Abstract
A natural product library consisting of the culture extracts of 814 actinomycete strains was screened for antifungal compounds that disrupt the cell integrity of plant pathogenic fungi using an adenylate kinase (AK) assay system. The culture extract of Streptomyces xanthocidicus strain S3 exhibited high AK activity against various plant pathogens. The active ingredients, AT-1 and AT-2, were isolated from the culture extract using a series of chromatographic procedures. Based on MS, UV, and NMR spectrometric analyses, the structures of AT-1 and AT-2 were determined as the pentaene macrolides, AB023a and takanawaene C. AB023a and takanawaene C displayed broad-spectrum antifungal activity against Aspergillus oryzae, Botrytis cinerea, Colletotrichum coccodes, C. gloeosporioides, C. orbiculare, Cylindrocarpon destructans, and Fusarium oxysporum f. sp. lycopersici, showing minimum inhibitory concentrations of 1–32 μg/mL. Treatment of AB023a and takanawaene C successfully inhibited anthracnose development on pepper plants in a concentration-dependent manner without phytotoxicity. The disease control efficacy of both compounds was comparable to that of the commercial fungicide chlorothalonil. Collectively, these results suggest that the polyene macrolides produced by S. xanthocidicus strain S3 can be used as natural fungicides for plant disease control.
Similar content being viewed by others
Introduction
Colletotrichum species, causal pathogens of anthracnose, have a broad host range and are known to be widely distributed in temperate regions [1]. Generally, Colletotrichum spp. can attack leaves, stems, and both pre- and post-harvest fruits to cause dark, sunken, circular spots, leaf blight, and defoliation, leading to direct marketable yield and economic losses [2, 3]. Synthetic fungicides have been intensively used to reduce the loss incurred from Colletotrichum spp. However, the increased environmental risks associated with their use and the emergence of fungicide-resistant strains have raised public concerns, prompting the development of new control measures. One such measure involves the utilization of natural product-based fungicides with a novel mode of action and enhanced efficacy.
Natural products are a rich source of biologically active compounds that have the potential to be developed into new medicinal and agricultural drugs [4,5,6]. As a source of fungicidal agents, they offer several merits in terms of biocompatibility, degradability, and structural diversity in comparison to synthetic compounds [7]. The discovery of streptomycin from Streptomyces griseus and penicillin from Penicillium notatum has opened new avenues in which natural products are perceived as sources of drugs [8]. Early research has led to the discovery of numerous novel antibiotics and the development of commercial antifungal and antibacterial agents. By the end of 2013, the FDA had approved 547 natural products, including unmodified natural products, semisynthetic derivatives, and synthetic structures with natural product-derived pharmacophores [9]. In addition, antifungal agents originating from natural products have been used to protect crops in agricultural fields. Kasugamycin is used per se as an active ingredient in commercial fungicides, and strobilurin has become a lead compound in numerous QoI fungicides, one of the two most popular fungicide classes used in agricultural fields [10]. Blasticidin S, kasugamycin, validamycin, streptomycin, polyoxin, midiomycin, and oxytetracycline have been marketed as fungicides for plant disease control [11].
Targeting the integrity of the fungal cell envelope, which consists of cell walls and membranes, has emerged as a crucial strategy for the discovery of potent antifungal agents owing to its lethal effect against fungal pathogens [12, 13]. As part of our efforts to find natural antifungal agents to control plant diseases, we adopted an adenylate kinase (AK) assay to screen a natural product library for disruptors of fungal cell envelopes. An AK-based cell lysis assay enables the screening of antifungal compounds targeting cell envelopes via detection of ATP leakage from cells. Therefore, high AK activity indicates substantial damage to the cell envelope, leading to increased ATP release from the cell. In the screening process, the culture extract of S. xanthocidicus strain S3 showed potent AK activity compared with that of caspofungin, which inhibits fungal 1,3-β-glucan synthase, an essential component of fungal cell wall biosynthesis [14]. In this study, we identified active ingredients of the culture extracts of S. xanthocidicus strain S3 via chromatographic and spectroscopic analyses and examined their antifungal activity against plant pathogenic fungi and disease control efficacy on anthracnose in pepper plants.
