Abstract
Recently, focus has been placed on renewable sources, as they can be provided in large quantities at the lowest possible cost, in order to create nanoparticles. One of these sources is Zygnema moss which used in the present investigation to create Copper oxide nanoparticles (CuONPs). Several phenols and flavonoids were identified the extract of Zygnema sp. via analysis of High performance liquid chromatography. These constituents served as reducing and stabilizing agents for CuONPs. Characterization of CuONPs was performed via UV-visible spectrum that demonstrated peak at 252 nm, Transmission electron microscopy that showed spherical CuONPs with mean diameter of 30.06 nm, Fourier transform infrared spectroscopy that confirm that presence of several functional groups aided to formation of CuONPs. The crystallographic pattern of CuONPs was recorded via X-ray diffraction analysis. Antimicrobial potential of CuONPs was compared to copper acetate and antibiotic/antifungal drug. CuONPs exhibited more inhibition zones against S. aureus (32 ± 0.1 mm), E. coli (36 ± 0.1 mm), S. typhi (27 ± 0.2 mm), E. faecalis (37 ± 0.1 mm), C. albicans (34 ± 0.3 mm) than copper acetate and antibiotic/antifungal drug. Promising MIC values of were recorded against S. aureus, E. coli, and S. typhi. CuONPs at 200 ppm inhibited the growth of C. lunata, F. oxysporium, A. flavus, and Mucor circinelloid with inhibtion of 76.92, 73.33, 63.63, and 53.84%, respectively regarded the control 100% growth. The photocatalytic role of CuONPs was recorded for degradation of reactive red (RR195) and reactive blue (RB) dyes with maximum degradation of 84.66% and 90.82%, respectively at 75 min. Moreover, the optimal dyes degradation was 84.66 and 90.82%, respectively at 40 °C.
Similar content being viewed by others
Introduction
The formulation of nanoparticles (NPs) via green methods is an attractive field in several applications. Although there is numerous methods in the synthesis of NPs such chemical and physical, however biological processes represent a promising alternative compared to other methods [1,2,3,4,5]. Biological methods possess many advantages during the synthesis of NPs such as avoidance the generation of chemical pollutants, low cost, and simple process. Bacteria, fungi, plants, and algae become play a vital role in the synthesis of NPs [6, 7]. Investigators highlight the ability of algae as a viable biological source for the creation of NPs. The present investigation focused on the algae in the synthesis of NPs, because as mentioned in other studies, algae characterized by quick growth, high yield of biomass. Besides, algae may develop in both effluent and clean water, which promote its ecological friendliness. These creators characterized by the existence of several constituents such as alkaloids, amino acids, vitamins, sugars, flavonoids are used reducing and stabilizing agents for NPs synthesis [5, 8].
Nanoparticles are most important in many fields, such as medicine, drug delivery, antisensory, tissue biotechnology, cosmetics, and gene engineering utilizations [9]. Copper oxide has drawn the most attention out of all the metal oxide nanoparticles because copper-based compounds have strong biocidal qualities and can be utilized in pesticide formulations as well as other health-related utilizations.
The release of dyes into water bodies causes a number of environmental issues that get worse every year [10]. Accordingly, it is a challenge for investigations to find new or develop approaches for dye degradation [5, 11, 12]. Due to their small band gaps, a wide range of semiconductors and metal oxides function well as photocatalysts under various lighting conditions. For example, CuO is linked to a narrow band gap between 2.1 and 2.71 eV [13].
According to Pourmoslemi et al. [14], CuONPs respond appropriately to mechanical, optical, and photolytic applications. It is well known that algae can accumulate heavy metals and that they can also miraculously change them into more malleable forms. Algae have been expected to serve as model organisms for the production of various types of nanomaterials, especially metallic NPs, due to these alluring properties [15]. Algae such as Sargassum polycystum [16], Cystoseira trinodis and Bifurcaria bifurcate [17], Macrocystis pyrifera [18] were applied for CuONPs synthesis.
In the current decade several attempts from the investigators to treat and management the microbial infections of human as well as plants by metal oxides NPs. Some metal oxides NPs is harmless and revealed activity against pathogens. Some unique features were associated to CuONP such as biocompatibility, small size of particles, high surface area, and great chemical and biological potential which aids to inhibit and kill microbial cells. Activity of formulated CuONPs was recorded against wide array of pathogenic bacterial including gram-positive and gram-negative such as Clostridium difficile, Pseudomonas aeruginosa, Escherichia coli, and Staphylococcus aureus [19].
According to El-Batal et al. [20] study, growth of plant pathogenic fungi such as Alternaria solani, Fusarium oxysporum, Penicillium citrinum, and Aspergillus niger were influenced by CuONPs. Other destructive crop fungi such as and Phoma destructive, Curvularia lunata and Alternaria alternata were inhibited by green synthesized CuONPs [21].
