Abstract
Introduction
Coronavirus Disease-2019 (SARS-CoV-2) started its devastating trajectory into a global pandemic in Wuhan, China, in December 2019. Ever since, several variants of SARS-CoV-2 have been identified. In the present review, we aimed to characterize the different variants of SARS-CoV-2 and explore the related morbidity and mortality.
Methods
A systematic review including the current evidence related to different variants of SARS-CoV-2 and the related morbidity and mortality was conducted through a systematic search utilizing the keywords in the online databases including Scopus, PubMed, Web of Science, and Science Direct; we retrieved all related papers and reports published in English from December 2019 to September 2020.
Results
A review of identified articles has shown three main genomic variants, including type A, type B, and type C. we also identified three clades including S, V, and G. Studies have demonstrated that the C14408T and A23403G alterations in the Nsp12 and S proteins are the most prominent alterations in the world, leading to life-threatening mutations.The spike D614G amino acid change has become the most common variant since December 2019. From missense mutations found from Gujarat SARS-CoV-2 genomes, C28854T, deleterious mutation in the nucleocapsid (N) gene was significantly associated with patients' mortality. The other significant deleterious variant (G25563T) is found in patients located in Orf3a and has a potential role in viral pathogenesis.
Conclusion
Overall, researchers identified several SARS-CoV-2 variants changing clinical manifestations and increasing the transmissibility, morbidity, and mortality of COVID-19. This should be considered in current practice and interventions to combat the pandemic and prevent related morbidity and mortality.
Similar content being viewed by others
Introduction
In December 2019, a novel coronavirus (CoV) emerged from Hubei province in China among people visiting the Huanan seafood market in Wuhan. The virus is transmitted through human-to-human contact and rapidly spread across the world, and soon turned into a pandemic [1, 2]. Its symptoms can be divided to two main groups of majors (fever, cough, dyspnea) and minors (anosmia, dysgeusia, headache, gastrointestinal symptoms, skin lesions) [3,4,5,6]. Ever since, it has caused worldwide social and economic disruption. There are no universally recognized clinically useful antiviral drugs against COVID-19 so far (actually remdesivir showed good efficacy in many trials, but it needs to be discussed more). Although there are several effective vaccines to prevent infection with SARS-CoV-2, efforts to develop medications and vaccines are continuing [7].
It appeared that there are several variants of SARS-CoV-2. Since the beginning of the pandemic; there have been tremendous efforts to determine the genetic diversity of virus and discover the variations such as immune targets change (such as the spike glycoprotein), primer-binding and probe-binding sites change (which can reduce the sensitivity of diagnostic tests), and genetic variations (which might affect transmissibility and virulence) [8,9,10,11].
SARS-CoV-2 genome consists of approximately 29,903 nucleotides and organized in the following order from 5' to 3': open reading frame (ORF) 1ab (replicas), structural spike glycoprotein (S), ORF3a protein, a structural envelope protein (E), structural membrane glycoprotein (M), ORF6 protein, ORF7a protein, ORF7b protein, ORF8 protein, structural nucleocapsid-phosphoprotein (N), and ORF10 protein. ORF1ab is a large polyprotein (∼ 21,291 nucleotides) encoding sixteen non-structural proteins: leader protein, nsp2, nsp3, nsp4, 3C-like proteinase, nsp6, nsp7, nsp8, nsp9, nsp10, RNA-dependent RNA polymerase (RdRp), helicase, 3'–5' exonuclease, endoRNAse, 2'-o-ribose methyltransferase, and nsp11 [12].
Different variants have been discovered besides the wild-type, with a certain amount of nucleotide deletion in the ORF8 (which is the biological function of the ORF8 protein in SARS-CoV-2 remains unclear). The most known one is the ∆382 variant, a 382-nucleotide deletion in the ORF7b and ORF8, removing its transcription-regulatory sequence (this omission stops ORF8 transcription). This variant was successfully early transmitted during the epidemic, but was unknown until March 2020. These different variants have been found around the world in countries such as Bangladesh (345 nucleotides), Australia (138 nucleotides), and Spain (62 nucleotides). An identical Δ382 variant was also detected in February 2020 in a traveler who returned from Wuhan, China, to Taiwan [13, 14].
In the last pandemic of 2002–2003, the SARS-CoV was responsible for zoonotic transmission from civets to humans. After a short time, the virus's wild-type mutated, and a new variant emerged, which had a 29-nucleotide deletion in the ORF8, known as the Δ29. Subsequently, there were some reports of 82-nucleotide deletion and 415-nucleotide deletions in the same region. The influence of these deletions on the pandemic is still unknown. But some in vitro studies have shown that Δ29 replicates less efficiently and causes milder clinical illness than the wild-type [15, 16].
The relations between the magnitude of nucleotide deletion in ORF8 with its virulence and the ORF8 function are still unknown. However, a recent study suggested that ORF8 mediates immune evasion by downregulating MHC-I molecules. In vitro studies have also shown that these deletions do not affect replicative fitness, but it can affect the transcription of some essential and defensive regions such as ORF6 and N genes (known as SARS-CoV interferon antagonists); thus, it can create a more fragile variant compared to the wild-type [17,18,19].