Materials and methods
Construction of a microbial culture extract library
Microorganisms were isolated from soil samples collected in Jeju island. Soil samples (1 g) collected from 5 to 20 cm depth of the soil surface were mixed with 10 mL of sterilized water in 15 mL falcon tube. The tubes containing mixtures were then shaken at 28 °C at 200 rpm for 1 h. The soil suspensions were filtered using Whatman no. 1 filter paper. The filtered soil suspensions were serially diluted using tenfold up to 10–5. Soil samples containing 100 μL of tenfold serial dilutions were plated onto humic acid vitamin agar medium (1.0 g humic acid, 0.5 g Na2HPO4, 1.71 g KCl, 0.05 g MgSO4·7H2O, 0.01 g FeSO4·7H2O, 0.02 g CaCO3, 0.5 mg thiamine-HCl, 0.5 mg riboflavin, 0.5 mg niacin, 0.5 mg pyridoxine–HCl, 0.5 mg inositol, 0.5 mg Ca-pantothenate, 0.5 mg p-aminobenzoic acid, 0.25 mg biotin, 50 mg cycloheximide and 18 g agar in 1 L water, adjusted to pH 7.2). The plates were incubated at 28 °C for 4–14 days. Microbial colonies with different morphological type which appeared on the plates were selected and transferred into trypticase soy agar (TSA). The isolates were stored in 20% glycerol suspension at − 80 °C. To construct of a microbial culture extract library, selected microorganisms were cross-hatch streaked three TSA plate (20 mL of medium in a 9 cm diameter). After incubation for 3–5 days at 28 °C, the cultures were thinly sliced and extracted with 50 mL of methanol in a sonication bath for 90 min. The culture extracts were centrifuged at 10,000 × g for 30 min, and evaporated under reduced pressure. The concentrated culture extracts were dissolved in 1 mL dimethyl sulfoxide (DMSO).
Adenylate kinase assay
The AK assay was performed as described previously [13, 15]. Adenylate kinase assay was evaluated using conidial suspension of Fusarium oxysporum f. sp. lycopersici (4 × 106 conidial/mL). 25 μL of the conidial suspension was added into each well of a 96-well plate containing 25 μL of quadruple-strength potato dextrose, 48 μL of sterile water, and 2 μL of each microbial extract. Caspofungin (40 μg/mL) and DMSO (2%) were used as positive and negative controls, respectively. The 96-well plate was incubated at 28 °C for 4 h and then putted a plate at room temperature for 1 h. The mixture (50 μL) from each well of 96-well plate was transferred to a white-walled 96-well plate and treated with AK detection regent (50 μL) (Toxilight kit, Lonza, Basel, Switzerland). After incubation for 15 min, relative luminescence units (RLU) were measured using a multi-label plate reader Victor 3 (Perkin Elmer Inc., Waltham, MA, USA).
16S rRNA gene sequencing and phylogenetic analysis of the S3 strain
The actinomycete, strain S3, was grown on TSA at 28°C for five days. The boiling method described by Metsä-Ketelä et al. [16] was used for the extraction of genomic DNA from strain S3. The colonies were transferred to a microtube and suspended in 100 μL sterilized water. The cell suspension was boiled in a 100 °C water bath for 15 min and centrifuged at 12,225 × g for 10 min. The supernatant was transferred to a microtube and used as a template for PCR. Primers used for amplification of the 16S rRNA gene of strain S3 were as follows: forward primer fD1 (5′-AGAGTTTGATCCTGGCTCAG-3′) and reverse primer rP2 (5′-ACGGCTACCTTGTTACGACTT-3′). PCR amplification was performed using 2 × PreMIX-HF (Macrogen Co., Seoul, Korea). PCR reaction mixtures consisted of 100 ng template DNA, primers fD1 and rP2 (each at a concentration of 10 pmol), 0.2 U Taq-HF DNA polymerase, Taq-HF buffer, 4 mM MgCl2, and a mixture of 2 × deoxynucleoside triphosphates (0.4 mM each). The final volume of the mixture was adjusted to 20 μL by adding triple distilled water. The PCR mixture was subjected to an initial denaturing step at 94 °C for 5 min and thermocycling conditions of 25 cycles consisting of 1 min at 94 °C, 1 min annealing at 56 °C, and 1 min and 30 s extension at 72 °C. Finally, the reaction sequence was terminated after a 3 min extension at 72 °C. The amplified product was examined via electrophoresis on a 1% agarose gel and visualized using UV fluorescence after staining with ethidium bromide. Purification of the amplified product was performed using the LaboPass PCR product purification kit (CosmoGen, Korea) according to the manufacturer’s instructions.
