non-random), duration of the study, type of the serological test, race, and ethnicity, age, gender (male, and female), number of positive tests and number of performed tests was designed. Then, information extraction based on the checklist was independently performed by the two authors (AM and MA), and disputes, if any, were resolved by the third person (YM).
Quality assessment
In this study, to evaluate the quality of included articles, the Joanna Briggs Institute (JBI) critical appraisal checklist was used for observational studies. JBI critical appraisal tools have been developed by the JBI and collaborators and approved by the JBI Scientific Committee following extensive peer review.
Statistical analysis
According to the extracted information, the Metaprop command was used to calculate the pooled prevalence, and the results were analysed [16]. Cochrane Q and I2 tests were used to investigate the heterogeneity and variance between the studies selected for meta-analysis [17,18,19,20]. Funnel Plot and Egger test were used to evaluate the publication bias [19, 20]. Also, the meta-regression analysis and diagram were used to examine the association between important variables with the estimated pooled prevalence. Statistical analysis was performed using STATA 16.0.
Results
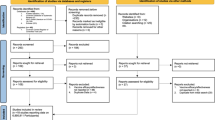
As a result of searching the electronic databases, 3413 studies were obtained, and after removing duplicates, 2507 studies remained. After eliminating studies conducted before 2019, 1926 titles remained for review. In the last stage, after reviewing titles, abstracts, and full texts and considering the inclusion and exclusion criteria, 88 studies were selected for inclusion in the study (Fig. 1).
All 88 studies entered at different time intervals examined the prevalence of positive tests in various communities (Table 1). In total, 414,773 serological tests were performed in all studies. Studies have been reviewed in different countries and were also divided according to WHO classifications. In total, studies have been conducted in 34 countries, with 26 in the United States, 7 in Italy, 5 in France, 4 in each country of Japan, the United Kingdom, Brazil, and China, 3 in each country of Spain, Germany, and Denmark, and 2 in each country of Belgium, Iran, Greece, and Sweden, and 1 in each one of the other countries. According to the WHO classification, there were four studies in the Eastern Mediterranean, 4 in Africa, 31 in America, 35 in Europe, and 12 in Western Pacific.
The quality assessment checklist of the observational studies showed that most of these studies had a good quality. Except for a few of the studies had unknown parts in the checklist (Table 2).
Seropositive in Eastern Mediterranean population
Four studies with a total sample size of 5298 cases determined the prevalence of SARS-CoV-2 in this area. The lowest correlation belonged to the study of Banjar et al. with a prevalence of 1% (95% CI 1 to 2%), and the highest prevalence belonged to the study of Younas et al. with a prevalence of 34% (95% CI 29 to 39%). After combining the results of these studies, the pooled estimate was equal to 15%, with a 95% confidence interval of 5 to 29% (Figs. 2 and 7). The highest value was in Pakistan with a prevalence of 24% (95% CI 19 to 39%), and the lowest was in Saudi Arabia with a prevalence of 1% (95% CI 1 to 2%) (Table 3).
Seropositive in Africa population
Four studies were performed to determine the prevalence of SARS-CoV-2 positive serological tests in this area. The lowest correlation belonged to the study of Halatoko et al. with a prevalence of 1% (95% CI 0 to 2%), and the highest prevalence belonged to the study of Chibwana et al. with a prevalence of 17% (95% CI 14 to 20%). After combining the results of these studies, the pooled estimate was equal to 6%, with a 95% confidence interval of 1 to 13% (Figs. 3 and 7). Also, among the countries in this region, the highest value was related to Malawi with a prevalence of 17% (95% CI 14 to 20%) and the lowest to Togo with a prevalence of 1% (95% CI 0 to 2%) (Table 3).
Seropositive in America population
Thirty-one studies determined the prevalence of SARS-CoV-2 positive serological tests in this area, with the lowest correlation belonging to the study of Ng et al. with a prevalence of 0% (95% CI 0 to 1%) and also the study of Silveira et al. with a prevalence of 0% (95% CI 0 to 1%). The highest prevalence belonged to the study of Racine-Brzostek et al., with a prevalence of 35% (95% CI 33 to 37%). After combining the results of these studies, the pooled estimate was equal to 8%, with a 95% confidence interval of 6 to 10% (Figs. 4 and 7). According to the analysis, among the countries in this region, the highest value was related to Colombia with a prevalence of 29% (95% CI 23 to 31%) and the lowest to Brazil with a prevalence of 7% (95% CI 2 to 12). %) (Table 3).
In the subgroup analysis related to this area, the prevalence was also examined based on the population type (healthy and unhealthy), the diagnostic test type (ELISA–CLISA–LFIA), the sampling type (random and non-random), time (months after pandemic), the perspective (local–regional–national), and the type of the study (cohort–cross-sectional). According to the classification based on the type of population, the results showed that the serological test's positivity was 5% in healthy people (95% CI 4 to 6%). In addition, the evaluation results differed according to the test type, and the prevalence of positive tests was 12% for ELISA (95% CI 10 to 15%), 6% for CLISA (95% CI 4 to 8), and 6% for LFIA (95% CI 4 to 9%). The results showed that the highest prevalence occurred in the diagnostic subgroup of ELISA. Also, depending on the type of sampling, in randomized studies, the prevalence was 9% (95% CI 7 to 11%), and in non-randomized studies, the prevalence was 10% (95% CI 7 to 13%). This indicated a higher prevalence in the non-randomized group. Based on the months after pandemic, the prevalence were 7% for 4 month (95% CI 3 to 12%), 8% for 5 month (95% CI 5 to 13%), 9% for 6 month (95% CI 6 to 14%), and 11% for 7 month (95% CI 0 to 32%). Over time, this prevalence increased. Prevalence based on perspective was 12% for local (95% CI 6 to 19%), 6% for regional (95% CI 4 to 10%), and 3% for national (95% CI 4 to 10%), which was higher in local studies. Also, prevalence was 7% for cohort (95% CI 2 to 14%), and 9% for cross-sectional (95% CI 6 to 12%). Prevalence was higher in cross-sectional studies (Table 4).
Seropositive in European population
In addition, 35 studies determined the prevalence of SARS-CoV-2 positive serological tests in this area with the lowest correlation belonging to the study of Fischer et al. with a prevalence of 01% (95% CI 01 to 01%) and also the study of Merkely et al. with a prevalence of 01% (95% CI 01 to 011%). The highest correlation belonged to the study of Clarke et al., with a prevalence of 36% (95% CI 31 to 41%). After combining the results of these studies, the pooled estimate was equal to 5% with a 95% confidence interval of 4 to 6% (Figs. 5 and 7). In addition, the highest value was related to the United Kingdom among the countries in this region, with a prevalence of 20% (95% CI 4 to 45%). The lowest was associated with Greece, with a prevalence of 1% (95% CI 0 to 2%) (Table 3).