Besides ORF8, other genome parts can be affected by the mutations, and new variants could emerge. Studies have revealed that the highly mutable spike (S) protein of the virus is associated with the elevated human-to-human transmission rate through interaction with the host's ACE2 receptor. S protein is one of the well-characterized proteins of the Coronaviridae family; this ∼ 1255 amino acid transmembrane protein helps the virus to attach and enter the host [12, 20].
There are also reports about the mutations in other parts such as nsp2 and nsp12 (RdRp). The SARS-CoV-2 nsp12 is RNA-dependent RNA polymerase (RdRp) consisting of 932 amino acids located in the polyprotein, from 4393 to 5324 aa. Structurally, the SARS-CoV-2 nsp12 protein is categorized into N-terminal (1–397aa) and a polymerase domain (398–919aa). These mutations have been observed in patients from India, Germany, and Iran [20].
In this article, we aimed to report on and compare the morbidity and mortality of the different variants of SARS-CoV-2 with that of the wild-type to realize whether they could be an immense threat to humans.
Methods
This study is a systematic review of current evidence conducted in September 2020. The authors aimed to study the effect of different variants of SARS-CoV-2 on mortality and morbidity. Our study is consistent with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist to ensure reported results' reliability and validity.
Sources of data
We retrieved all the relevant papers and reports published in English from December 2019 to September 2020 through a systematic search using keywords in the online databases of PubMed, Web of Science, Scopus, and Science Direct. We updated our search on late February 2021. Our search strategy employed multiple combinations of keywords, as follows:
-
"SARS-CoV-2" OR "Coronavirus" OR "COVID-19" OR "2019-nCoV" OR "Novel Coronavirus"[Title/abstract]
-
"Variants "OR "Variation" OR "Strains" OR "Types" OR "Minority Variants" OR "Genomic Variants" OR "Genetic Variation" OR "Genomic Diversity"OR "Characterizations" [Title/abstract]
-
[A] and [B]
Selection of the study
Three independent investigators reviewed the extracted papers' full text and selected the most pertinent papers according to the eligibility criteria. Then we pulled the relevant data and organized them in some tables. We included the original and peer-reviewed English papers fulfilling the eligibility criteria in the final report. Besides, the following exclusion criteria were applied in the present study:
-
Non-human studies, including animal experiments, in vitro observations, or papers with a limited report on COVID-19, and those without reference to this review's keywords.
-
Papers with inaccessibility to their full texts.
-
Any duplicated and suspicious outcomes in databases.
Extraction of data
The authors’ names, publication date, article types (e.g., case reports), country of origin, sample size, gender, age, and clinical symptoms were recorded in an information sheet. This information was collected by two independent researchers and subsequently organized in tables. All authors cross-checked the selected articles to avoid any duplications or overlap in the content.
Assessment of quality
This study adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist to ensure the quality and accuracy of selected papers and outcomes. Two independent researchers examined the quality of the articles and the probable risk of bias. A third researcher addressed any disagreement. The full text of selected articles was read, and the key findings are summarized in tables.
Results
We identified a total of 56 relevant articles by title and abstract. Of 56 articles, 50 were related to the genomic variations in SARS-CoV-2. The included studies were conducted in 16 countries, and one of the articles was a report on multinational scientific collaborations [1] (Table 1 illustrates a summary of the findings). We summarized each study’s main findings in the two categories: genomic variants and other results.
Studies have revealed that the highly mutable spike (S) protein of the virus is associated with the elevated human-to-human transmission rate through interaction with the host’s ACE2 receptor. A review of identified articles has shown three main genomic variants, including type A, type B, and type C. we also identified three clades including S, V, and G. Studies have demonstrated that the C14408T and A23403G alterations in the Nsp12 and S proteins are the most prominent alterations in the world, leading to miserable mutations.The spike D614G amino acid change has become the most common variant since December 2019. From missense mutations found from Gujarat SARS-CoV-2 genomes, C28854T, deleterious mutation in the nucleocapsid (N) gene was significantly associated with patients’ mortality. The other significant deleterious variant (G25563T) is found in patients located in Orf3a and has a potential role in viral pathogenesis.
Discussion
Since the emergence of COVID-19, understanding the virus behavior has been received much attention from the scientific community. The different viral behavior has been attributed to the virus’s difference in types and strains [75]. Therefore, in the present review, we characterized the different variants of SARS-CoV-2 and discussed the findings in four sections, including the different types of SARS-CoV-2 variants, their effects on viral transmission, clinical manifestations, morbidity as well as mortality, and the other relevant findings.
Different variants and strains
This review has focused on the different variations of SARS-CoV-2 and their impact on virus behavior. Alternation of the SARS-CoV-2 genome, through mutation and recombination, potentially leads to changes in the viral life cycle, including transitivity, cellular tropism, and severity of the disease. The diverse clinical outcomes in COVID-19 patients happen due to SARS-CoV-2 genome mutations. The mutation of single-stranded RNA viruses is much faster than the human genome's mutation rate, about 10–6–10–4 and 10–8, respectively [76, 77]. This leads to numerous quasi-species in each infected one, which may justify the observed difference in symptoms and disease severity [78]. Altered ACE2 binding interactions or shifted tissue tropism may happen due to a mutation among viral progeny that causes aggressive and immense infections [20].