The 16S rRNA gene sequence of strain S3 was analyzed using an ABI Prism 3700 automatic DNA sequencer (Applied Biosystems, CA, USA) and aligned using the DNASTAR software program (DNASTAR Inc., WI, USA). The aligned sequences were compared with Streptomyces species submitted to the GenBank database using BLAST network services at the National Center for Biotechnology Information (NCBI). Phylogenetic analysis of the 16S rRNA gene sequences of strain S3 was carried out using MEGA software and the neighbor-joining method [17]. Bootstrap values are expressed as percentages of 1,000 replicates.
Production and purification of active compounds from the strain S3
The S3 strain was cultured on TSA for five days at 28 °C. Single colonies on plates were selected and pre-cultured in 1-L Erlenmeyer flasks containing 100 mL trypticase soy broth at 28 °C for three days on a rotary shaker at 200 rpm. The culture was used as an inoculum (1% of culture volume) for a 2.5 L culture using 25 flasks that each contained 100 mL production medium (30 g glucose, 30 g soybean meal, 5 g cottonseed flour, 1 g yeast extract, 3 g CaCO3, and 1 L distilled water, pH 8.0). After incubation at 28 °C for five days in a rotary shaker (200 rpm), the culture broth (2.5 L) was centrifuged at 10,000 × g for 30 min and extracted with an equal volume of n-butanol. The n-butanol extracts were concentrated under reduced pressure and the remaining residue dissolved in 1 L of 2% aqueous methanol.
The crude n-butanol extract was purified using flash column chromatography with a 500 mm × 50 mm i.d. glass column packed with Diaion HP-20 resin (Mitsubishi Chemical, Tokyo, Japan) and a 200 mm × 30 mm i.d. glass column packed with 20–63 μm Lichropep RP-18 resin (Merck, Darmstadt, Germany). The crude extract was poured and absorbed onto Diaion HP-20 resin and then eluted with a stepwise gradient of 0, 20, 40, 60, 80, and 100% methanol in water, then washed by acetone. The eluted fractions were evaporated in vacuo and their antifungal activities against F. oxysporum f. sp. lycopersici evaluated using the disk diffusion method [18]. The active 100% methanol fraction was dried and redissolved in 500 mL of 2% aqueous methanol, and then subjected to C18 flash column chromatography. The column was eluted with a stepwise gradient of 0, 20, 40, 60, 80, and 100% methanol in water. The active 80% methanol fraction was concentrated in vacuo, dissolved in a small amount of methanol, and further purified using a high performance liquid chromatography (HPLC) system (Varian, Palo Alto, CA, USA) equipped with J’sphere ODS-H80 column (250 × 10 mm, 4 μm; YMC Co., Ltd., Kyoto, Japan). The HPLC analysis was conducted at a flow rate of 2 mL/min using a linear gradient elution of 20–95% methanol in water over 10 min, followed by isocratic elution with 95% methanol in water for 20 min. Each peak was monitored at 254 nm and collected individually. Finally, two antifungal peaks AT-1 (2.9 mg) and AT-2 (2.1 mg) were obtained at respective retention time of 17.7 min and 19.1 min, respectively.