According to Sandesh et al. [22], about 10,000 kinds of dyes are manufactured across the world. These dyes if released to the water sources, the entrance of sunlight into the water are decrease; and therefore it suppresses the biota growth, gas solubility and photosynthesis. Reactive blue (RB49) and reactive red (RR195) are the major cause of contamination, particularly in developing states [23]. Therefore dyes degradation is a challenge for investigators. Several methods were employed for dyes degradation such as ion exchange, ozonolysis, chemical oxidation, photocatalytic degradation [24]. But, the photocatalytic degradation of dyes via a semiconductor photocatalyst such as metal NPs is considered green and economical approach [25]. Algae are commonly employed in industrial applications; however, their utilization for NPs creation at the international level has not been extensively employed [26]. Therefore, the present research aimed to synthesis of CuONPs from Zygnema sp. with antimicrobial and dyes removal applications.
Materials and methods
Collection of Zygnema sp. for CuONPs synthesis
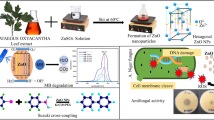
The used alga for synthesis of CuONPs was Zygnema sp. It collected from a pond located at Governorate of Monufia, Egypt (30° 62′8014″ N, 116° 31′ 070334″ E), examined and identified at genus level. Then it cultured and purified on Axenic Chu-10 medium which contains the following constituents: 0.10 mg/L, 0.40 mg/L, 0.40 mg/L, 0.20 mg/L, 0.25 mg/L, 0.30 mg/L, 0.30 mg/L, 0.50 mg/L, 0.50, 0.01 mg/L, 0.02 mg/L of K2HPO4, KNO3, CaCl2, Na2SiO3, MgSO4·7H2O, citric acid, ferric citrate, MnCl2·4H2O, H3BO3, Na2MoO4·2H2O, and CuSO4·5H2O, respectively. After the growth developed, the biomass was harvested and then air dried for 5 days under shade condition and then grinded to obtain a fine powder for further investigation [16].
HPLC analysis of Phenolic and flavonoid constituents of Zygnema sp. extract
Methanol (500 ml) was used to extract the 100 g powder of Zygnema sp. To find the flavonoid and phenolic contents, the extract was put through high performance liquid chromatography (HPLC; Agilent 1260 Infinity HPLC Series, Agilent Technologies, Santa Clara, CA, USA). A Quaternary pump and a Zorbax Eclipse supplemented with a C18 column (100 mm × 4.6 mm i.d.) were used to strengthen the HPLC. In HPLC, 20 µL of the extract were injected. For the separation of the phenolic constituents at 30 °C, three gradient elutions were used: methyl alcohol (B), acetonitrile (C), and HPLC grade water 0.2% H3PO4 (v/v). At 284 nm, the detector wavelength was applied. In order to separate the flavonoid constituents, the Knauer HPLC was strengthened with a binary pump. The gradient elutions that were applied consisted of methanol and 0.5% H3PO4 in water (50:50%) at a flow rate of 0.7 mL/min. An injection of 20 µL of Zygnema sp. extract was made. To detect flavonoids, a detector wavelength of 284 nm was used. The presence of standard constituents was necessary for the identification of constituents [27].
Copper oxide nanoparticles synthesis via Zygnema sp. extract
One mM aqueous of copper acetate was prepared as a precursor of CuONPs synthesis. Ten mL of Zygnema sp. extract (5% g/v) was added to100 mL of the prepared precursor in a glass flask with 250 mL capacity as positive reaction for CuONPs synthesis. The reaction mixture was vigorous stirred for 18 h at 80 °C. If the color shifted from bright blue to dark brown after 7 h, it was a symbol for formation of CuONPs. Aqueous solution of copper acetate (1mM) without Zygnema sp. extract was applied as negative control as in the circumstances of the positive reaction. The solution containing CuONPs was centrifuged for 15 min at 10,000 rpm. The precipitated CuONPs were re-dispersed and then cleaned via the deionized water to eliminate any undesirable remains. The collected CuONPs were oven-dried to achieve the characterization phases [28].
CuONPs characterization
At a wavelength ranged from 200 to 700 nm of UV-visible spectrophotometer (Nicolet evolution 100, Cambridge, MA, USA) was applied to detect the synthesized CuONPs. A transmission electron microscope (TEM) ( JEOL JEM-2100, Tokyo, Japan) was applied to detect the size and shape of the synthesized CuONPs. In aqueous solution, the synthesized CuONPs were suspended, followed by making a suspended drop onto the grids of TEM, and followed by drying before check. The analysis of FT-IR (Agilent system Cary 630 FT-IR model) with the Potassium bromide (KBr) pellet was done on the synthesized CuONPs to recognize the functional groups that aide to the formation of CuONPs. In the range of 4000 –400 cm-1, the frequencies were taken with 4 cm-1 resolution. The crystallinity of the synthesized CuONPs was detected by X-ray diffractometer X’Pert Pro (Philips, Eindhoven, Netherlands) (XRD) with ranged temperature from 4 to 70 °C of 2θ. The source of X-ray was Ni-filtered Cu Ka of radiation with 40 kV and 30 mA as voltage current, respectively.