In the subgroup analysis related to this area, the prevalence was also examined based on the population type (healthy and unhealthy), the diagnostic test type (ELISA–CLISA–LFIA–VN–ECLIA), and the sampling type (random and non-random), time (months after pandemic), the perspective (local–regional–national), and the type of the study (cohort–cross-sectional). The classification results by the population type showed the positivity of the serological test in the healthy and unhealthy populations at 5% (95% CI 4 to 6%) and 20% (95% CI 16 to 23%), respectively. Prevalence in the unhealthy population was higher. The results obtained based on the type of the diagnostic test were different, and the prevalence of positive tests was 6% for ELISA (95% CI 4 to 8%), 6% for CLISA (95% CI 3 to 9%), 4% for LFIA (95% CI 2 to 8%), 7% for VN (95% CI 5 to 8%), and 1% for ECLIA (95% CI 1 to 3%). The highest value was evaluated in VN type. Also, depending on the type of sampling, the prevalence in randomized studies was 5% (95% CI 4 to 6%), and in non-randomized studies, it was 6% (95% CI 3 to 8%). Prevalence was higher in non-randomized studies (Table 4). For the months after pandemic, the prevalence were 23% for 2 month (95% CI 19 to 28%), 5% for 3 month (95% CI 4 to 7%), 4% for 4 month (95% CI 2 to 7%), 6% for 5 month (95% CI 5 to 8%), 3% for 6 month (95% CI 2 to 6%), and 5% for 7 month (95% CI 3 to 7%).The highest prevalence was in the 2 months after the pandemic. Prevalence based on perspective was 8% for local (95% CI 6 to 11%), 6% for regional (95% CI 3 to 8%), and 3% for national (95% CI 2 to 4%) indicating higher prevalence in local studies. Prevalence based on type of study was 5% for cohort (95% CI 2 to 8%), and 6% for cross-sectional (95% CI 5 to 7%). Prevalence was higher in cross-sectional studies (Table 4).
Seropositive in Western Pacific population
Finally, 12 studies determined the prevalence of SARS-CoV-2 positive serological tests in this area, with the lowest correlation belonging to the study of Coatsworth et al. with a prevalence of 0% (95% CI 0 to 1%) and the highest correlation belonging to the study of Pan et al. with a prevalence of 33% (95% CI 27 to 40%). After combining the results of these studies, the pooled estimate was equal to 3%, with a 95% confidence interval of 2 to 4% (Figs. 6 and 7). Finally, among the countries in this region, the highest value was related to Taiwan with a prevalence of 33% (95% CI 23 to 40%), and the lowest was associated with Malaysia with a prevalence of 0% (95% CI 0 to 2%) (Table 3).
In the subgroup analysis related to this region, the prevalence was also examined based on the population type (healthy and unhealthy), the diagnostic test type (ELISA–CLISA–LFIA–VN), and the sampling type (random and non-random). The classification results based on the population type showed that the serological test was positive in 3% of the healthy population (95% CI 2 to 5%) and 2% of the unhealthy population (95% CI 1 to 3%). It was higher in the healthy population than in the unhealthy one. The results obtained based on the type of diagnostic test were different. The prevalence of positive tests was 7% for ELISA (95% CI 3 to 10%), 1% for CLISA (95% CI 0 to 2%), 4% for LFIA (95% CI 3 to 5%) and 1% for VN (95% CI 0 to 2%). The highest value was observed in the ELISA group. Also, depending on the type of sampling, the prevalence was 4% in randomized studies (95% CI 2 to 5%), and in non-randomized studies, the prevalence was 2% (95% CI 0 to 4%). The prevalence in the randomized group was higher than that in the non-randomized one (Table 4).
Meta-regression results
In this part, we analyzed the changes in SARS-CoV-2 seroprevalence in different WHO regions and worldwide based on the year from 2020 to 2021. The result in America (B: − 0.03, SE: 0.05, P: 0.469), Europe (B: − 0.01, SE: 0.02, P: 0.401), Western Pacific (B: − 0.01, SE: 0.01, P: 0.430), Eastern Mediterranean (B: − 0.19, SE: 0.08, P: 0.033) and around the World (B: − 0.03, SE: 0.02, P: 0.122) was decreasing which in Western Pacific and World was significant. However, the result in Africa (B: 0.01, SE: 0.02, P: 0.854) was increased (Fig. 8).
Meta-regression analysis of estimated pooled prevalence in WHO regions and around the world from 2020 to 2021. America (B: − 0.03, SE: 0.05, P: 0.469). Europe (B: − 0.01, SE: 0.02, P: 0.401). Western Pacific (B: − 0.01, SE: 0.01, P: 0.430). Eastern Mediterranean (B: − 0.19, SE: 0.08, P: 0.033). Africa (B: 0.01, SE: 0.02, P: 0.854). World (B: − 0.03, SE: 0.02, P: 0.122)
Discussion
Due to the current Covid-19 pandemic, the prevalence and incidence of this disease are increasing worldwide. Because antibodies are produced in response to many pathogens, including Covid-19, and have a higher advantage than other diagnostic methods in determining the serology prevalence, here we have globally collected verified data (by September 2020) to contribute to a comprehensive understanding of the current pandemic by conducting a comprehensive review of the prevalence of Covid-19 serology in different populations and geographical areas. In this meta-analysis, the cumulative prevalence was calculated at 414,773 based on the studied research, and 25,065 people in the world were infected with Covid-19 by the date of this study.
The results obtained based on the study region showed that among the six regions of the WHO, Eastern Mediterranean and Western Pacific had the highest (15%) and lowest (3%) prevalence, respectively. The largest sample size and number of studies were related to the European Region, accompanied by other development characteristics in this region. It is also impossible to accurately assess the Covid-19 prevalence based on just one study at the local level. Still, one can imagine the general situation from these few studies, especially globally. Although the exact protective effect of antibodies against mutant variants has not been determined so far [21], it can be said that the differences observed in seroprevalence are probably related to differences in the disease transmission status in the community due to behavioral differences, the public health status, local resources, and environmental issues. Of course, there are other issues, such as altitude and climatic differences, and the relevant evidence is not yet complete [22, 23]. Differences in the volume, time, single approach, sampling method, missing samples, sample size, selection bias, greater participation of symptomatic individuals, the inclusion of minority populations, lack of validity and reliability of questionnaires in determining symptoms, accuracy of diagnostic kits, rate of decrease in the antibody titer, possible reinfection, the persistence of the virus in a large population of the society, and diversity of geographical and demographic characteristics (age, sex, race, ethnicity, etc.) were among the limiting factors in most studies [24,25,26].
