Abstract
RNA interference (RNAi) is a gene regulatory mechanism that involves the interaction of small interfering RNAs (siRNAs) and RNA-induced silencing complex (RISC). Dicer cleaves exogenous double-stranded RNA (dsRNA) into siRNAs, which get incorporated into RISC and bind to complementary sequences on the target mRNA to induce its degradation. In this study, we adopted RNAi technology using dsRNAs to suppress Phytophthora capsici, which causes diseases in solanaceous crops, including pepper. We designed and synthesized dsRNAs targeting the P. capsici effector genes PcNLP2 and PcNLP6, respectively. These genes encode necrosis and ethylene-inducing peptide 1-like proteins in P. capsici, which are known to promote oomycete infection. Nicotiana benthamiana leaves were first infiltrated with dsRNAs and inoculated with P. capsici 2 days later. We confirmed significant suppression of P. capsici and PcNLP2, PcNLP6 expression in dsRNA-treated leaves. In addition, we found that downregulation of PcNLP2 and PcNLP6 distinctly affected the expression of some defense-related genes. These results suggest that dsRNA mediated RNAi technology can be used to suppress various pathogens, and may contribute toward crop protection.
Similar content being viewed by others
Introduction
Pepper is an important crop used worldwide as a vegetable, spice, and a source of pharmacological agents. Phytophthora capsici is an oomycete plant pathogen that infects solanaceous crops like pepper, causing root rot leading to yield losses [1, 2]. Criollo de Morelos 334 is a pepper landrace known to be resistant to P. capsici but it is not used commercially [2, 3]. Moreover, the continuous use of chemical fungicides containing mefenoxam cause P. capsici to develop resistance to them [4, 5]. Therefore, new strategies are necessary to manage P. capsici.
RNA interference (RNAi), induced by exogenous double-stranded RNA (dsRNA), can be used for crop protection and other processes as a gene silencing mechanism in plants [6]. Dicer-like endonucleases cleave dsRNA into 20–25-nucleotide small interfering RNAs (siRNAs) in plant cellular system. The guide strand of siRNAs is incorporated into the Argonaute protein to form RNA-induced silencing complex (RISC), which post-transcriptionally silences target genes through sequence-specific base pairing [6,7,8]. Target gene silencing via exogenous dsRNA is influenced by the length of dsRNA, regions in the target mRNA, and the method of application [9,10,11,12,13,14]. In previous studies, we suppressed green fluorescent protein and pepper mottle virus in plants using dsRNAs varying in their length and position of their targets [12,13,14]. These results suggest that P. capsici can be effectively suppressed via exogenous dsRNA.
Plant pathogens carry various effector proteins that contribute to microbial fitness and pathogen virulence by manipulating plant immune responses at infection interface [15,16,17,18]. Necrosis and ethylene-inducing peptide 1-like proteins (NLPs) secreted by oomycetes induce leaf necrosis distinct from immune-related programmed cell death in plants. They have been suggested to play roles of both an immune response trigger and a toxin-like virulence factor [19]. Most of the NLPs in P. sojae are highly expressed during cyst germination and infection stages [20]. In P. capsici, PcNLP2 and PcNLP6 were highly expressed at infection stage. Moreover, the largest necrotic area in Capsicum annuum and Nicotiana benthamiana leaves were produced in agroinfection assays that delivered PcNLP2 and PcNLP6, respectively, indicating the importance of these genes for virulence during infection stages [21]. Thus, inhibiting PcNLP2 and PcNLP6 may suppress P. capsici infection in plants.
The aim of this study was to suppress P. capsici via RNAi using dsRNAs targeting PcNLP2 and PcNLP6. For efficient application of dsRNAs, location on the target gene and time of application are crucial factors [12, 13]. Therefore, we designed and synthesized two distinct dsRNAs targeting PcNLP2 and PcNLP6, respectively, and applied them at the optimum time for effective P. capsici suppression.
Materials and methods
Plant materials and growth conditions
The wild-type N. benthamiana was used in this study. Its seeds were sown in 200-plug tray filled with horticultural soil (Seoulbio, Republic of Korea). Two-week-old N. benthamiana plants were transplanted into pots and grown at 25 ℃ under 16 h of light per day and 50% humidity in a growth chamber. Three-week-old N. benthamiana plants were selected for the following experiments.