Activity of created CuONPs against bacteria and Candida albicans
Various bacteria namely Staphylococcus aureus ATCC6538, Escherichia coli ATCC8739, Salmonella typhi (ATCC 6539), Enterococcus faecalis (ATCC 10,541), besides unicellular yeast (Candida albicans ATCC 10,221)) were utilizes as test microorganisms. The tested bacteria were inoculated on the surface of nutrient agar in the petri dishes, while Candida albicans was inoculated on the surface of malt extract agar in the petri dishes by a method of streaking. Wells (6 mm) were done in the inoculated agar layer, and then 100 µL (100 ppm) of CuONPs were loaded in each well. Amoxicillin at (20 µg/mL) was applied as standard antibiotic, while clotrimazole at 1000 µg/mL was applied as standard anti-yeast. The activity of CuONPs was compared to copper acetate at the same concentration and condition. The prepared plates were kept for 20 min at 5 ℃ to permit the diffusion of examined materials before microbial growth. Subsequently, it incubated for 24 h, at 37℃, followed by measuring the appeared inhibition zones around the wells [5].
Measurement of minimum inhibitory concentration (MIC) and minimum bactericidal/fungicidal concentration (MBC/MFC)
The microdilution method was employed, per the CLSI, to determine the MIC. First, Müeller-Hinton broth was added to each well on the microdilution plates. Afterwards, ZnONP concentration was introduced. The microbial suspensions were adjusted to 0.5 on the McFarland scale, diluted, and added to the wells to reach a final concentration of 2 × 105 CFU/mL. After that, the plates were incubated at 37 2 °C for 24 h. Using a spectrophotometric analysis at 620 nm, the standard medication’s minimum inhibitory concentration (MIC) that might inhibit microbial growth was found. MBC/MFC was established after the MIC data was obtained. An aliquot of 10 µL was taken from each well that showed no signs of microbial growth. The aliquot was then aseptically removed and placed on Müeller-Hinton agar. For 24 h at 35 °C, the petri plates were incubated. MBC/MFC had the lowest dose at which no grew of microorganisms [29].
Activity of created CuONPs against fungi
Various fungi namely Aspergillus flavus, Curvularia lunata, Fusarium oxysporium, and Mucor circinelloid were employed in the testing process. Agar culture medium devoid of CuONPs, copper acetate, and copper oxychloride ( Commercial fungicide) was present in Petri dishes, along with a fortified medium with varying doses of each tested compound (50, 100, and 200 ppm). After being placed in the center of the agar plate, six mm of fungal disc were incubated for 6 days at 28 °C. By comparing the colony radius of the tested fungi to the control cultures, the growth development of the fungi was estimated [30].
Photo-catalytic degradation of RR195 and RB using CuONPs
The photo-catalytic activity of CuONPs was tested to degrade two dyes namely RR195 and RB at different times (from 15 to 105 min) and temperatures (from 10 to 50 ℃). The photo-catalytic reaction consists of 50 mL of dye (10 mg/mL) and 50 µg/mL of CuONPs. The mixture of the photo-catalytic reaction was incubated at different times in the existence of sunlight. Also, with same trial, photo-catalytic reaction was incubated at different temperatures at optimum period of previous experiment. After each period, and at each temperature the solution of the photo- reaction was centrifuged at 10,000 rpm for 5 min. UV-Vis spectroscopy (JENWAY 6305 Spectrophotomete) was used to measure the optical density of the obtained supernatant at 538 nm and 554 nm for the degradation of RR195 and RB dyes, respectively [31]. According the next formula, the degradation efficacy (DE) of CuONPs to dyes was recorded
Results and discussion
As mentioned in several investigation the synthesis of metal NPs via biological sources depend on the reducing and stabilizing agents which exist in these sources [6, 28, 32]. Therefore the present research spotlighted the constituents of Zygnema sp. (Fig. 1) extract via HPLC analysis as visualized in Table 1; Fig. 2. The obtained data in Table (1) indicted the presence of 12 compounds associated to phenols and flavonoids with different concentrations, retention times, and area in the extract. Gallic acid, followed by chlorogenic acid, methyl gallate, naringenin, and caffeic acid represent the greatest constituents with concentrations of 101.77, 88.28, 40.92, 19.33, and 18.24 µg/mL, respectively. While cinnamic acid, coumaric acid, and rutin were detected in low concentrations less than 10 µg/mL. HPLC analysis indicated that some compounds including catechin, ellagic acid, ferulic acid, daidzein, kaempferol, and hesperetin not detected according the injected standards. Gentscheva et al. [33] reported the presence of naringenin and quercetin in several extracts of algae.
Characterization of CuONPs
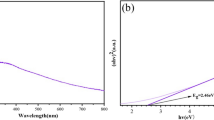
The UV-visible spectrum appeared the absorption peak at 252 nm (Fig. 3), theses peak was attributed to the formation of CuONPs. Our results may agreement with findings of other scientific papers, where the absorption peaks were nearly 258 nm for CuONPs synthesis by green microalga Botryococcus braunii [34]. The absorption peak of the synthesized CuONPs was compared to the absorption peak of the extract (Fig. 3).