In the present study, the lowest Covid-19 seroprevalence was in Western Pacific and African countries, followed by European and American ones, and was slightly higher in the Eastern Mediterranean. However, within each of the World Health Organization's geographical areas, there were significant differences. For example, the estimated prevalence in Taiwan (33%) was much higher than that of other Western Pacific countries. The same difference existed in Europe, so the United Kingdom, with an estimated prevalence of 20%, was significantly different from its neighbors. In contrast, the differences in the Americas and Africa were relatively small, and the Covid-19 seroprevalence was moderate in these regions. Finally, in the Eastern Mediterranean region, Covid-19 seroprevalence was relatively high in Iran and Pakistan, except in Saudi Arabia. Similar studies that have mainly classified the prevalence based on countries' income reported that in some cases, middle-income countries and, in other instances, high-income countries had reported a higher prevalence [27, 28]. So, we could not find a precise correlation between the income level of countries and the Covid-19 seroprevalence, which may be due to differences in the time of epidemic changes in these countries, sampling and laboratory methods, disease control policies, and vaccination in different populations.
Studies used different serological tests. Due to the many reasons presented for the difference in Covid-19 seroprevalence in additional studies and populations, it was impossible to precisely determine the effect of the test type on this rate. Various studies showed that the type of used antigen, the number of passed days since the onset of the patient’s initial symptoms, and the performance of the serological test itself affected the sensitivity and specificity of various tests [29,30,31]. The reported sensitivity for different tests was from 66 to 97%, while the specificity of all tests was reported to be higher than 95% [32, 33].
Different demographic subgroups such as healthy and unhealthy individuals and the randomized and non-randomized sampling, in general, can affect the difference in seroprevalence. As stated in the present study, studies reported lower and higher seroprevalence in different geographic perspectives and time from the beginning of the pandemic areas in each category. For example, in the Western Pacific countries, the seroprevalence of healthy populations was higher than that of unhealthy ones. In cases with the random sampling method, it was more than the non-random one. Also, in our study, the seroprevalence increased from local to national perspectives, respectively, due to the impact of more facilities, effective health policies, and easier access to health care services at the national level. In general, the samples taken in our study were in the time period from 2 January to 21 September 2020. In this period, clinical management of the disease was based on symptomatic therapies. Still, non-pharmaceutical interventions (NPIs) such as physical distance in all settings, hand hygiene and use of protective equipment self and large-scale isolation, and closure of borders, schools, and workplaces play a critical role in preventing and controlling disease transmission. Therefore, problems with infrastructure, imports of some drugs, and strategies such as quarantine, proper promotion, or non-observance of the mentioned factors can change the prevalence of the disease months from the beginning of the pandemic. For example, the prevalence peaked in Western Pacific and European countries in April 2020.
Also, specific mutations in the SARS-CoV-2 genome over time impacted diagnostics, transmissibility, and treatment. And the first variant (alpha) was identified in late 2020, so the obtained seroprevalence pattern cannot be justified by Covid-19 variants [34, 35]. Hence, there were no effective and available vaccines or drugs against Covid-19 in our study period. The first public vaccine was given to a 91-year-old woman in The UK named Margaret Keenan on 8th December 2020 [36]; the results of the current meta-analysis may be less justified by vaccination and viral variants, so conducting such seroprevalence studies would need to be done again carefully.
In the meta-regression performed based on the observed changes in Covid-19 seroprevalence over time, it was found that other countries showed a downward trend despite our expectation of this increase over time, except in the subgroup of African countries in Covid-19 seroprevalence. This may be due to differences in sampling times in different countries due to the peak of the disease and changes in prevention systems in these countries on the one hand and the instability of Covid-19 specific antigens over time on the other hand.
One of the strengths of this study was the global review of Covid-19 seroprevalence studies. Also, in this research, studies were aggregated by different regions of the World Health Organization, while in similar studies, classification was more based on the income level of countries [27, 28]. Also, in this study, changes in the seroprevalence time of populations were presented first. On the other hand, one of the weaknesses of the research was the lack of a sample study from all people and countries of the world to better estimate global seroprevalence. Also, some countries had only one study on the existing cases, and others reported several ones. Indeed, the prevalence of Covid-19 varies in different subgroups and varies according to epidemic changes and prevention policies. Therefore, with a small number of studies, the demographic and temporal generalizability of the findings is problematic. Also, different sampling methods, tests, different times passed from the onset of symptoms in different people, and other antigens make it challenging to interpret the findings uniformly. The probability of underestimating seroprevalence in the world is high. If the prevalence is higher with confirmed cases, a lower death rate can be found in all cases of infection [26]. According to the findings of the studies, the highest prevalence was seen in ethnic and racial minorities such as Blacks and South Asians than Whites. Factors related to this finding include various determinants of health inequality, including discrimination, access to health care, the employment status and its related factors, financial and educational gaps, the housing status and the number of household members, and in general, occupational, social, and environmental variables [37,38,39].
Conclusion
The present research performed on 88 studies showed that the seroprevalence of Covid-19 has been between 3 and 15% worldwide, and even considering the low estimate of this rate and the increasing vaccination in the world, a large number of people are still susceptible to Covid-19. Countries need to implement prevention policies with greater sensitivity and follow-up, especially those with low Covid-19 serology prevalence and vaccination coverage.
Availability of data and materials
Input data for the analyses are available from the corresponding author on request.
Abbreviations
- WHO:
-
World Health Organization
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- RT-PCR:
-
Real-time reverse transcription-polymerase chain reaction
- PCR:
-
Polymerase chain reaction
- Nab:
-
Neutralizing antibodies
- LFIA:
-
Lateral flow immunoassays
- ELISA:
-
Enzyme-linked immunoassays
- FIA:
-
Fluorescence immunoassays
- CLIA:
-
Chemiluminescence assays
- PsVN:
-
Pseudo-virus neutralization assays
- VN:
-
Virus neutralization assays
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- JBI:
-
Joanna Briggs Institute
- CI:
-
Confidence interval
- CINAHL:
-
Cumulative Index to Nursing and Allied Health Literature
- EMBASE:
-
Excerpta Medica dataBASE
References
Bubba L, Simmonds P, Fischer TK, Harvala H. Mapping of serological testing and SARS-CoV-2 seroprevalence studies performed in 20 European countries, March–June 2020. J Glob Health. 2021;11:05014. https://doi.org/10.7189/jogh.11.05014.
Galanis P, Vraka I, Fragkou D, Bilali A, Kaitelidou D. Seroprevalence of SARS-CoV-2 antibodies and associated factors in healthcare workers: a systematic review and meta-analysis. J Hosp Infect. 2021;108:120–34. https://doi.org/10.1016/j.jhin.2020.11.008.