Spectroscopic analyses of purified antifungal compounds
The ultra-violet (UV) sbsoption spectra of the antifungal compounds were measured in an Optizen POP UV–Vis spectrophotometer (Mecasys Co., Ltd., Daejeon, Korea). The high resolution mass spectrometry (HR-MS) data of the antifungal compounds was obtained using a Q-ToF mass spectrometer (Waters, Milford, MA, USA) with a negative electrospray ionization (ESI) mode. For analysis of nuclear magnetic resonance (NMR), the active compounds were dissolved in dimethyl sulfoxide-d6 (99.9% D; Cambridge Isotope Laboratories, Andover, MA, USA). The 1H and 13C NMR spectra were obtained using a Varian 500 MHz NMR spectrometer.
Broth microdilution assay of AB023a and takanawaene C on various plant pathogenic fungi
The minimum inhibitory concentration (MIC) values of AT-1 and AT-2 were evaluated for various fungal and oomycete plant pathogens using the CLSI M38-A protocol in a 96-well plate [19]. Spore suspensions (1 × 105 spores/mL) of Alternaria brassicicola, Aspergillus oryzae, Botrytis cinerea, Cladosporium cucumerinum, Colletotrichum coccodes, C. gloeosporioides, C. orbiculare, Cylindrocarpon destructans, Fusarium oxysporum f. sp. cucumerinum, F. oxysporum f. sp. lycopersici, Rhizopus stolonifer var. stolonifer, and Phytophthora capsici were added to potato dextrose broth in a 96-well plate. Compounds were serially diluted two-fold to yield different concentrations of 1–128 μg/mL. The MIC value of each compound was determined by visual observation of microbial growth at 1–3 days after treatment.
Pepper anthracnose control efficacy test of AB023a and takanawaene C
The disease control efficacy of AT-1 and AT-2 was evaluated against pepper anthracnose caused by C. coccodes. Pepper seeds (Capsicum annuum L. cv. Nockwang) were sown in 72-hole plastic tray (hole size: W58 × L58 × H63 mm) containing the sterilized commercial soil (Baroker, Seoul Bio Co., Eumsung, Korea) and grown in a greenhouse at 25 ± 3 °C until the four-leaf stage of pepper seedlings (about 4 weeks). Test compounds were dissolved in methanol (25 mg/mL) and diluted to concentrations of 1, 10, 50, 100, and 500 μg/mL in 0.05% Tween 20 solution. Each solution was sprayed onto the leaf surface of pepper plants. The commercial fungicide chlorothalonil and 2% methanol were used as positive and negative controls, respectively. After 24 h after treatment, the spore suspension (4 × 106 conidia/mL) of C. coccodes was sprayed on the leaf surface of pepper plants. Next, the inoculated plants were placed in a dew chamber at 28 °C for 24 h and thereafter transferred to greenhouse. At seven days after inoculation, disease severity (diseased leaf area / total leaf area × 100%) was estimated using an image analysis program Matrox Inspector 2.2 [20]. Data (n = 12) were analyzed by one-way analysis of variance (ANOVA) and the significance of mean differences between treatments was determined by Duncan’s least significant range test (P < 0.05) using R software (version 4.2.3; available online: https://www.r-project.org/) [21].
Results
Adenylate kinase activity of Streptomyces xanthocidicus strain S3
Among 814 actinomycete culture extracts, the S3 culture extract was one of the most effective in inducing AK release from the mycelia of F. oxysporum f. sp. lycopersici. The S3 extract showed potent AK-releasing activity that was 86.6% similar to that of caspofungin (positive control) (Additional file 1: Fig. S1). S3 extract, like caspofungin, could disrupt the integrity of the fungal cell wall. Therefore, strain S3 was selected for further studies to identify its active ingredients and antifungal efficacy against plant pathogenic fungi.
Identification of Streptomyces xanthocidicus strain S3.