Evolutionary benefits such as changing a primary epitope to escape from the host immune system or changing virulence factors to enhance transmission of the virus can occur due to gene mutations. Natural selection or vaccine selective pressure can cause these mutations and subsequently lead to new viral strains [79]. Preliminary studies at the beginning of the outbreak identified two major genotypes of SARS-CoV-2 among a Chinese population, type Ι, and type ΙΙ [18]. The prevalence of the aggressive form had decreased in the early months due to the start of treatment, and its mild form became the common variant [24].
Further studies reported the identification of three major variant types (A, B, C) of SARS-CoV-2, based on amino acid changes [22]. Forster et al. confirmed those three major variant types by phylogenetic analysis of 160 viral genomes [32]. Interestingly, variant A is the conventional type; type B viruses prevailed in East Asia, while both type A and C viruses have been dominant in America and Europe. After two mutations, including the synonymous mutation T8782C and the non-synonymous mutation C28144T, by replacing serine with leucine in type A, type B is formed. Type C is also derived from type B by the non-synonymous mutation G26144T, in which valine replaces glycine [32, 80, 81]. In other words, the S variant (Type A) with two mutations at 8782C>T and 28144 T>C was mainly identified in East Asia. Still, outside Asia, significant and long mutations were observed with the length of the branches. The G variant was dominant in Europe and was rare in Asia [70]. Bhowmik et al. reported two D and E subgrouping of the influential group A. Moreover, they stated that the SARS-CoV-2 genome is around 29,903 nucleotides. The highly mutable spike (S) protein of the virus is probably related to the increased human-to-human transmission rate through interaction with the host's ACE2 receptor [20]. Ugurel et al. reported C14408T variant on Nsp12 and A23403G variation on S protein, and both cause significant mutations and changes in virus variants worldwide [66].
Recent studies around the world have identified eight strains of SARS-CoV-2. However, they have a significant sequence similarity [50]. Also, Liu et al. have been recognized four distinct groups of common mononucleotide types (SNVs) in more than 28,000 high-quality, high-coverage SARS-CoV-2 complete genome sequences, demonstrating different viral strains [46]. These reports are consistent with the findings of two studies in Italy and the United States, where about 4–10 non-synonymous stable mutations were reported in the SARS genome [11, 50]. Eke, one of the mutations in S protein (D614G), has been seen repeatedly in Europe and the United States since the onset of the infection, apparently because it has dramatically increased the transmission ability of SARS-CoV-2. Thus, it became the most common variant [41, 56].
Although the mutation of the SARS-CoV-2 appears to be stable, consecutive consideration of virus mutations remains essential. A large study by Poterico and colleagues characterized two novel mutations in the S region across 691 complete viral genomes of SARS-CoV-2 from around the world. They also highlighted that the virus had acquired about 27 mutations, and most of South American countries’ trains are nearly related to European viral isolates [57]. Meanwhile, a unique mutation 24351C (A930V (T)) in the spike surface glycoprotein was reported in one of the Wuhan strains in India [24]. In a study conducted in Singapore, the cause of SARS attenuation was attributed to the 382-nucleotide deletion in ORF8 of the viral genome [23]. In a survey conducted in mid-March in Mexico, evidence of local translocation of strains with an H49Y mutation in Spike protein strains was reported [63]. According to the findings of Castello et al., the first three cases of ORF amino acid are classified as S type in position 28 144; nevertheless, the fourth case is a G type in position 23 403 [29].
The effect of the variants on the viral transmission
Several studies have reported the association between the transmissibility and different variants and mutations [18, 20, 25, 27, 44, 51, 56, 57, 63, 75]. Some mutations facilitate the transmission of SARS-CoV-2 between animal species and humans. The G-U transversion excess might play a role in the bat to human transmission [51]. Besides, the S1/S2 junction region's specific motif may have caused the viral exchange between species [44].
Regarding the viral transmission between humans, some fundamental facts are noteworthy. The rapid viral replication might cause rapid morbidity and mortality and hinder the viral passage to healthy individuals. Viruses causing slower replications and asymptomatic or mild disease can allow the transmission for a more extended period [27]. Furthermore, mutations altering viral structure might increase virulence or helping the pathogen escape the immune system, resulting in higher transmission rates [75]. Type II SARS-CoV-2 strains possibly spreading through the Huanan seafood market were also considered more prevalent than type I viruses, probably due to being more contagious [18]. Several mutations are proposed to increase transmissibility [20, 57, 63, 75]. Variants possessing immensely mutable S proteins might be more contagious due to their interaction with the host ACE2 [20]. Concurrently, a specific mutation (D614G) in the S protein might speed up the viral transmission [75, 82]. The role of QHD43416 p.Asp614Gly variant in many strains is controversial and not fully understood [56, 82]. H49Y mutation in the S protein may also be responsible for local transmissions in earlier stages. It was proposed as a potential marker to trace the viral spreading between the countries and regions [63]. ORF1ab (nt8750) and N (nt29063) are also the identified responsible genes for the higher transmissibility of clade G strain [57].