Qin X, Shen J, Dai E, Li H, Tang G, Zhang L, et al. The seroprevalence and kinetics of IgM and IgG in the progression of COVID-19. BMC Immunol. 2021;22(1):14. https://doi.org/10.1186/s12865-021-00404-0.
World Health Organization. COVID-19 weekly epidemiological update, 3 November 2020. https://apps.who.int/iris/bitstream/handle/10665/336478/nCoV-weekly-sitrep01Nov20-eng.pdf.
Shakiba M, Nazemipour M, Heidarzadeh A, Mansournia MA. Prevalence of asymptomatic COVID-19 infection using a seroepidemiological survey. Epidemiol Infect. 2020;148: e300. https://doi.org/10.1017/s0950268820002745.
Tsai PH, Lai WY, Lin YY, Luo YH, Lin YT, Chen HK, et al. Clinical manifestation and disease progression in COVID-19 infection. J Chin Med Assoc JCMA. 2021;84(1):3–8. https://doi.org/10.1097/jcma.0000000000000463.
Pollán M, Pérez-Gómez B, Pastor-Barriuso R, Oteo J, Hernán MA, Pérez-Olmeda M, et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396(10250):535–44.
McConnell D, Hickey C, Bargary N, Trela-Larsen L, Walsh C, Barry M, et al. Understanding the challenges and uncertainties of seroprevalence studies for SARS-CoV-2. Int J Environ Res Public Health. 2021. https://doi.org/10.3390/ijerph18094640.
Anka AU, Tahir MI, Abubakar SD, Alsabbagh M, Zian Z, Hamedifar H, et al. Coronavirus disease 2019 (COVID-19): An overview of the immunopathology, serological diagnosis and management. Scand J Immunol. 2021;93(4): e12998. https://doi.org/10.1111/sji.12998.
Chvatal-Medina M, Mendez-Cortina Y, Patiño PJ, Velilla PA, Rugeles MT. Antibody responses in COVID-19: a review. Front Immunol. 2021;12: 633184. https://doi.org/10.3389/fimmu.2021.633184.
Higgins V, Fabros A, Kulasingam V. Quantitative measurement of anti-SARS-CoV-2 antibodies: analytical and clinical evaluation. J Clin Microbiol. 2021. https://doi.org/10.1128/jcm.03149-20.
Clarke C, Prendecki M, Dhutia A, Ali MA, Sajjad H, Shivakumar O, et al. High prevalence of asymptomatic COVID-19 infection in hemodialysis patients detected using serologic screening. J Am Soc Nephrol. 2020;31(9):1969–75.
James J, Rhodes S, Ross CS, Skinner P, Smith SP, Shipley R, et al. Comparison of serological assays for the detection of SARS-CoV-2 antibodies. Viruses. 2021. https://doi.org/10.3390/v13040713.
Moher D, Altman DG, Liberati A, Tetzlaff J. PRISMA statement. Epidemiology. 2011;22(1):128.
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–73.
Nyaga VN, Arbyn M, Aerts M. Metaprop: a stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72(1):1–10.
Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Commun Health. 2013;67(11):974–8.
Biggerstaff BJ, Jackson D. The exact distribution of Cochran’s heterogeneity statistic in one-way random effects meta-analysis. Stat Med. 2008;27(29):6093–110.
Guzzo RA, Jackson SE, Katzell RA. Meta-analysis analysis. Res Organ Behav. 1987;9(1):407–42.
Hedges LV. Meta-analysis. J Educ Stat. 1992;17(4):279–96.
Younas A, Waheed S, Khawaja S, Imam M, Borhany M, Shamsi T. Seroprevalence of SARS-CoV-2 antibodies among healthy blood donors in Karachi, Pakistan. Transfus Apher Sci. 2020;59(6): 102923. https://doi.org/10.1016/j.transci.2020.102923.
Yao Y, Pan J, Liu Z, Meng X, Wang W, Kan H, et al. No association of COVID-19 transmission with temperature or UV radiation in Chinese cities. Eur Respir J. 2020;55(5):2000517.
Briz-Redón Á, Serrano-Aroca Á. The effect of climate on the spread of the COVID-19 pandemic: a review of findings, and statistical and modelling techniques. Prog Phys Geogr. 2020;44(5):591–604.
Anna F, Goyard S, Lalanne AI, Nevo F, Gransagne M, Souque P, et al. High seroprevalence but short-lived immune response to SARS-CoV-2 infection in Paris. Eur J Immunol. 2021;51(1):180–90. https://doi.org/10.1002/eji.202049058.
Rosenberg ES, Tesoriero JM, Rosenthal EM, Chung R, Barranco MA, Styer LM, et al. Cumulative incidence and diagnosis of SARS-CoV-2 infection in New York. Ann Epidemiol. 2020;48:23-9.e4. https://doi.org/10.1016/j.annepidem.2020.06.004.
Sood N, Simon P, Ebner P, Eichner D, Reynolds J, Bendavid E, et al. Seroprevalence of SARS-CoV-2–specific antibodies among adults in Los Angeles County, California, on April 10–11, 2020. JAMA. 2020;323(23):2425–7. https://doi.org/10.1001/jama.2020.8279.
Rostami A, Sepidarkish M, Leeflang MM, Riahi SM, Shiadeh MN, Esfandyari S, et al. SARS-CoV-2 seroprevalence worldwide: a systematic review and meta-analysis. Clin Microbiol Infect. 2021;27(3):331–40.
Bobrovitz N, Arora RK, Cao C, Boucher E, Liu M, Donnici C, et al. Global seroprevalence of SARS-CoV-2 antibodies: a systematic review and meta-analysis. PLoS ONE. 2021;16(6): e0252617.
Liu G, Rusling JF. COVID-19 antibody tests and their limitations. ACS Sens. 2021;6(3):593–612.
Zhang JJ, Lee KS, Ong CW, Chan MY, Ang LW, Leo YS, et al. Diagnostic performance of COVID-19 serological assays during early infection: a systematic review and meta-analysis of 11 516 samples. Influenza Other Respir Viruses. 2021. https://doi.org/10.1111/irv.12841.
Higgins V, Fabros A, Kulasingam V. Quantitative measurement of anti-SARS-CoV-2 antibodies: analytical and clinical evaluation. J Clin Microbiol. 2021;59(4):e03149-e3220.
Zhang ZL, Hou YL, Li DT, Li FZ. Diagnostic efficacy of anti-SARS-CoV-2 IgG/IgM test for COVID-19: a meta-analysis. J Med Virol. 2021;93(1):366–74.
Chua CR, de Santos los ED, Escasa KV, Estolas RL, Feliciano J, Ortega SA, et al. Diagnostic accuracy of COVID-19 antibody tests authorized by FDA Philippines: a systematic review and meta-analysis. SciMed J. 2021;3(4):283–301.