Shape and size of the synthesized CuONPs were visualized in Fig. 4, where the shape was spherical with diameter mean of 30.06 nm. The diameter of synthesized CuONPs by B. braunii was ranged from 10 to 70 nm [35]. The differences in the size of synthesized CuONPs among the present investigation and other studies may be due to type of creator, reaction conditions, reducing and stabilizing agents. The different genera of the same group of algae (brown algae) giving different diameters of CuONPs, for instance, 6 to 7.8 nm from Cystoseira trinodis, 5 to 45 nm from Bifurcaria bifurcata [17], 2 and 50 nm from Macrocystis pyrifera [18].
The use of FTIR was used to confirm that functional groups existed on the surface of biosynthetic materials. Figure 5 displayed the spectrum of CuONPs. The different functional groups that are present in the synthesized CuO-NPs are represented by the detected peaks in the spectra. The absorption peaks of the synthesized CuO-NPs are predicted to be in the range of 3385, 2358, 1637, and 491 cm1. They attribute the peaks at 3385 and 2358 cm− 1 to OH and -COO stretching, respectively. The peak at 1637 cm− 1 is caused by the vibration patterns of aromatic and alkyl nitro compounds. The 491 cm− 1 absorption peak is associated with the metal-oxygen (Cu-O) vibration pattern [28].
The crystalline structure of formulated CuONPs was recorded XRD analysis (Fig. 6). The primary strong angles in the diffractogram of created CuONPs were visible in the XRD patterns, indicating that CuONPs were crystallographic in nature [36, 37]. CuONPs diffraction peaks are depicted in Fig. 6, which also displays the diffraction characteristics with respect to 2θ at 33.7°, 35.6°, 37.8°, 48.1°, 51.7°, 61 °, and 66.3°, which correspond to the Bragg’s reflections at 110, 111, -111, -202, 020, -113, and 022, respectively. With a standard card, all of the peaks matched the Joint Committee on Powder Diffraction Standards (JCPDS) of CuO-NPs. File No. for JCPDS: 01-1117 [38, 39]. CuO-NPs diffractogram do not reveal the existence of any other impurities. It ensures that the CuONPs obtained are pure.
Antimicrobial activity of CuONPs
Table 2 and Fig. 7 demonstrated the inhibitory potential of CuONPs against various bacteria as well as unicellular yeast (C. albicans), besides the effect antibiotic/antifungal (standard drug) and copper acetate. The bacterial growth was prevented by CuONPs with inhibition zones of 32 ± 0.1, 36 ± 0.1, 27 ± 0.2, and 37 ± 0.1 mm, while the appeared zones caused by copper acetate were 28 ± 0.2, 30 ± 0.2, 23 ± 0.1, and 33 ± 0.1 mm against S. aureus, E. coli, S. typhi, and E. faecalis, respectively. Moreover, C. albicans was more inhabited by CuONPs than that by copper acetate. S. aureus, E.coli, K. pneumoniae, and B. subtilis in recent study were inhibited by the created CuONPs by mixed algae with inhibition zones of 22, 23, 25, and 26 mm, respectively [28]. The size of created CuONPs plays an important role in the microbial growth inhibition. The effect of standard antibiotic/antifungal was less than that CuONPs but more than that copper acetate. MIC of CuONPs was less than that copper acetate in case S. aureus, E. coli, S. typhi, while it was equal in case of E. faecalis (7.8 µg/mL) and C. albicans (31.25 µg/mL). Furthermore, MBC/MFC value of CuONPs was less than that copper acetate for all tested microorganisms except S. typhi. Several bacteria including Edwardsiella tarda, Proteus mirabilis, Aeromonas hydrophila, Vibrio anguillarum, and S. aureus were inhibited by CuONPs [36]. Abdelghany et al. [7] recorded different inhibition zones ranged from 28.10 to 33.83 mm against the exposed Staphylococcus aureus, Bacillus subtilis, Klebsiella pneumonia, Escherichia coli, Salmonella typhimurium and Pseudomonas aeruginosa to ZnONPs. The entry of CuNPs in the bacterial cells across cell membrane depends on the particle size. Some chemical groups associated to cell membrane such as amine and carboxylic group’s aids to attract the ions of Cu. Akintelu et al. [40] mentioned that the efficacy of CuONPs seriously differs with the shape and size of the particles.
The actual substances in some applied fungicides and fertilizers are copper in the form organic and inorganic, and therefore numerous investigations suggest the applied of CuONPs at certain doses to suppress phytopathogens progress [41]. Figure 8 showed the effect of CuONPs at different concentrations compared to copper acetate and commercial fungicide against the growth of C. lunata, F. oxysporium, A. flavus and Mucor circinelloid. The three tested compounds showed fungal inhibition but with different levels of inhibition based on the fungal species and type of compound. The decreasing of colony radius increased with increasing the dose of tested compounds. The colony radius of C. lunata, F. oxysporium, A. flavus, and M. circinelloid decreased from 6.5 to 1.50, 7.5 to 2.0, 5.5 to 2.0, and 6.5 to 3 cm, with inhibtion % of 76.92, 73.33, 63.63, and 53.84%, respectively when exposed to 200 ppm of CuONPs. CuONPs exhibited more inhibition of fungal growth than the effect of copper acetate but less than that of chemical fungicide. M. circinelloid was the most tolerance to CuONPs, copper acetate, and chemical fungicide at all tested doses if compared to other tested fungi, this may due to the cell wall composition or the ability of the fungus to accumulate the copper inside the cell. Based on the previous study, Fusarium oxysporum was inhibited by Botryococcus braunii mediated CuONPs [35]. According to Gaba et al. [42], CuONPs block metabolic pathways responsible for fungal spores germination. Antifungal activity of CuONPs was documented recently [43]. Vasantharaj et al. [44] recorded great antimicrobial activity of CuONPs compared to copper compound existed in non-nano shape. Recently, Abdelhady et al. [45] mentioned that the Exserohilum rostratum mediated ZnONPs at 100 µg/mL suppress the growth of Fusarium nygamai (colony diameter was 2.0 cm) compared to chemical fungicide Revanol 50% where the colony diameter was 3.85 cm.