Variant effects on symptoms, morbidity, and mortality
Pneumonia and lung involvement is often the main clinical sign of COVID-19. Recent evidence also demonstrates gastrointestinal symptoms and asymptomatic infections [83]. The percentage of people infected with the coronavirus who remain asymptomatic during infection has not yet been accurately assessed and reported. Symptomatic patients often have clinical symptoms of fatigue, cough, nasal congestion, fever, and other signs of upper respiratory tract infections that usually appear after a week. The condition can develop into severe disease with dyspnea and severe chest symptoms [84, 85], and the respiratory tract infections are known as the primary clinical signs of COVID-19 [86].
In COVID-19, pneumonia usually manifests in the second or third week of symptomatic infection. Prominent signs of viral pneumonia include reduced oxygen saturation, blood gas deviations. Changes can be seen through chest X-rays and other imaging techniques, leading to the deterioration of vital signs and death. Lymphopenia (abnormally low level of lymphocytes in the blood) is common in these patients, and inflammatory markers (C-reactive protein and pro-inflammatory cytokines) could also increase [85, 87]. Furthermore, specific genetic mutations in the coronavirus can even increase mortality [85, 87].
Other relevant findings
COVID-19 mortality rates differ substantially depending on the country. This difference in mortality rates depends on various factors in each country, including the adequacy of health care delivery, political decisions, and epidemiological characteristics of the affected population. The frequency of diagnostic and screening measures in asymptomatic or mildly symptomatic patients may also affect morbidity and mortality [88, 89].
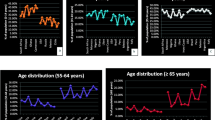
Studies have demonstrated a steadfast and transparent pattern of age-based exponential enhancement in mortality, regardless of geographic area, in patients with COVID-19. Age-related mortality changes are relatively common for COVID-19 because other significant causes of mortality, especially chronic diseases such as cardiovascular disease, could also be increased by advanced ages [90]. Promislow et al. have shown that the mortality rate doubling time (MRDT) of all-cause fatality 9 years in the United States was close to that of COVID-19 in New York City [91]. In other words, there is no significant relationship between age and increased death in patients with COVID-19. However, many scientists and the media have paid particular attention to age as a risk factor for mortality in COVID-19. Nonetheless, the age-related pattern of death from COVID-19 is different from other respiratory viral infections. The pattern of morbidity and mortality is higher in the elderly than in young people [90].
Limitations
Although this systematic review produces valuable knowledge regarding the COVID-19 variants and related morbidity and mortality, there were some shortcomings. First, the number of published reports is still limited. The knowledge regarding the different strains and variants and their effects on the symptoms, morbidity, and mortality is not entirely described yet. Furthermore, various countries ought to report their data to identify the worldwide distribution of these variants. Researchers might also strive to discover various novel mutations resulting in different viral behaviors in the future.
Conclusion
Overall, researchers identified several SARS-CoV-2 variants changing clinical manifestations and increasing the transmissibility, morbidity, and mortality of COVID-19; however, many observations produced controversial results. Variants with asymptomatic disease or milder disease can increase their transmission by extending the duration of contact between sick and healthy people. Mutations causing increased virulence and immune escapes might also cause an elevated transmissibility level. As the vaccine inoculations are increasing worldwide, we encourage researching for potential mutations that might escape vaccine-induced immunity. The current practice and interventions should consider these findings to combat the COVID-19 pandemic and prevent related morbidity and mortality.
Availability of data and materials
The authors stated that all information provided in this article could be shared.
References
Esmaeil M, Farzane B, Mohammad AS, Tayebeh N, Hamid H, SeyedAhmad S. Olfactory and gustatory dysfunctions due to the coronavirus disease (COVID-19) a review of current evidence. Eur Arch Oto-Rhino-Laryngol. 2020. https://doi.org/10.1007/s00405-020-06120-6.
Mehraeen E, Hayati B, Saeidi S, Heydari M, Seyed AS. Self-care instructions for people not requiring hospitalization for coronavirus disease 2019 (COVID-19). Arch Clin Infect Dis. 2020. https://doi.org/10.5812/archcid.102978.
De Vito A, Fiore V, Princic E, Geremia N, Panu Napodano CM, Muredda AA, et al. Predictors of infection, symptoms development, and mortality in people with SARS-CoV-2 living in retirement nursing homes. PLoS ONE. 2021;16(3):e0248009-e.
De Vito A, Fau GN, Fiore V, Fau FV, Princic E, Fau PE, Babudieri S, Fau BS, Madeddu G, Madeddu G. Clinical features, laboratory findings and predictors of death in hospitalized patients with COVID-19 in Sardinia Italy. Eur Rev Med Pharmacol Sci. 2020;24(14):7861–8.
Vaira LA-OX, Deiana G, Fois AG, Pirina P, Madeddu G, De Vito A, et al. Objective evaluation of anosmia and ageusia in COVID-19 patients: single–center experience on 72 cases. Head neack. 2020;42:1252–8.
Zinellu A, Arru F, De Vito A, Sassu A, Valdes G, Scano V, et al. The De Ritis ratio as prognostic biomarker of in-hospital mortality in COVID-19 patients. Eur J Clin Invest. 2021;51(1):13427.
SeyedAlinaghi S, Ghadimi M, Hajiabdolbaghi M, Rasoolinejad M, Abbasian L, Nezhad MH, et al. Prevalence of COVID-19-like symptoms among people living with HIV, and using antiretroviral therapy for prevention and treatment. Curr HIV Res. 2020;18:373–80.