Khandia R, Singhal S, Alqahtani T, Kamal MA, Nahed A, Nainu F, et al. Emergence of SARS-CoV-2 Omicron (B. 1.1. 529) variant, salient features, high global health concerns and strategies to counter it amid ongoing COVID-19 pandemic. Environ Res. 2022;209: 112816.
https://www.who.int/activities/tracking-SARS-CoV-2-variants. Accessed 28 May 2022.
Yousaf M. COVID-19 vaccination campaign: a tale of a volunteer on the frontline in England. Br Med Pract. 2021;14(2):a011.
Ward H, Atchison CJ, Whitaker M, Ainslie KEC, Elliot J, Okell LC, et al. Antibody prevalence for SARS-CoV-2 in England following first peak of the pandemic: REACT2 study in 100,000 adults. MedRxiv. 2020.
Feehan AK, Fort D, Garcia-Diaz J, Price-Haywood E, Velasco C, Sapp E, et al. Point prevalence of SARS-CoV-2 and infection fatality rate in Orleans and Jefferson Parish, Louisiana, May 9–15, 2020. medRxiv. 2020. https://doi.org/10.1101/2020.06.23.20138321.
Havers FP, Reed C, Lim T, Montgomery JM, Klena JD, Hall AJ, et al. Seroprevalence of antibodies to SARS-CoV-2 in 10 sites in the United States, March 23-May 12, 2020. JAMA Intern Med. 2020;180(12):1576–86.
Herzog S, De Bie J, Abrams S, Wouters I, Ekinci E, Patteet L, et al. Seroprevalence of IgG antibodies against SARS coronavirus 2 in Belgium–a serial prospective cross-sectional nationwide study of residual samples. medRxiv. 2020. https://doi.org/10.1101/2020.06.08.20125179.
Amorim Filho L, Szwarcwald CL, Mateos SdOG, Leon AC, Medronho RdA, Veloso VG, et al. Seroprevalence of anti-SARS-CoV-2 among blood donors in Rio de Janeiro, Brazil. Rev Saude Publica. 2020;54:69.
Silveira MF, Barros AJD, Horta BL, Pellanda LC, Victora GD, Dellagostin OA, et al. Population-based surveys of antibodies against SARS-CoV-2 in Southern Brazil. Nat Med. 2020;26(8):1196–9.
Torres JP, Piñera C, De La Maza V, Lagomarcino AJ, Simian D, Torres B, et al. Severe acute respiratory syndrome coronavirus 2 antibody prevalence in blood in a large school community subject to a coronavirus disease 2019 outbreak: a cross-sectional study. Clin Infect Dis. 2020. https://doi.org/10.1093/cid/ciaa955.
Chang L, Hou W, Zhao L, Zhang Y, Wang Y, Wu L, et al. The prevalence of antibodies to SARS-CoV-2 among blood donors in China. Nat Commun. 2021;12(1):1383. https://doi.org/10.1038/s41467-021-21503-x.
To KK, Cheng VC, Cai J-P, Chan K-H, Chen L-L, Wong L-H, et al. Seroprevalence of SARS-CoV-2 in Hong Kong and in residents evacuated from Hubei province, China: a multicohort study. Lancet Microbe. 2020;1(3):e111–8. https://doi.org/10.1016/S2666-5247(20)30053-7.
Liang W, Lin Y, Bi J, Li J, Liang Y, Wong S-S, et al. Serosurvey of SARS-CoV-2 among hospital visitors in China. Cell Res. 2020;30(9):817–8. https://doi.org/10.1038/s41422-020-0371-0.
Jerkovic I, Ljubic T, Bašic Ž, Kružic I, Kunac N, Bezic J, et al. SARS-CoV-2 Antibody Seroprevalence in Industry Workers in Split-Dalmatia and Šibenik-Knin County, Croatia. J Occup Environ Med. 2021;63(1):32–7. https://doi.org/10.1097/jom.0000000000002020.
Erikstrup C, Hother CE, Pedersen OBV, Mølbak K, Skov RL, Holm DK, et al. Estimation of SARS-CoV-2 infection fatality rate by real-time antibody screening of blood donors. Clin Infect Dis. 2020;72(2):249–53. https://doi.org/10.1093/cid/ciaa849.
Petersen MS, Strøm M, Christiansen DH, Fjallsbak JP, Eliasen EH, Johansen M, et al. Seroprevalence of SARS-CoV-2–specific antibodies, Faroe Islands. Emerg Infect Dis. 2020;26(11):2760.
Ward H, Atchinson C, Whitaker M, Ainslie K, Elliott J, Okell L. Antibody prevalence for SARS-CoV-2 following the peak of the pandemic in England: REACT2 study in 100,000 adults. medRxiv. 2020. https://doi.org/10.1101/2020.08.12.20173690.
Gallian P, Pastorino B, Morel P, Chiaroni J, Ninove L, de Lamballerie X. Lower prevalence of antibodies neutralizing SARS-CoV-2 in group O French blood donors. Antiviral Res. 2020;181: 104880. https://doi.org/10.1016/j.antiviral.2020.104880.
Grzelak L, Temmam S, Planchais C, Demeret C, Huon C, Guivel-Benhassine F, et al. SARS-CoV-2 serological analysis of COVID-19 hospitalized patients, pauci-symptomatic individuals and blood donors. medRxiv. 2020. https://doi.org/10.1101/2020.04.21.20068858.
Fischer B, Knabbe C, Vollmer T. SARS-CoV-2 IgG seroprevalence in blood donors located in three different federal states, Germany, March to June 2020. Eurosurveillance. 2020;25(28):2001285. https://doi.org/10.2807/1560-7917.
Weis S, Scherag A, Baier M, Kiehntopf M, Kamradt T, Kolanos S, et al. Seroprevalence of SARS-CoV-2 antibodies in an entirely PCR-sampled and quarantined community after a COVID-19 outbreak—the CoNAN study. medRxiv. 2020. https://doi.org/10.1101/2020.07.15.20154112.
Bogogiannidou Z, Vontas A, Dadouli K, Kyritsi MA, Soteriades S, Nikoulis DJ, et al. Repeated leftover serosurvey of SARS-CoV-2 IgG antibodies, Greece, march and april 2020. Eurosurveillance. 2020;25(31):2001369. https://doi.org/10.2807/1560-7917.ES.2020.25.31.2001369.
Merkely B, Szabó AJ, Kosztin A, Berényi E, Sebestyén A, Lengyel C, et al. Novel coronavirus epidemic in the Hungarian population, a cross-sectional nationwide survey to support the exit policy in Hungary. GeroScience. 2020;42(4):1063–74.
Shakiba M, Hashemi Nazari SS, Mehrabian F, Rezvani SM, Ghasempour Z, Heidarzadeh A. Seroprevalence of COVID-19 virus infection in Guilan province, Iran. medRxiv. 2020. https://doi.org/10.1101/2020.04.26.20079244.