Photocatalytic degradation of dyes by CuONPs
CuONPs were tested at various times between 15 and 105 min and between 10 and 50 °C for their capacity to remove two dyes, RR195 and RB. The decolorization percentages of RR195 and RB increased over time, reaching a maximum degradation of 84.66% and 90.82%, respectively, at 75 min (Fig. 9). The degradation of dyes using CuONPs under solar light may be caused by the plasmon resonance effect of the CuONPs’ increased surface. Due to the prolonged duration, the reaction solution’s dye release was accompanied by a decrease in its decolorization. Per Ali et al. [46], ZnONPs degraded RR 195 at a maximum rate of 91–94% after 70 min.
Figure (9) illustrated how varying temperatures affected ZnONPs’ ability to photocatalytically degrade dyes. In contrast to low temperatures of 10 and 20 °C, high temperatures of 40 °C and 50 °C were ideal for dye degradation. At 40 °C, the optimal percentage of dye degradation was 84.66 and 90.82%, while at 10 °C, the degradation was 31.55% and 42.33% for RR195 and RB, respectively. The rate of degradation dropped at 50 °C, possibly as a result of weak adsorption forces and a decline in the bond between the adsorbent and the adsorbate. Furthermore, et al. [47] noted that as temperature rises, dye dissociation and solubility increase, minimizing the interaction between the adsorbate and the adsorbent. Metal particles particularly NPs offer more active sites for dyes absorption, enhancing the effectiveness of the degradation process [48]. In the present investigation, a good finding was obtained employing 50 µg/mL of CuONPs. The result of the effect of CuONPs on the dyes degradation, control tests were performed in the dark with presence and absence of CuONPs. In the lack of CuONPs, negligible dye degradation was observed, at the same time in the dark, CuONPs exert negligible result on degradation rate of dyes (data not tabulated). These findings were matching with the finding of Dulta et al. [49], but with other dyes namely methyl red and methylene blue. The optimum degradation of methylene blue was recorded at180 min and 60 min using CuONPs and Sn3O4 NPs according to Sonia et al. [50] and Balakumaran et al. [51], respectively. The influence of reactive oxygen species (ROS) is investigated in the visible light photodegradation of dyes. Tao et al. [2024] mentioned that ROS play a vital role in the mineralization of dyes is hydroxyl radical (•OH) besides the combination of singlet oxygen (1O2) and •OH has a synergistic influence on the dyes mineralization. The mechanism of photodegradation is mostly dependent on the kind of ROS included in the process which in turn associated to the source of light irradiation, photocatalyst, besides the kind of involved dyes [53].
The present investigation validated a renewable, ecofriendly and appropriate source for synthesis of CuONPs utilizing green alga Zygnema sp. Via HPLC analysis, extract of Zygnema sp. was rich with numerous contents of phenols and flavonoids that can reduce ions of copper into CuONPs. Green synthesized CuONPs were characterized via UV-visible spectroscopy, FT-IR, TEM, and XRD. The synthesized CuONPs displayed activity against growth of various bacteria, and fungi as well as unicellular yeast (C. albicans). The environmental role of the synthesized CuONPs was demonstrated through the degradation process of reactive dyes of RR195 and RB that reached to 84.66% and 90.82%, at 75 min. From the results, it conclude that CuONPs can have vital biotechnological utilizations.
Data availability
All data that support the findings of this study are available within the article.