Jary A, Leducq V, Malet I, Marot S, Klement-Frutos E, Teyssou E, et al. Evolution of viral quasispecies during SARS-CoV-2 infection. Clin Microbiol Infect. 2020;26(11):1560-e1.
Álvarez-Díaz DA, Franco-Muñoz C, Laiton-Donato K, Usme-Ciro JA, Franco-Sierra ND, Flórez-Sánchez AC, et al. Molecular analysis of several in-house rRT-PCR protocols for SARS-CoV-2 detection in the context of genetic variability of the virus in Colombia. Infect Genet Evolut. 2020;84:104390.
Lokman SM, Rasheduzzaman M, Salauddin A, Barua R, Tanzina AY, Rumi MH, et al. Exploring the genomic and proteomic variations of SARS-CoV-2 spike glycoprotein: a computational biology approach. Infect Genet Evolut. 2020;84:104389.
Korber B, Fischer WM, Gnanakaran S, Yoon H, Theiler J, Abfalterer W, et al. Tracking changes in SARS-CoV-2 Spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182:812–27.
Wu F, Zhao S, Yu B, Chen Y-M, Wang W, Song Z-G, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–9.
Su YCF, Anderson DE, Young BE, Linster M, Zhu F, Jayakumar J, et al. Discovery and genomic characterization of a 382-nucleotide deletion in ORF7b and ORF8 during the early evolution of SARS-CoV-2. MBio. 2020. https://doi.org/10.1128/mBio.01610-20.
Gong Y-N, Tsao K-C, Hsiao M-J, Huang C-G, Huang P-N, Huang P-W, et al. SARS-CoV-2 genomic surveillance in Taiwan revealed novel ORF8-deletion mutant and clade possibly associated with infections in middle East. Emerg Microb Infect. 2020;9(1):1457–66.
Consortium CSME. Molecular evolution of the SARS coronavirus during the course of the SARS epidemic in China. Science. 2004;303(5664):1666–9.
Cui J, Li F, Shi Z-L. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2018;17(3):181–92.
Muth D, Corman VM, Roth H, Binger T, Dijkman R, Gottula LT, et al. Attenuation of replication by a 29 nucleotide deletion in SARS-coronavirus acquired during the early stages of human-to-human transmission. Sci Rep. 2018;8(1):1–1.
Zhang L, Yang J-R, Zhang Z, Lin Z. Genomic variations of SARS-CoV-2 suggest multiple outbreak sources of transmission. medRxiv. 2020;7:154.
Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, White KM, et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583(7816):459–68.
Bhowmik D, Pal S, Lahiri A, Talukdar A, Paul S. Emergence of multiple variants of SARS-CoV-2 with signature structural changes. bioRxiv. 2020;91:157.
Alouane T, Laamarti M, Essabbar A, Hakmi M, Bouricha EM, Chemao-Elfihri M, et al. Genomic diversity and hotspot mutations in 30,983 SARS-CoV-2 genomes: moving toward a universal vaccine for the “confined virus”? Pathogens. 2020;9(10):829.
Al-Tawfiq JA, Leonardi R, Fasoli G, Rigamonti D. Prevalence and fatality rates of COVID-19: what are the reasons for the wide variations worldwide? Travel Med Infect Dis. 2020;35:101711.
Armengaud J, Delaunay-Moisan A, Thuret JY, Van Anken E, Acosta-Alvear D, Aragón T, et al. The importance of naturally attenuated Sars-Cov-2 in the fight against Covid-19. Environ Microbiol. 2020;22(6):1997–2000.
Bajaj A, Purohit HJ. Understanding SARS-CoV-2: genetic diversity, transmission and cure in human. Indian J Microbiol. 2020;160:398–401.
Laha S, Chakraborty J, Das S, Manna SK, Biswas S, Chatterjee R. Characterizations of SARS-CoV-2 mutational profile, spike protein stability and viral transmission. Infect Genet Evolut. 2020;85:104445.
Biswas SK, Mudi SR. Genetic variation in SARS-CoV-2 may explain variable severity of COVID-19. Med Hypotheses. 2020;143:109877.
Blackstone NW, Blackstone SR, Berg AT. Variation and multilevel selection of SARS-CoV-2. Evolution. 2020;74:2429–34.
Canhui C, Huang L, Liu K, Ma K, Tian Y, Qin Y, et al. Amino acid variation analysis of surface spike glycoprotein at 614 in SARS-CoV-2 strains. Genes Dis. 2020;7:567–77.
Castillo AE, Parra B, Tapia P, Acevedo A, Lagos J, Andrade W, et al. Phylogenetic analysis of the first four SARS-CoV-2 cases in Chile. J Med virol. 2020;92:1562–6.
Everett J, Hokama P, Roche AM, Reddy S, Hwang Y, Kessler L, et al. SARS-CoV-2 genomic variation in space and time in hospitalized patients in philadelphia. MBio. 2021. https://doi.org/10.1128/mBio.03456-20.
Forni D, Cagliani R, Pontremoli C, Mozzi A, Pozzoli U, Clerici M, et al. Antigenic variation of SARS-CoV-2 in response to immune pressure. Mol Ecol. 2020;9:7861.