The partial 16S rRNA region (1335 nucleotides) of the S3 strain was analyzed by comparison with sequences in GenBank through nBLAST (http://www.ncbi.nlm.nih.gov/ BLAST). In the phylogenetic analysis of 16S rRNA gene sequences (Additional file 1: Fig. S2), the S3 strain was most closely related to Streptomyces xanthocidicus IFO 13469 (99.48% sequence identity). Thus, the S3 stain was identified as S. xanthocidicus and deposited in GenBank under the accession number of KF041002.
Structure elucidation of AB023a and takanawaene C
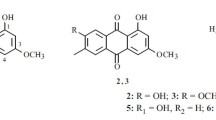
Through a series of chromatographic steps, the antifungal compounds AT-1 (2.9 mg) and AT-2 (2.1 mg) produced by the S3 strain were obtained as yellow powders. The UV spectra of AT-1 and AT-2 in methanol showed the characteristic strong UV absorption maxima of 317, 331, and 349 nm indicating the presence of conjugated pentaene moiety (Fig. 1). The HR-ESI–MS of AT-1 and AT-2 showed intense [M–H]− molecular ions at m/z 549.3434 (calculated m/z 549.3427) and m/z 577.3766 (calculated m/z 577.3740) corresponding to molecular formula of C31H50O8 and C33H54O8, respectively (Fig. 1). The 1H and 13C NMR data of AT-1 and AT-2 are summarized in Table 1. The UV, MS, and NMR analytic data for AT-1 and AT-2 were identical to those of the previously reported pentaene macrolides AB023a and takanawaene C, respectively (Fig. 2) [22, 23].
In vitro and in vivo antifungal activities of AB023a and takanawaene C
The MICs of AB023a and takanawaene C against fungal and oomycete plant pathogens are summarized in Table 2. Both AB023a (MICs of 2–16 μg/mL) and takanawaene C (MICs of 1–2 μg/mL) showed broad-spectrum antifungal activity against all tested fungi except R. stolonifer var. stolonifer. Among tested fungi, three Colletotrichum species and B. cinerea were most sensitive fungi against AB023a. The MIC values of takanawaene C were 1–2 μg/mL indicating that takanawaene C is a more potent antifungal compound than AB023a. On the other hand, both compounds showed no significant activity against the oomycete P. capsici (MIC > 128 μg/mL) (Table 2).
The efficacy of AB023a and takanawaene C in controlling pepper anthracnose was evaluated. Both compounds were effective to protect pepper plants from an attack of C. coccodes (Fig. 3). Treatment of AB023a and takanawaene C significantly inhibited anthracnose development on pepper plants in a concentration-dependent manner. Disease severity was 2.36% in untreated pepper plants, whereas significant reduction in lesion area on pepper leaves started to appear in plants treated with 50 μg/mL of each compound (Fig. 3). At the highest concentration of 500 μg/mL, AB023a and takanawaene C completely controlled the development of pepper anthracnose without phytotoxic effect (Fig. 3). The disease control efficacy of both compounds was comparable to that of the commercial fungicide chlorothalonil (Fig. 3).
In vivo disease control efficacies of AB023a, takanawaene C, and chlorothalonil against anthracnose caused by Colletotrichum coccodes in pepper plants. A anthracnose development on pepper leaves treated with different concentrations of the antifungal compounds. Disease severity (diseased leaf area/total leaf area × 100%) of leaves treated with B AB023a, C takanawaene C, D chlorothalonil at the indicated concentrations. Disease severity was rated on 7 days after inoculation. Different letters on the graph indicate significant differences between treatments (n = 12 replicates; P < 0.05)
Discussion
As part of our efforts to find natural antimicrobial agents to control plant diseases, we screened a natural product library consisting of microbial culture extracts for fungal cell envelope disruptors using an AK-based cell lysis assay. In this study, the culture extract of S. xanthocidicus strain S3 showed the AK-releasing activity similar to that of caspofungin (a fungal 1,3-β-glucan inhibitor) (Additional file 1: Fig. S1). Compounds with AK releasing activity disrupt cell membrane permeability and integrity, and cause leakage of intracellular contents, and leading to cell death (strong antifungal activity). Therefore, the polyene macrolides AB023a and takanawaene C could be the active ingredients for the promising AK-releasing activity of the S3 culture extract.