References
Abdelghany TM (2013) Stachybotrys chartarum: a novel biological agent for the extracellular synthesis of silver nanoparticles and their antimicrobial activity. Indones J Bi Otechnol 18(2):75–82
Yahya R, Al-Rajhi AMH, Alzaid SZ, Al Abboud MA, Almuhayawi MS, Al Jaouni SK, Selim S, Ismail KS, Abdelghany TM (2022) Molecular docking and efficacy of Aloe vera gel based on chitosan nanoparticles against Helicobacter pylori and its antioxidant and anti-inflammatory activities. Polym (Basel) 14(15):2994. https://doi.org/10.3390/polym14152994
Abdelghany TM, Al-Rajhi AMH, Almuhayawi MS et al (2023) Green fabrication of nanocomposite doped with selenium nanoparticle–based starch and glycogen with its therapeutic activity: antimicrobial, antioxidant, and anti-inflammatory in vitro. Biomass Conv Bioref 13:445. https://doi.org/10.1007/s13399-022-03301-7
Abdelghany TM, Al-Rajhi AMH, Almuhayawi MS et al (2023) Green fabrication of nanocomposite doped with selenium nanoparticle–based starch and glycogen with its therapeutic activity: antimicrobial, antioxidant, and anti-inflammatory in vitro. Biomass Conv Bioref 13:431–443. https://doi.org/10.1007/s13399-022-03257-8
Qanash H, Bazaid AS, Alharazi T et al (2023) Bioenvironmental applications of myco-created bioactive zinc oxide nanoparticle-doped selenium oxide nanoparticles. Biomass Conv Bioref. https://doi.org/10.1007/s13399-023-03809-6
Al-Rajhi AM, Salem SS, Alharbi AA, Abdelghany TM (2022) Ecofriendly synthesis of silver nanoparticles using Kei-apple (Dovyalis caffra) fruit and their efficacy against cancer cells and clinical pathogenic microorganisms. Arab J Chem 15(7):103927. https://doi.org/10.1016/j.arabjc
Abdelghany TM, Al-Rajhi AMH, Yahya R et al (2023) Phytofabrication of zinc oxide nanoparticles with advanced characterization and its antioxidant, anticancer, and antimicrobial activity against pathogenic microorganisms. Biomass Conv Bioref 13:417–430. https://doi.org/10.1007/s13399-022-03412-1
Abdelghany TM, Al-Rajhi AMH, Al Abboud MA et al (2018) Recent advances in green synthesis of silver nanoparticles and their applications: about future directions. Rev BioNanoSci 8:5–16. https://doi.org/10.1007/s12668-017-0413-3
Ganash M, Abdel Ghany TM, Omar AM (2018) Morphological and biomolecules dynamics of phytopathogenic fungi under stress of silver nanoparticles. BioNanoScience (2018) 8:566–573 https://doi.org/10.1007/s12668-018-0510-y
Mehra S, Singh M, Chadha P (2021) Adverse impact of textile dyes on the aquatic environment as well as on human beings. Toxicol Int 28:165–176
Abdelghany T, Abboud M, Alawlaqi M, Shater AR (2019) Dead biomass of thermophilic aspergillus fumigatus for Congo red biosorption. Egypt J Experimental Biology (Botany) 15(1):1–6. https://doi.org/10.5455/egyjebb.20181206084342
Ihsanullah I, Jamal A, Ilyas M, Zubair M, Khan G, Atieh MA (2020) Bioremediation of dyes: Current status and prospects, J. Water Process Eng 2020, 38, Article ID 101680
Karthikeyan C, Arunachalam, Ramachandran K, Al-Mayouf AM, Karuppuchamy S (2020) Recent advances in semiconductor metal oxides with enhanced methods for solar photocatalytic applications. J Alloy Compd 828. article ID 154281
Pourmoslemi S, Bayati N, Mahjub R (2022) Application of Box–Behnken design to optimize a sol-gel synthesis method for Ag and Zn doped CuO nanoparticles with antibacterial and photocatalytic activity. J Sol-Gel Sci Technol 104:319–329
Fawcett D, Verduin JJ, Shah M, Sharma SB, Poinern GEJ (2017) A review of current research into the biogenic synthesis of metal and metal oxide nanoparticles via marine algae and seagrasses, J. Nanosci 2017, article ID 8013850. https://doi.org/10.1155/2017/8013850
Ramaswamy SVP, Narendhran S, Sivaraj R (2016) Potentiating effect of ecofriendly synthesis of copper oxide nanoparticles using brown alga: antimicrobial and anticancer activities. Bull Mater Sci 39:361–364
Gu H, Chen X, Chen F, Zhou X, Parsaee Z (2018) Ultrasound-assisted biosynthesis of CuO-NPs using brown alga Cystoseira Trinodis: characterization, photocatalytic AOP, DPPH scavenging and antibacterial investigations. Ultrason Sonochem 41:109–119
Araya-Castro K, Chao T-C, Durán-Vinet B, Cisternas C, Ciudad G, Rubilar O (2021) Green synthesis of copper oxide nanoparticles using protein fractions from an aqueous extract of Brown Algae Macrocystis pyrifera. Processes 9(1):78. https://doi.org/10.3390/pr9010078
Camacho-Flores BA, Martínez-Álvarez O, Arenas-Arrocena MC, Garcia-Contreras R, Argueta-Figueroa L, de La Fuente-Hernández J, Acosta-Torres LS (2015) Copper: synthesis techniques in Nanoscale and powerful application as an Antimicrobial Agent. J Nanomater 2015. https://doi.org/10.1155/2015/415238