Forster P, Forster L, Renfrew C, Forster M. Phylogenetic network analysis of SARS-CoV-2 genomes. Proc Natl Acad Sci. 2020;117(17):9241–3.
Gómez-Carballa A, Bello X, Pardo-Seco J, Martinón-Torres F, Salas A. Mapping genome variation of SARS-CoV-2 worldwide highlights the impact of COVID-19 super-spreaders. Genome Res. 2020;30(10):1434–48.
Goren A, Wambier C, McCoy J, Shapiro J, Vaño-Galván S, Herrera S, et al. Clock genes may drive seasonal variation in SARS-CoV-2 infectivity: are we due for a second wave of COVID-19 in the fall? J Biol Regul Homeost Agents. 2020;34(4):1455–7. https://doi.org/10.23812/20-359-L-35.
Graudenzi A, Maspero D, Angaroni F, Piazza R, Ramazzotti D. Mutational signatures and heterogeneous host response revealed via large-scale characterization of SARS-CoV-2 genomic diversity. bioRxiv. 2020;110:375.
Islam OK, Al-Emran HM, Hasan MS, Anwar A, Jahid MIK, Hossain MA. Emergence of European and north American mutant variants of SARS-CoV-2 in South–East Asia. Transbound Emerg Dis. 2020;68:824–32.
Jain A, Rophina M, Mahajan S, Krishnan BB, Sharma M, Mandal S, et al. Analysis of the potential impact of genomic variants in SARS-CoV-2 genomes from India on molecular diagnostic assays. bioRxiv. 2020;7:200636.
Joshi M, Puvar A, Kumar D, Ansari A, Pandya M, Raval J, et al. Genomic variations in SARS-CoV-2 genomes from Gujarat: underlying role of variants in disease epidemiology. bioRxiv. 2020;555:549.
Junejo Y, Ozaslan M, Safdar M, Khailany RA, Rehman S, Yousaf W, et al. Novel SARS-CoV-2/COVID-19: origin, pathogenesis, genes and genetic variations, immune responses and phylogenetic analysis. Gene Rep. 2020;20:100752.
Kouriba B, Dürr A, Rehn A, Sangaré AK, Traoré BY, Bestehorn-Willmann MS, et al. First phylogenetic analysis of Malian SARS-coV-2 sequences provides molecular insights into the genomic diversity of the Sahel region. Viruses. 2020;12(11):1251.
Koyama T, Platt D, Parida L. Variant analysis of SARS-CoV-2 genomes. Bull World Health Organ. 2020;98(7):495.
Kozlovskaya L, Piniaeva A, Ignatyev G, Selivanov A, Shishova A, Kovpak A, et al. Isolation and phylogenetic analysis of SARS-CoV-2 variants collected in Russia during the COVID-19 outbreak. Int J Infect Dis. 2020;99:40–6.
Latini A, Agolini E, Novelli A, Borgiani P, Giannini R, Gravina P, et al. COVID-19 and genetic variants of protein involved in the SARS-CoV-2 entry into the host cells. Genes. 2020;11(9):1010.
Lau S-Y, Wang P, Mok BW-Y, Zhang AJ, Chu H, Lee AC-Y, et al. Attenuated SARS-CoV-2 variants with deletions at the S1/S2 junction. Emerg Microb Infect. 2020;9(1):837–42.
Lee JW, Lee IH, Sato T, Kong SW, Iimura T. Genetic variation analyses indicate conserved SARS-CoV-2-host interaction and varied genetic adaptation in immune-response factors in modern human evolution. Dev Growth Differ. 2021;63:219–27.
Liu S, Shen J, Fang S, Li K, Liu J, Yang L, et al. Genetic spectrum and distinct evolution patterns of SARS-CoV-2. medRxiv. 2020;5:536.
Lokman SM, Rasheduzzaman M, Salauddin A, Barua R, Tanzina AY, Rumi MH, et al. Exploring the genomic and proteomic variations of SARS-CoV-2 spike glycoprotein a computational biology approach. Infect Genet Evol. 2020;84:104389.
Muhammad Ansori AN, Dhea Kharisma V, Sabilil Muttaqin S, Antonius Y, Parikesit AA. Genetic variant of SARS-CoV-2 isolates in Indonesia: spike glycoprotein gene. J Pure Appl Microbiol. 2020;14(1):971–8.
Mukherjee M, Goswami S. Global cataloguing of variations in untranslated regions of viral genome and prediction of key host RNA binding protein-microRNA interactions modulating genome stability in SARS-CoV-2. PLoS ONE. 2020;15(8):e0237559.
Pachetti M, Marini B, Benedetti F, Giudici F, Mauro E, Storici P, et al. Emerging SARS-CoV-2 mutation hot spots include a novel RNA-dependent-RNA polymerase variant. J Transl Med. 2020;18:1–9.
Panchin AY, Panchin YV. Excessive G–U transversions in novel allele variants in SARS-CoV-2 genomes. PeerJ. 2020;8:e9648.
Pardo-Seco J, Gómez-Carballa A, Bello X, Martinón-Torres F, Salas A. Pitfalls of barcodes in the study of worldwide SARS-CoV-2 variation and phylodynamics. Zool Res. 2021;42(1):87.