AB023a and takanawaene C are 28-membered pentaene macrolides. Various 28-membered pentaene macrolides have been reported so far. Filipin [24], fungichromin [25], chainin [26] and elizabethin [27] are methylpentaenes having a methyl group substituted at C-16. Strevertene [28] with a carboxyl group substituted at C-14 has also been reported. Bortolo et al. [23] first isolated and characterized AB023a and AB023b from the broth of Streptomyces sp. SD581. AB023a and AB023b are 28-membered pentaene macrolides with no substituents on the conjugated double-bond chain, and the two compounds differ only by a substitution at C-27. Later, takanawaenes A, B, and C were reported to have a proton or methyl group at C-14, with differences in methyl, ethyl, and isobutyl groups at C-27 [22]. These macrolides were reported to disrupt the fungal plasma membrane; they bind ergosterol, a major fungal sterol in the plasma membrane, and cause cell death via membrane pore formation [29].
In previous reports [30, 31], the polyene macrolides AB023a and takanawaene C have been described as broad-spectrum antifungal agents. Similarly, in our study, both compounds showed inhibitory activity against a wide range of plant pathogenic fungi (Table 2). Notably, the fungal growth of Colletothrichum spp. was completely inhibited at very low concentrations of 1–2 μg/mL (Table 2). Furthermore, takanawaenee C showed two- to eight-fold lower MIC values than AB023a (Table 2), suggesting that the alkyl substituents at C-27 could play a important role in determining antifungal property of 28-membered pentaene macrolides. Kim et al. [31] reported the antifungal activity of AB023a and takanawaenes A/B/C against Aspergillus niger, Mucor racemosus, Candida albicans, and S. cerevisiae using a paper disk-agar diffusion assay. Although these polyene macrolides differed in methyl, ethyl, and isobutyl groups at C-27 position, there was no substantial difference in antifungal activity [31]. Therefore, further studies are required to determine structure–activity relationship of AB023a and takanawaenes.
Despite the long-standing use of various polyene macrolides in the clinical and food industries, their recognition as fungicides for plant disease control remains limited. These compounds are susceptible to inactivation or degradation under harsh conditions commonly encountered during crop cultivation, including high temperatures, dry soil, exposure to heavy metals, and ultraviolet radiation. This vulnerability arises from their inherent structural characteristics [32]. Phytotoxicity of polyenes can be another limiting factor to their use as control agents of plant disease [33]. Nevertheless, recent reports have shown promising results for several pentaene macrolides as control agents for plant diseases. Strevertene A, strevertene B [34] and fillipin III [24] were reported to strongly inhibit tomato Fusarium wilt caused by F. oxysporum f. sp. lycopersici at concentrations of 1 and 10 μg/mL, respectively. More recently, fungichromin isolated from the culture filtrate of S. padanus strain PMS-702 was reported to be effective against downy mildew on cucumber leaves caused by Pseudoperonospora cubensis [35]. These results demonstrate the potential of pentaene macrolides as agricultural fungicides for plant disease control. In this study, treatment with 100 μg/mL AB023a and takanawaene C showed a control efficacy of 87.3% and 89.8% against anthracnose on pepper plants, respectively. Furthermore, even seven days after treatment, both compounds effectively controlled disease development. In addition to this disease control effect, they did not show any phytotoxicity on pepper plants, even at a dose of 500 μg/mL, the highest concentration tested. These results demonstrate that AB023a and takanawene C have a certain level of structural stability and selectivity and could be used as plant disease control agents.
To our knowledge, this is the first study to evaluate the potential of AB023a and takanawaene C as disease control agents in planta, although further studies on their formulation and application methods are required for their development as commercial fungicides.
Availability of data and materials
All data generated or analyzed during the present study are included in this published article.