El-Batal AI, El-Sayyad GS, Mosallam FM, Fathy RM (2020) Penicillium Chrysogenum-Mediated Mycogenic Synthesis of Copper Oxide Nanoparticles Using Gamma Rays for in Vitro Antimicrobial Activity against some plant pathogens. J Cluster Sci 31:79–90. https://doi.org/10.1007/s10876-019-01619-3
Kanhed P, Birla S, Gaikwad S, Gade A, Seabra AB, Rubilar O, Duran N, Rai M (2014) Vitro Antifungal efficacy of copper nanoparticles against selected crop pathogenic Fungi. Mater Lett 115:13–17. https://doi.org/10.1016/j.matlet.2013.10.011
Sandesh K, Kumar G, Chidananda B, Ujwal P (2019) Optimization of Direct Blue-14 dye degradation by Bacillus fermus (Kx898362) an alkaliphilic plant endophyte and assessment of degraded metabolite toxicity. J Hazard Mater 364:742–751
Mishra S, Mohanty P, Maiti A (2019) Bacterial mediated bio-decolourization of wastewater containing mixed reactive dyes using jack-fruit seed as co-substrate: process optimization. J Clean Prod 235:21–33
Safajou H, Ghanbari M, Amiri O, Khojasteh H, Namvar F, Zinatloo-Ajabshir S, Salavati-Niasari M (2021) Green synthesis and characterization of RGO/Cu nanocomposites as photocatalytic degradation of organic pollutants in waste-water. Int J Hydrog Energy 46:20534–20546
Aroob S, Carabineiro SAC, Taj MB, Bibi I, Raheel A, Javed T, Yahya R, Alelwani W, Verpoort F, Kamwilaisak K et al (2023) Green Synthesis and photocatalytic dye degradation activity of CuO nanoparticles. Catalysts 13(3):502. https://doi.org/10.3390/catal13030502
Shanmugam BS, Pitchiah S, Suresh V, Ramasamy P (2023) Biosynthesis of copper nanoparticles from seaweed Ulva lactuca and their in Vitro Antioxidative potential. Cureus 15(11):e48985. https://doi.org/10.7759/cureus.48985
Alsalamah SA, Alghonaim MI, Jusstaniah M, Abdelghany TM, Anti-Yeasts (2023) Antioxidant and Healing properties of Henna pre-treated by Moist Heat and Molecular Docking of its major constituents, Chlorogenic and ellagic acids, with Candida albicans and Geotrichum candidum proteins. Life 13(9):1839. https://doi.org/10.3390/life13091839
Alsalamah SA, Alghonaim MI, Mohammad AM, Abdel Ghany TM (2023) Algal Biomass Extract as Mediator for copper oxide nanoparticle synthesis: applications in control of Fungal, bacterial growth, and Photocatalytic degradations of dyes. BioResources 18(4):p7474. https://doi.org/10.15376/biores.18.4.7474-7489
French GL (2006) Bactericidal agents in the treatment of MRSA infections—the potential role of daptomycin. J Antimicrob Chemother 58:1107
Abdelghany TM, Hassan MM, El-Naggar MA, El-Mongy A, M (2020) GC/MS analysis of Juniperus procera extract and its activity with silver nanoparticles against aspergillus flavus growth and aflatoxins production. Biotechnol Rep 27:e00496
Farias S, Oliveira DD, Souza AA, Souza SMA, Morgado AF (2017) Removal of reactive blue 21 and reactive red 195 dyes using horseradish peroxidase as catalyst. Braz J Chem Eng 34:701–707
Alghonaim MI, Alsalamah SA, Mohammad AM et al (2024) Green synthesis of bimetallic Se@TiO2NPs and their formulation into biopolymers and their utilization as antimicrobial, anti-diabetic, antioxidant, and healing agent in vitro. Biomass Conv Bioref. https://doi.org/10.1007/s13399-024-05451-2
Gentscheva G, Milkova-Tomova I, Pehlivanov I, Gugleva V, Nikolova K, Petkova N, Andonova V, Buhalova D, Pisanova E (2022) Chemical characterization of selected algae and cyanobacteria from Bulgaria as sources of compounds with antioxidant activity. Appl Sci 12(19) article ID 9935. https://doi.org/10.3390/app12199935
Kimber RL, Lewis EA, Parmeggiani F, Smith K, Bagshaw H, Starborg T, Lloyd JR (2018) Biosynthesis and characterization of copper nanoparticles using Shewanella oneidensis: application for click chemistry. Small 14(10):1703145
Arya A, Gupta K, Chundawat TS, Vaya D (2018) Biogenic synthesis of copper and silver nanoparticles using green alga Botryococcus braunii and its antimicrobial activity, Bioinorg Chem Appl 2018, article ID 7879403. https://doi.org/10.1155/2018/7879403
Nabila MI, Kannabiran K (2018) Biosynthesis, characterization and antibacterial activity of copper oxide nanoparticles (CuONPs) from actinomycetes. Biocatal Agric Biotechnol 15:56–62. https://doi.org/10.1016/j.bcab.2018.05.011
Hammad EN, Salem SS, Zohair MM, Mohamed AA, El-Dougdoug W (2022) Purpureocillium lilacinum mediated biosynthesis copper oxide nanoparticles with promising removal of dyes. Biointerface Res Appl Chem 12(2):1397–1404
Saied M, Hasanin M, Abdelghany TM, Amin BH, Hashem AH (2023) Anticandidal activity of nanocomposite based on nanochitosan, nanostarch and mycosynthesized copper oxide nanoparticles against multidrug-resistant Candida. Int J Biol Macromol 242:124709