Percivalle E, Cambiè G, Cassaniti I, Nepita EV, Maserati R, Ferrari A, et al. Prevalence of SARS-CoV-2 specific neutralising antibodies in blood donors from the Lodi Red Zone in Lombardy, Italy, as at 06 April 2020. Eurosurveillance. 2020;25(24):2001031. https://doi.org/10.2807/1560-7917.
Valenti L, Bergna A, Pelusi S, Facciotti F, Lai A, Tarkowski M, et al. SARS-CoV-2 seroprevalence trends in healthy blood donors during the COVID-19 Milan outbreak. MedRxiv. 2020. https://doi.org/10.1101/2020.05.11.20098442.
Fiore JR, Centra M, De Carlo A, Granato T, Rosa A, Sarno M, et al. Results from a survey in healthy blood donors in South Eastern Italy indicate that we are far away from herd immunity to SARS-CoV-2. J Med Virol. 2021;93(3):1739–42. https://doi.org/10.1002/jmv.26425.
Doi A, Iwata K, Kuroda H, Hasuike T, Nasu S, Kanda A, et al. Seroprevalence of novel coronavirus disease (COVID-19) in Kobe, Japan. medRxiv. 2020. https://doi.org/10.1101/2020.04.26.20079822.
Takita M, Matsumura T, Yamamoto K, Yamashita E, Hosoda K, Hamaki T, et al. Geographical profiles of COVID-19 outbreak in Tokyo: an analysis of the primary care clinic–based point-of-care antibody testing. J Prim Care Commun Health. 2020;11:2150132720942695.
Takita M, Matsumura T, Yamamoto K, Yamashita E, Hosoda K, Hamaki T, et al. Preliminary results of seroprevalence of SARS-CoV-2 at community clinics in Tokyo. medRxiv. 2020. https://doi.org/10.1101/2020.04.29.20085449.
Uyoga S, Adetifa IMO, Karanja HK, Nyagwange J, Tuju J, Wanjiku P, et al. Seroprevalence of anti–SARS-CoV-2 IgG antibodies in Kenyan blood donors. Science. 2021;371(6524):79–82. https://doi.org/10.1126/science.abe1916.
Song S-K, Lee D-H, Nam J-H, Kim K-T, Do J-S, Kang D-W, et al. IgG Seroprevalence of COVID-19 among individuals without a history of the coronavirus disease infection in Daegu, Korea. J Korean Med Sci. 2020. https://doi.org/10.3346/jkms.2020.35.e269.
Kammon AM, El-Arabi AA, Erhouma EA, Mehemed TM, Mohamed OA. Seroprevalence of antibodies against SARS-CoV-2 among public community and health-care workers in Alzintan City of Libya. medRxiv. 2020. https://doi.org/10.1101/2020.05.25.20109470.
Snoeck CJ, Vaillant M, Abdelrahman T, Satagopam V, Turner J, Beaumont K, et al. Prevalence of SARS-CoV-2 infection in the Luxembourgish population: the CON-VINCE study. Medrxiv. 2020. https://doi.org/10.1101/2020.05.11.20092916.
Sam I-C, Chong YM, Tan CW, Chan YF. Low postpandemic wave SARS-CoV-2 seroprevalence in Kuala Lumpur and Selangor, Malaysia. J Med Virol. 2021;93(2):647–8. https://doi.org/10.1002/jmv.26426.
Lundkvist Å, Hanson S, Olsen B. Pronounced difference in covid-19 antibody prevalence indicates cluster transmission in Stockholm, Sweden. Infect Ecol Epidemiol. 2020;10(1):1806505.
Stringhini S, Wisniak A, Piumatti G, Azman AS, Lauer SA, Baysson H, et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet. 2020;396(10247):313–9. https://doi.org/10.1016/S0140-6736(20)31304-0.
Bendavid E, Mulaney B, Sood N, Shah S, Bromley-Dulfano R, Lai C, et al. Covid-19 antibody seroprevalence in Santa Clara County, California. Int J Epidemiol. 2021;50(2):410–9.
Biggs HM, Harris JB, Breakwell L, Dahlgren FS, Abedi GR, Szablewski CM, et al. Estimated community seroprevalence of SARS-CoV-2 antibodies—two Georgia counties, April 28–May 3, 2020. Morb Mortal Wkly Rep. 2020;69(29):965.
Bryan A, Pepper G, Wener MH, Fink SL, Morishima C, Chaudhary A, et al. Performance characteristics of the abbott architect SARS-CoV-2 IgG assay and seroprevalence in Boise, Idaho. J Clin Microbiol. 2020;58(8):e00941-e1020.
Dietrich ML, Norton EB, Elliott D, Smira AR, Rouelle JA, Bond NG, et al. SARS-CoV-2 seroprevalence rates of children in louisiana during the state stay at home order. medRxiv. 2020. https://doi.org/10.1101/2020.07.07.20147884.
McLaughlin CC, Doll MK, Morrison KT, McLaughlin WL, O’Connor T, Sholukh AM, et al. High community SARS-CoV-2 antibody seroprevalence in a ski resort community, Blaine County, Idaho, US. Preliminary results. Medrxiv. 2020. https://doi.org/10.1101/2020.07.19.20157198.
Menachemi N, Yiannoutsos CT, Dixon BE, Duszynski TJ, Fadel WF, Wools-Kaloustian KK, et al. Population point prevalence of SARS-CoV-2 infection based on a statewide random sample—Indiana, April 25–29, 2020. Morb Mortal Wkly Rep. 2020;69(29):960.
Dianna Ng, Goldgof G, Shy B, Levine A, Balcerek J, Bapat SP, et al. SARS-CoV-2 seroprevalence and neutralizing activity in donor and patient blood from the San Francisco Bay Area. MedRxiv. 2020. https://doi.org/10.1038/s41467-020-18468-8.
Akinbami LJ, Vuong N, Petersen LR, Sami S, Patel A, Lukacs SL, et al. SARS-CoV-2 seroprevalence among healthcare, first response, and public safety personnel, detroit metropolitan area, Michigan, USA, May-June 2020. Emerg Infect Dis. 2020;26(12):2863–71. https://doi.org/10.3201/eid2612.203764.
Berardis S, Verroken A, Vetillart A, Struyf C, Gilbert M, Gruson D, et al. SARS-CoV-2 seroprevalence in a Belgian cohort of patients with cystic fibrosis. J Cyst Fibros. 2020;19(6):872–4. https://doi.org/10.1016/j.jcf.2020.08.005.
Borges LP, Martins AF, de Melo MS, de Oliveira MGB, de Rezende Neto JM, Dósea MB, et al. Seroprevalence of SARS-CoV-2 IgM and IgG antibodies in an asymptomatic population in Sergipe, Brazil. Rev Panam Salud Publica. 2020. https://doi.org/10.26633/RPSP.2020.108.