References
Talhinhas P, Baroncelli R (2021) Colletotrichum species and complexes: geographic distribution, host range and conservation status. Fungal Divers 110:109–198
Biju CN, Ravindran P, Peeran MF et al (2017) Significance of microsclerotia in the epidemiology of black pepper anthracnose and an approach for disease management in nurseries. J Phytopathol 165:342–353
Kang BK, Kim JH, Lee KH et al (2009) Effects of temperature and moisture on the survival of Colletotrichum acutatum, the causal agent of pepper anthracnose in soil and pepper fruit debris. Plant Pathol J 25:128–135
Shin Y-J, Shin J, Jang H et al (2022) Decursinol chloroacrylates useful as fungicides. Appl Biol Chem 65:1–9
Liu H, Wu H, Wang Y et al (2021) Enhancement on antioxidant and antibacterial activities of Brightwell blueberry by extraction and purification. Appl Biol Chem 64:78
Long X, Kim Y-K, Yu T et al (2021) The protective effect of Jangkanghwan (Korean traditional food) on lipopolysaccharide-induced disruption of the colonic epithelial barrier. Appl Biol Chem 64:1–12
Harvey AL, Edrada-Ebel R, Quinn RJ (2015) The re-emergence of natural products for drug discovery in the genomics era. Nat Rev Drug Discov 14:111–129
Demain AL (2006) From natural products discovery to commercialization: a success story. J Ind Microbiol Biotechnol 33:486–495
Patridge E, Gareiss P, Kinch MS et al (2016) An analysis of FDA-approved drugs: natural products and their derivatives. Drug Discov Today 21:204–207
Knight S, Anthony V, Brady A et al (1997) Rationale and perspectives on the development of fungicides. Annu Rev Phytopathol 35:349–372
Valarmathi P (2020) Antibiotics-miracle drugs as crop protectants: a review. Agric Rev 41:43–50
Jeon B, Kim J, Han J et al (2016) Antifungal activity of rimocidin and a new rimocidin derivative BU16 produced by Streptomyces mauvecolor BU16 and their effects on pepper anthracnose. J Appl Microbiol 120:1219–1228
Kim HY, Kim JD, Hong JS et al (2013) Identification of antifungal niphimycin from Streptomyces sp. KP 6107 by screening based on adenylate kinase assay. J Basic Microbiol 53:581–589
Mccormack PL, Perry CM (2005) Caspofungin: a review of its use in the treatment of fungal infections. Drugs 65:2049–2068
Krysan DJ, Didone L (2008) A high-throughput screening assay for small molecules that disrupt yeast cell integrity. SLAS Discov 13:657–664
Metsä-Ketelä M, Halo L, Munukka E et al (2002) Molecular evolution of aromatic polyketides and comparative sequence analysis of polyketide ketosynthase and 16S ribosomal DNA genes from various Streptomyces species. Appl Environ Microbiol 68:4472–4479
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Weinstein MP, Patel JB, Bobenchik AM et al (2019) Performance Standards for Antimicrobial Disk Susceptibility Tests: Approved Standard, 29th ed. Clinical and Laboratory Standards Institute Supplement M100, Wayne, PA, USA, 32
Rex JH (2008) Reference method for broth dilution antifungal susceptibility testing of filamentous fungi: approved standard. Clinical and Laboratory Standards Institute, Wayne
Kwack MS, Kim EN, Lee H et al (2005) Digital image analysis to measure lesion area of cucumber anthracnose by Colletotrichum orbiculare. J Gen Plant Pathol 71:418–421
R Core Team (2013) R: a language and environment for statistical computing. R foundation for statistical computing, Vienna
Fukuda T, Kim Y-P, Iizima K et al (2003) Takanawaenes, novel antifungal antibiotics produced by Streptomyces sp. K99–5278 II. structure elucidation. J Antibiot 56:454–458
Bortolo R, Spera S, Guglielmetti G et al (1993) Ab023, novel polyene antibiotics II. Isolation Struct Determinat J Antibiot 46:255–264