Parlikar A, Kalia K, Sinha S, Patnaik S, Sharma N, Vemuri SG, et al. Understanding genomic diversity, pan-genome, and evolution of SARS-CoV-2. Peer J. 2020;8:e9576.
Peñarrubia L, Ruiz M, Porco R, Rao SN, Juanola-Falgarona M, Manissero D, et al. Multiple assays in a real-time RT-PCR SARS-CoV-2 panel can mitigate the risk of loss of sensitivity by new genomic variants during the COVID-19 outbreak. Int J Infect Dis. 2020;97:225–9.
Peñarrubia L, Ruiz M, Porco R, Rao SN, Vella SA, Juanola-Falgarona M, et al. In response to: multiple assays in a real-time RT-PCR severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) panel can mitigate the risk of loss of sensitivity by new genomic variants during the COVID-19 outbreak. Intern J Infect Dis. 2021;105(241):2.
Portelli S, Olshansky M, Rodrigues CHM, D’Souza EN, Myung Y, Silk M, et al. Exploring the structural distribution of genetic variation in SARS-CoV-2 with the COVID-3D online resource. Nat Genet. 2020;52:999–1001.
Poterico JA, Mestanza O. Genetic variants and source of introduction of SARS-CoV-2 in South America. J Med Virol. 2020;10:2139–45.
Romero PE. Comment on “Genetic variants and source of introduction of SARS-CoV-2 in South America.” J Med Virol. 2020;93:28–9.
Sapoval N, Mahmoud M, Jochum M, Liu Y, Elworth RL, Wang Q, et al. Hidden genomic diversity of SARS-CoV-2: implications for qRT-PCR diagnostics and transmission. Genome Res. 2021;31:635–44.
Sarkar R, Mitra S, Chandra P, Saha P, Banerjee A, Dutta S, et al. Comprehensive analysis of genomic diversity of SARS-CoV-2 in different geographic regions of India: an endeavour to classify Indian SARS-CoV-2 strains on the basis of co-existing mutations. Adv Virol. 2021;166(3):801–12.
Shen Z, Xiao Y, Kang L, Ma W, Shi L, Zhang L, et al. Genomic diversity of severe acute respiratory syndrome-coronavirus 2 in patients with coronavirus disease. Clin Infect Dis. 2020;4:536.
Singh PK, Kulsum U, Rufai SB, Mudliar SR, Singh S. Mutations in SARS-CoV-2 leading to antigenic variations in spike protein: a challenge in vaccine development. J Lab Phys. 2020;12(2):154–60.
Taboada B, Vazquez-Perez JA, Muñoz-Medina JE, Ramos-Cervantes P, Escalera-zamudio M, Boukadida C, et al. Genomic analysis of early SARS-coV-2 variants introduced in Mexico. J Virol. 2020;94(18):e01056–20. https://doi.org/10.1128/JVI.01056-20.
Thielen PM, Wohl S, Mehoke T, Ramakrishnan S, Kirsche M, Falade-Nwulia O, et al. Genomic diversity of SARS-CoV-2 during early introduction into the united states national capital region. MedRxiv. 2020;181:997.
Toyoshima Y, Nemoto K, Matsumoto S, Nakamura Y, Kiyotani K. SARS-CoV-2 genomic variations associated with mortality rate of COVID-19. J Hum Genet. 2020;65:1075–82.
Ugurel OM, Ata O, Turgut-Balik D. An updated analysis of variations in SARS-CoV-2 genome. Turkish J Biol Turk Biyoloji Dergisi. 2020;44(3):157–67.
van Dorp L, Acman M, Richard D, Shaw LP, Ford CE, Ormond L, et al. Emergence of genomic diversity and recurrent mutations in SARS-CoV-2. Infect Genet Evol. 2020;83:104351.
Wang C, Liu Z, Chen Z, Huang X, Xu M, He T, et al. The establishment of reference sequence for SARS-CoV-2 and variation analysis. J Med Virol. 2020;92(6):667–74.
Xiao M, Liu X, Ji J, Li M, Li J, Yang L, et al. Multiple approaches for massively parallel sequencing of SARS-CoV-2 genomes directly from clinical samples. Genome Med. 2020;12(1):1–15.
Yap PSX, Tan TS, Chan YF, Tee KK, Kamarulzaman A, Teh CSJ. An Overview of the Genetic Variations of the SARS-CoV-2 genomes isolated in Southeast Asian countries. J Microbiol Biotechnol. 2020;30(7):962–6.
Zhu Z, Liu G, Meng K, Yang L, Liu D, Meng G. Rapid spread of mutant alleles in worldwide SARS-CoV-2 strains revealed by genome-wide single nucleotide polymorphism and variation analysis. Genome Biol Evol. 2021;13(2):15.
Novazzi F, Genoni A, Spezia PG, Focosi D, Zago C, Colombo A, et al. Introduction of SARS-CoV-2 variant of concern 20h/501Y. V2 (B. 1.351) from Malawi to Italy. Emerg Microb Infect. 2021;10(1):710–2.