Caban-Martinez AJ, Schaefer-Solle N, Santiago K, Louzado-Feliciano P, Brotons A, Gonzalez M, et al. Epidemiology of SARS-CoV-2 antibodies among firefighters/paramedics of a US fire department: a cross-sectional study. Occup Environ Med. 2020;77(12):857–61. https://doi.org/10.1136/oemed-2020-106676.
De Carlo A, Caputo SL, Paolillo C, Rosa AM, D’orsi U, De Palma M, et al. SARS-CoV-2 serological profile in healthcare professionals of a Southern Italy hospital. Int J Environ Res Public Health. 2020;17(24):1–11. https://doi.org/10.3390/ijerph17249324.
Dingens AS, Crawford KHD, Adler A, Steele SL, Lacombe K, Eguia R, et al. Serological identification of SARS-CoV-2 infections among children visiting a hospital during the initial Seattle outbreak. Nat Commun. 2020;11(1):4378. https://doi.org/10.1038/s41467-020-18178-1.
Flannery DD, Gouma S, Dhudasia MB, Mukhopadhyay S, Pfeifer MR, Woodford EC, et al. SARS-CoV-2 seroprevalence among parturient women in Philadelphia. Sci Immunol. 2020. https://doi.org/10.1126/sciimmunol.abd5709.
Halatoko WA, Konu YR, Gbeasor-Komlanvi FA, Sadio AJ, Tchankoni MK, Komlanvi KS, et al. Prevalence of SARS-CoV-2 among high-risk populations in Lomé (Togo) in 2020. PLoS ONE. 2020. https://doi.org/10.1371/journal.pone.0242124.
Hunter BR, Dbeibo L, Weaver CS, Beeler C, Saysana M, Zimmerman MK, et al. Seroprevalence of severe acute respiratory coronavirus virus 2 (SARS-CoV-2) antibodies among healthcare workers with differing levels of coronavirus disease 2019 (COVID-19) patient exposure. Infect Control Hosp Epidemiol. 2020;41(12):1441–2. https://doi.org/10.1017/ice.2020.390.
Khan SMS, Qurieshi MA, Haq I, Majid S, Bhat AA, Nabi S, et al. Seroprevalence of SARS-CoV-2 specific IgG antibodies in District Srinagar, northern India—a cross-sectional study. PLoS ONE. 2020. https://doi.org/10.1371/journal.pone.0239303.
Kobashi Y, Shimazu Y, Nishikawa Y, Kawamura T, Kodama T, Obara D, et al. The difference between IgM and IgG antibody prevalence in different serological assays for COVID-19; lessons from the examination of healthcare workers. Int Immunopharmacol. 2020;92: 107360. https://doi.org/10.1016/j.intimp.2020.107360.
Lastrucci V, Lorini C, Riccio MD, Gori E, Chiesi F, Sartor G, et al. Sars-cov-2 seroprevalence survey in people involved in different essential activities during the general lock-down phase in the province of Prato (Tuscany, Italy). Vaccines. 2020;8(4):1–9. https://doi.org/10.3390/vaccines8040778.
Mahajan S, Srinivasan R, Redlich CA, Huston SK, Anastasio KM, Cashman L, et al. Seroprevalence of SARS-CoV-2-specific IgG antibodies among adults living in connecticut: post-infection prevalence (PIP) study. Am J Med. 2020. https://doi.org/10.1016/j.amjmed.2020.09.024.
Mansour M, Leven E, Muellers K, Stone K, Mendu DR, Wajnberg A. Prevalence of SARS-CoV-2 antibodies among healthcare workers at a tertiary academic hospital in New York City. J Gen Intern Med. 2020;35(8):2485–6. https://doi.org/10.1007/s11606-020-05926-8.
Mattern J, Vauloup-Fellous C, Zakaria H, Benachi A, Carrara J, Letourneau A, et al. Post lockdown COVID-19 seroprevalence and circulation at the time of delivery, France. PLoS ONE. 2020;15(10): e0240782. https://doi.org/10.1371/journal.pone.0240782.
McDade TW, McNally EM, Zelikovich AS, D’Aquila R, Mustanski B, Miller A, et al. High seroprevalence for SARS-CoV-2 among household members of essential workers detected using a dried blood spot assay. PLoS ONE. 2020;15(8): e0237833. https://doi.org/10.1371/journal.pone.0237833.
Naranbhai V, Chang CC, Beltran WFG, Miller TE, Astudillo MG, Villalba JA, et al. High seroprevalence of anti-SARS-CoV-2 antibodies in Chelsea, Massachusetts. J Infect Dis. 2020;222(12):1955–9. https://doi.org/10.1093/infdis/jiaa579.
Oliveira L, Tiyo BT, Silva LTD, Fonseca LAM, Rocha RC, Santos VAD, et al. Prevalence of anti-SARS-CoV-2 antibodies in outpatients of a large public university hospital in Sao Paulo, Brazil. Rev Inst Med Trop Sao Paulo. 2020;62: e91. https://doi.org/10.1590/s1678-9946202062091.
Pollán M, Pérez-Gómez B, Pastor-Barriuso R, Oteo J, Hernán MA, Pérez-Olmeda M, et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet (London, England). 2020;396(10250):535–44. https://doi.org/10.1016/s0140-6736(20)31483-5.
Psichogiou M, Karabinis A, Pavlopoulou ID, Basoulis D, Petsios K, Roussos S, et al. Antibodies against SARS-CoV-2 among health care workers in a country with low burden of COVID-19. PLoS ONE. 2020;15(12): e0243025. https://doi.org/10.1371/journal.pone.0243025.
Racine-Brzostek SE, Yang HS, Chadburn A, Orlander D, An A, Campion TR, et al. COVID-19 Viral and serology testing in New York City health care workers. Am J Clin Pathol. 2020;154(5):592–5. https://doi.org/10.1093/ajcp/aqaa142.
Shields A, Faustini SE, Perez-Toledo M, Jossi S, Aldera E, Allen JD, et al. SARS-CoV-2 seroprevalence and asymptomatic viral carriage in healthcare workers: a cross-sectional study. Thorax. 2020;75(12):1089–94. https://doi.org/10.1136/thoraxjnl-2020-215414.
Sood N, Simon P, Ebner P, Eichner D, Reynolds J, Bendavid E, et al. Seroprevalence of SARS-CoV-2-specific antibodies among adults in Los Angeles County, California, on April 10–11, 2020. JAMA. 2020;323(23):2425–7. https://doi.org/10.1001/jama.2020.8279.