Shehabeldine AM, Amin BH, Hagras FA, Ramadan AA, Kamel MR, Ahmed MA, Atia KH, Salem SS (2023) Potential antimicrobial and antibiofilm properties of copper oxide nanoparticles: time-kill kinetic essay and ultrastructure of pathogenic bacterial cells. Appl Biochem Biotechnol 195(1):467–485
Akintelu S, Adewale AS, Folorunso FA, Folorunso, and Abel Kolawole Oyebamiji (2020). Green synthesis of copper oxide nanoparticles for biomedical applicationenvironmental remediation. Heliyon 6, no. 7 https://doi.org/10.1016/j.heliyon.2020.e04508
Faraz A, Faizan M, Hayat S, Alam P (2022) Foliar application of copper oxide nanoparticles increases the photosynthetic efficiency and antioxidant activity in Brassica juncea, J. Food Quality 2022, article ID 5535100. https://doi.org/10.1155/2022/5535100
Gaba S, Rai AK, Varma A, Prasad R, Goel A (2022) Biocontrol potential of mycogenic copper oxide nanoparticles against Alternaria brassicae, Front. Chem 10, article ID 966396. https://doi.org/10.3389/fchem.2022.966396
Atri A, Echabaane M, Bouzidi A, Harabi I, Soucase BM, Chaâbane RB (2023) Green synthesis of copper oxide nanoparticles using Ephedra Alata plant extract and a study of their antifungal, antibacterial activity and photocatalytic performance under sunlight. Heliyon 9(2) article ID e13484. https://doi.org/10.1016/j.heliyon.2023.e13484
Vasantharaj S, Shivakumar P, Sathiyavimal S, Senthilkumar P, Vijayaram S, Shanmugavel M, Pugazhendhi A (2023) Antibacterial activity and photocatalytic dye degradation of copper oxide nanoparticles (CuONPs) using Justicia Gendarussa. Appl Nanosci 13(3):2295–2302. https://doi.org/10.1007/s13204-021-01939-9
Abdelhady MA, Abdelghany TM, Mohamed SH, Abdelbary SA (2024) Impact of Green Synthesized Zinc Oxide Nanoparticles for Treating Dry Rot in Potato Tubers. BioResources, 19(2). 2106-211910.15376/biores.19.2.2106-2119
Ali AA, Ahmed IS, Amin AS, Gneidy MM (2021) Auto-combustion fabrication and Optical properties of Zinc Oxide nanoparticles for degradation of reactive red 195 and Methyl Orange Dyes. J Inorg Organomet Polym 31:3780–3792. https://doi.org/10.1007/s10904-021-01975-6
Malekkiani M, Heshmati Jannat Magham A, Ravari F et al (2022) Facile fabrication of ternary MWCNTs/ZnO/Chitosan nanocomposite for enhanced photocatalytic degradation of methylene blue and antibacterial activity. Sci Rep 12:5927. https://doi.org/10.1038/s41598-022-09571-5
Kumar AP, Bilehal D, Tadesse A, Kumar D (2021) Photocatalytic degradation of organic dyes: Pd–Al2O3 and PdO–Al2O3 as potential photocatalysts. RSC Adv 11:6396–6406
Dulta K, Koşarsoy Ağçeli G, Chauhan P, Jasrotia R, Chauhan PK, Ighalo JO (2022) Multifunctional CuO nanoparticles with enhanced photocatalytic dye degradation and antibacterial activity. Sustainable Environ Res 32:1–15
Sonia S, Poongodi S, Kumar PS, Mangalaraj D, Ponpandian N, Viswanathan C (2015) Hydrothermal synthesis of highly stable CuO nanostructures for efficient photocatalytic degradation of organic dyes. Mat Sci Semicon Proc 30:585–591
Balakumaran MD, Ramachandran R, Balashanmugam P, Mukeshkumar DJ, Kalaichelvan PT (2016) Mycosynthesis of silver and gold nanoparticles: optimization, characterization and antimicrobial activity against human pathogens. Microbiol Res 182:8–20
Surfaces and Interfaces, 104150
Moulahi A (2023) Cu Doped NiS/ZnS nanocomposites for Photodegradation of Methyl Green, Methylene Blue and Congo Red pollutants. J Inorg Organomet Polym 33:3948–3960. https://doi.org/10.1007/s10904-023-02702-z
Acknowledgements
The authors wish to appreciate the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) for supported and funded the current study (Grant number IMSIU-RG23108).
Funding
This work was supported and funded by the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) (Grant number IMSIU-RG23108), Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
S.A.A. and T.A.M. Conceptualization and methodology; M.I.A. and M.M.B. formal analysis and investigation; S.A.A., T.A.M, M.I.A. and M.M.B writing—original draft preparation, writing—review and editing. All authors agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
All authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alsalamah, S.A., Alghonaim, M.I., Bakri, M.M. et al. Zygnema sp. as creator of copper oxide nanoparticles and their application in controlling of microbial growth and photo-catalytic degradation of dyes. Appl Biol Chem 67, 47 (2024). https://doi.org/10.1186/s13765-024-00891-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13765-024-00891-w