Faria NR, Mellan TA, Whittaker C, Claro IM, Candido DdS, Mishra S, et al. Genomics and epidemiology of the P. 1 SARS-CoV-2 lineage in Manaus Brazil. Science. 2021;372:815–21.
Ferreira I, Datir R, Papa G, Kemp S, Meng B, Rakshit P, et al. SARS-CoV-2 B 1617 emergence and sensitivity to vaccine-elicited antibodies. bioRxiv. 2021;35:108950.
Ovsyannikova IG, Haralambieva IH, Crooke SN, Poland GA, Kennedy RB. The role of host genetics in the immune response to SARS-CoV-2 and COVID-19 susceptibility and severity. Immunol Rev. 2020;296(1):205–19.
Sanjuán R, Domingo-Calap P. Mechanisms of viral mutation. Cell Mol Life Sci. 2016;73(23):4433–48.
Narasimhan VM, Rahbari R, Scally A, Wuster A, Mason D, Xue Y, et al. Estimating the human mutation rate from autozygous segments reveals population differences in human mutational processes. Nat Commun. 2017;8(1):1–7.
Karamitros T, Papadopoulou G, Bousali M, Mexias A, Tsiodras S, Mentis A. SARS-CoV-2 exhibits intra-host genomic plasticity and low-frequency polymorphic quasispecies. bioRxiv. 2020. https://doi.org/10.1101/2020.03.2020;27:v1.
Karlsson EK, Kwiatkowski DP, Sabeti PC. Natural selection and infectious disease in human populations. Nat Rev Genet. 2014;15(6):379–93.
SeyedAlinaghi S, Mehrtak M, MohsseniPour M, Mirzapour P, Barzegary A, Habibi P, et al. Genetic susceptibility of COVID-19: a systematic review of current evidence. Eur J Med Res. 2021;26(1):46. https://doi.org/10.1186/s40001-021-00516-8.
Cao Y, Li L, Feng Z, Wan S, Huang P, Sun X, et al. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. 2020;6(1):1–4.
Korber B, Fischer WM, Gnanakaran S, Yoon H, Theiler J, Abfalterer W, et al. Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182(4):812-27.e19.
Chan JF-W, Yuan S, Kok K-H, To KK-W, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the novel coronavirus indicating person to person transmission a study of a family cluster. Lancet. 2020;395(10223):514–23.
Asadollahi-Amin A, Hasibi M, Ghadimi F, Rezaei H, SeyedAlinaghi S. Lung Involvement Found on Chest CT Scan in a Pre-Symptomatic Person with SARS-CoV-2 Infection: a Case Report. Trop Med Infect Dis. 2020;5(2):56. https://doi.org/10.3390/tropicalmed5020056.
Velavan TP, Meyer CG. The COVID-19 epidemic. Trop Med Intern Health. 2020;25(3):278.
Sekhavati E, Jafari F, SeyedAlinaghi S, Jamalimoghadamsiahkali S, Sadr S, Tabarestani M, et al. Safety and effectiveness of azithromycin in patients with COVID-19: an open-label randomised trial. Int J Antimicrob Agents. 2020;56(4): https://doi.org/10.1016/j.ijantimicag.2020.106143.
Bermejo-Martin JF, Almansa R, Menéndez R, Mendez R, Kelvin DJ, Torres A. Lymphopenic community acquired pneumonia as signature of severe COVID-19 infection. J Infect. 2020;80(5):e23.
Anderson RM, Heesterbeek H, Klinkenberg D, Hollingsworth TDJTL. How will country-based mitigation measures influence the course of the COVID-19 epidemic? Lancet. 2020;395(10228):931–4.
Kim D-H, Choe YJ, Jeong J-Y. Understanding and interpretation of case fatality rate of coronavirus disease 2019. J Korean Med Sci. 2020. https://doi.org/10.3346/jkms.2020.35.e137.
Kang S-J, Jung SI. Age-related morbidity and mortality among patients with COVID-19. Infect Chemothe. 2020;52(2):154.
Promislow DE. A geroscience perspective on COVID-19 mortality. J Gerontol Ser A. 2020;75(9):e30-3.
Acknowledgements
The present study was conducted in collaboration with Khalkhal University of Medical Sciences, Iranian Institute for Reduction of High-Risk Behaviors, Tehran University of Medical Sciences, and Department of Global Health and Socioepidemiology, Kyoto University.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
The conception and design of the study: EM, SS. Acquisition of data: AK, ZP, OD. Analysis and interpretation of data: PM, FV, AB. Drafting the article: EM, MM, AMA, MH. Revising it critically for important intellectual content: MS, AS, FB, SS. Final approval of the version to be submitted: EM, OD, SJ. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The present study was extracted from the research project with code IR.KHALUMS.REC.1399.001 entitled "Investigation of effective drugs for people affected by Coronavirus disease 2019 (COVID-19) in Imam Khomeyni hospital " conducted at Khalkhal University of Medical Sciences in 2020. We thank all the participants for taking the time to contribute to the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that there is no conflict of interests regarding the publication of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
SeyedAlinaghi, S., Mirzapour, P., Dadras, O. et al. Characterization of SARS-CoV-2 different variants and related morbidity and mortality: a systematic review. Eur J Med Res 26, 51 (2021). https://doi.org/10.1186/s40001-021-00524-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-021-00524-8