Tang H, Tian JB, Dong JW, Tang XT, Yan ZY, Zhao YY, et al. Serologic detection of SARS-CoV-2 infections in hemodialysis centers: a multicenter retrospective study in Wuhan, China. Am J Kidney Dis. 2020;76(4):490-9.e1. https://doi.org/10.1053/j.ajkd.2020.06.008.
Banjar A, Al-Tawfiq JA, Alruwaily A, Alserehi H, Al-Qunaibet A, Alaswad R, et al. Seroprevalence of antibodies to SARS-CoV-2 among blood donors in the early months of the pandemic in Saudi Arabia. Int J Infect Dis. 2021;104:452–7. https://doi.org/10.1016/j.ijid.2021.01.028.
Coatsworth N, Myles PS, Mann GJ, Cockburn IA, Forbes AB, Gardiner EE, et al. Prevalence of asymptomatic SARS-CoV-2 infection in elective surgical patients in Australia: a prospective surveillance study. ANZ J Surg. 2021;91(1–2):27–32. https://doi.org/10.1111/ans.16564.
Ebinger JE, Botwin GJ, Albert CM, Alotaibi M, Arditi M, Berg AH, et al. Seroprevalence of antibodies to SARS-CoV-2 in healthcare workers: a cross-sectional study. BMJ Open. 2021;11(2): e043584. https://doi.org/10.1136/bmjopen-2020-043584.
Kantele A, Lääveri T, Kareinen L, Pakkanen SH, Blomgren K, Mero S, et al. SARS-CoV-2 infections among healthcare workers at Helsinki University Hospital, Finland, spring 2020: Serosurvey, symptoms and risk factors. Travel Med Infect Dis. 2021. https://doi.org/10.1016/j.tmaid.2020.101949.
Ladoire S, Goussot V, Redersdorff E, Cueff A, Ballot E, Truntzer C, et al. Seroprevalence of SARS-CoV-2 among the staff and patients of a French cancer centre after first lockdown: the canSEROcov study. Eur J Cancer. 2021;148:359–70. https://doi.org/10.1016/j.ejca.2021.02.027.
Laursen J, Petersen J, Didriksen M, Iversen K, Ullum H. Prevalence of SARS-CoV-2 IgG/IgM Antibodies among Danish and Swedish Falck Emergency and Non-Emergency Healthcare Workers. Int J Environ Res Public Health. 2021. https://doi.org/10.3390/ijerph18030923.
Lombardi A, Mangioni D, Consonni D, Cariani L, Bono P, Cantù AP, et al. Seroprevalence of anti-SARS-CoV-2 IgG among healthcare workers of a large university hospital in Milan, Lombardy, Italy: a cross-sectional study. BMJ Open. 2021. https://doi.org/10.1136/bmjopen-2020-047216.
Moncunill G, Mayor A, Santano R, Jiménez A, Vidal M, Tortajada M, et al. SARS-CoV-2 seroprevalence and antibody kinetics among health care workers in a Spanish hospital after 3 months of follow-up. J Infect Dis. 2021;223(1):62–71. https://doi.org/10.1093/infdis/jiaa696.
Pan SC, Huang YS, Hsieh SM, Chen YC, Chang SY, Chang SC. A cross-sectional seroprevalence for COVID-19 among healthcare workers in a tertially care hospital in Taiwan. J Formos Med Assoc. 2021. https://doi.org/10.1016/j.jfma.2021.01.002.
Pereckaitė L, Dambrauskienė A, Urbonienė D, Sadauskas S, Petrikonis K, Naudžiūnas A, et al. SARS-CoV-2 seroprevalence in healthcare workers of kaunas hospitals during the first wave of the COVID-19 pandemic. Medicina. 2021. https://doi.org/10.3390/medicina57020148.
Rogawski McQuade ET, Guertin KA, Becker L, Operario D, Gratz J, Guan D, et al. Assessment of seroprevalence of SARS-CoV-2 and risk factors associated with COVID-19 infection among outpatients in Virginia. JAMA Netw Open. 2021;4(2): e2035234. https://doi.org/10.1001/jamanetworkopen.2020.35234.
Venugopal U, Jilani N, Rabah S, Shariff MA, Jawed M, Mendez Batres A, et al. SARS-CoV-2 seroprevalence among health care workers in a New York City hospital: a cross-sectional analysis during the COVID-19 pandemic. Int J Infect Dis. 2021;102:63–9. https://doi.org/10.1016/j.ijid.2020.10.036.
Malagón-Rojas JN, Mercado-Reyes M, Toloza-Pérez YG, Barrera ELP, Palma M, Muñoz E, et al. Seroprevalence of the SARS-CoV-2 antibody in healthcare workers: a multicentre cross-sectional study in 10 Colombian cities. Occup Environ Med. 2021. https://doi.org/10.1136/oemed-2021-107487.
Poustchi H, Darvishian M, Mohammadi Z, Shayanrad A, Delavari A, Bahadorimonfared A, et al. SARS-CoV-2 antibody seroprevalence in the general population and high-risk occupational groups across 18 cities in Iran: a population-based cross-sectional study. Lancet Infect Dis. 2021;21(4):473–81.
Poulikakos D, Sinha S, Kalra PA. SARS-CoV-2 antibody screening in healthcare workers in a tertiary centre in North West England. J Clin Virol. 2020;129: 104545.
Amendola A, Tanzi E, Folgori L, Barcellini L, Bianchi S, Gori M, et al. Low seroprevalence of SARS-CoV-2 infection among healthcare workers of the largest children hospital in Milan during the pandemic wave. Infect Control Hosp Epidemiol. 2020;41(12):1468–9.
Brandstetter S, Roth S, Harner S, Buntrock-Döpke H, Toncheva AA, Borchers N, et al. Symptoms and immunoglobulin development in hospital staff exposed to a SARS-CoV-2 outbreak. Pediatr Allergy Immunol. 2020;31(7):841–7.
Chibwana MG, Jere KC, Kamngona R, Mandolo J, Katunga-Phiri V, Tembo D, et al. High SARS-CoV-2 seroprevalence in health care workers but relatively low numbers of deaths in urban Malawi. medRxiv. 2020. https://doi.org/10.1101/2020.07.30.20164970.
Acknowledgements
Not applicable.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
AA conceptualized the idea for this review, formulated the review question and objectives, assisted with the development of the final search strategy, contributed to the data analysis/interpretation, and writing the manuscript. YM, MA, and AM contributed to the conceptualization of the final review question, formulation of the review objectives, data analysis/interpretation, and writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This work was recorded in the Research of Kurdistan University of Medical Sciences.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Azami, M., Moradi, Y., Moradkhani, A. et al. SARS-CoV-2 seroprevalence around the world: an updated systematic review and meta-analysis. Eur J Med Res 27, 81 (2022). https://doi.org/10.1186/s40001-022-00710-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-022-00710-2