Kim JD, Han JW, Hwang IC et al (2012) Identification and biocontrol efficacy of Streptomyces miharaensis producing filipin III against Fusarium wilt. J Basic Microbiol 52:150–159
Xiong Z-Q, Zhang Z-P, Li J-H et al (2012) Characterization of Streptomyces padanus JAU4234, a producer of actinomycin X2, fungichromin, and a new polyene macrolide antibiotic. Appl Environ Microbiol 78:589–592
Pandey RC, Narasimhachari N, Rinehart KL Jr et al (1972) Polyene antibiotics. IV. Structure of chainin. J Am Chem Soc 94:4306–4310
Bird CW, Latif M (1981) Antibiotics from the newly isolated Streptomyces elizabethii. II. Isolation and characterisation of the antibiotics. J Chem Technol Biotechnol 31:368–370
Schlingmann G, Milne L, Borders DB et al (1999) Strevertenes, antifungal pentaene macrolides produced by Streptoverticillcum LL-30F848. Tetrahedron 55:5977–5990
Ghannoum MA, Rice LB (1999) Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev 12:501–517
Cidaria D, Borgonovi G, Pirali G (1993) AB023, novel polyene antibiotics I. Taxonomy of the producing organism, fermentation and antifungal activity. J Antibiot 46:251–254
Kim Y-P, Tomoda H, Iizima K et al (2003) Takanawaenes, novel antifungal antibiotics produced by Streptomyces sp. K99–5278 I. taxonomy, fermentation, isolation and biological properties. J Antibiot 56:448–453
Thoma K, Kübler N (1997) Photostability of antifungal agents. 2 photostability of polyene antibiotics. Die Pharmazie 52:294–302
Scacchi A, Andriollo N, Cassani G (1995) Detection, characterization and phytotoxic activity of AB021-a and-b, two new macrolide polyene antibiotics. Pestic Sci 45:49–56
Kim JD, Han JW, Lee SC et al (2011) Disease control effect of strevertenes produced by Streptomyces psammoticus against tomato Fusarium wilt. J Agric Food Chem 59:1893–1899
Fan Y-T, Chung K-R, Huang J-W (2019) Fungichromin production by Streptomyces padanus PMS-702 for controlling cucumber downy mildew. Plant Pathol J 35:341
Acknowledgements
Not applicable.
Funding
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry (IPET) and Korea Smart Farm R&D Foundation (KosFarm) through Smart Farm Innovation Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) and Ministry of Science and ICT (MSIT), Rural Development Administration (RDA) (Nos. 421006-03-1-HD040-KIST). This research was supported by the Korea Institute of Science and Technology (Grant Numbers: 2Z06831, 2Z06851), Korea University (Grant Number: K2207301), and Kyungnong Corporation's K2 research funding.
Author information
Authors and Affiliations
Contributions
JBJ: formal analysis, investigation, methodology, conceptualization, writing–original draft, writing–review, and editing. JEK: formal analysis, investigation, conceptualization. JDK: funding acquisition, writing–original draft, writing–review, and editing. BSK: funding acquisition, conceptualization, supervision, validation, writing–original draft, writing–review, and editing. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Adenylate kinase (AK) assay of the culture extract of strain S3 against Fusarium oxysporum f. sp. lycopersici. Error bar represent standard deviation. Figure S2. Neighbor-joining phylogenetic tree based on 16S rRNA gene sequence showing the phylogenetic relationships between Streptomyces xanthocidicus strain S3 and related Streptomyces species. Bootstrap values of 1000 replications are shown at the branch points. Scale bar, 0.01 substitutions per nucleotide position.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jeon, B.J., Kang, J.E., Do Kim, J. et al. Pentaene macrolides AB023a and takanawaene C produced by Streptomyces xanthocidicus strain S3 for controlling pepper anthracnose. Appl Biol Chem 66, 50 (2023). https://doi.org/10.1186/s13765-023-00813-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13765-023-00813-2