Design and synthesis of dsRNAs
For each of PcNLP2 and PcNLP6, two dsRNAs were designed to target the 5′ and 3′ regions of the mRNA. They had a length of 400 bp and 540 bp with overlaps of 59 bp and 63 bp between each dsRNA, respectively (Fig. 1). A 500 bp-long dsRNA targeting the Renilla luciferase gene was used as a mock. For dsRNA synthesis, their corresponding DNA templates, including the T7 promoter sequences (5′-TAATACGACTCACATATAAGAGAG-3′), were synthesized by polymerase chain reaction (PCR) using Phusion High-Fidelity DNA polymerase (Thermo Scientific, United States), according to the manufacturer’s protocol. The PCR products were then used for dsRNA synthesis using the MEGAscript RNAi Kit (Invitrogen, United States), following the manufacturer’s protocol (Additional file 1: Fig. S1). The primers for PCR are listed in Additional file 1: Table S1.
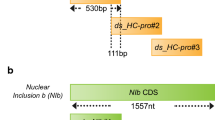
Design of double-stranded RNAs (dsRNAs) targeting PcNLP2 and PcNLP6. a dsRNA_PcNLP2_5′ and dsRNA_PcNLP2_3′ were designed to target the 5′ and 3′ regions of the PcNLP2 mRNA sequence, respectively. Each dsRNA was 400 bp in length, with a 59 bp overlap between them. b dsRNA_PcNLP6_5′ and dsRNA_PcNLP6_3′ were designed to target the 5′ and 3′ regions of the PcNLP6 mRNA sequence, respectively. Each dsRNA was 540 bp in length, with a 63 bp overlap between them
Maintenance of P. capsici
The KACC 40476 strain of P. capsici was kindly provided by Dr. Doil Choi’s Laboratory (Seoul National University, Seoul, Republic of Korea). It was grown on V8 juice agar media for 8 days in the dark at 23 °C.
Infiltration of dsRNAs and infection of N. benthamiana with P. capsici
According to our previous study [12], 2 mL of dsRNAs (25 ng/μL) were infiltrated 2 days before P. capsici infection into the abaxial side of N. benthamiana leaves using a 1 mL needle-free syringe. One day later, the mycelium of P. capsici on V8 juice agar media was scraped and incubated overnight at 23 °C under continuous light to form sporangia. The next day, the plate was filled with 10 mL of distilled water and incubated at 4 °C for 1 h to harvest the zoospores. The zoospore suspension was counted with a hemocytometer and adjusted to a concentration of 5 × 104 zoospores mL−1. The abaxial side of detached N. benthamiana leaves was infected with a 12 μL droplet of zoospore suspension. Infected leaves were incubated in the dark at 23 °C for about 24 h and P. capsici infection was confirmed via phenotypic observation before sampling the leaves.
Observation of chlorophyll fluorescence expression
Chlorophyll fluorescence was confirmed by the fluorescence in vivo imaging system FOBI (Neoscience, Republic of Korea) under blue light combined with a yellow filter. This method was used to confirm P. capsici infection because infected lesions do not exhibit chlorophyll fluorescence. The size of infected lesions was quantified using ImageJ [22].
Total RNA extraction
After observing chlorophyll fluorescence, N. benthamiana leaves were sampled and ground in liquid nitrogen. Total RNA was isolated using RiboEx (GeneAll, Republic of Korea) and treated with recombinant DNase I (Takara, Japan) to eliminate single-stranded and double-stranded DNA, according to the manufacturer’s instructions.
Quantitative real-time PCR (qRT-PCR)
Complementary DNA was synthesized from 1 μg of each RNA sample using PrimeScript Reverse Transcriptase (Takara, Japan) and oligo (dT) primers (Thermo Scientific, United States), according to the manufacturer’s instructions. qRT-PCR was performed using Light Cycler 480 SYBR Green I Master (Roche, United States) with SYBR Green detection and gene-specific primers. The Ct values for genes were obtained using NbL23 as a control [23], and relative expression values were calculated using the ΔΔCt method. The primers for defense-related genes were kindly provided by Dr. Doil Choi’s Laboratory (Seoul National University, Seoul, Republic of Korea) and sequences of all the primers are listed in Additional file 1: Table S2.
Results
Design of dsRNAs targeting PcNLP2 and PcNLP6 in P. capsici
In our previous study [12, 13], treating plants with dsRNAs targeting different regions in the target mRNA sequence yielded different effects on the target gene. Likewise, we first examined which region of the PcNLP2 and PcNLP6 mRNAs should be targeted to most effectively suppress P. capsici infection. We designed two dsRNAs for each gene: dsRNA_PcNLP2_5′ and dsRNA_PcNLP2_3′ targeting the 5′ and 3′ regions of PcNLP2, dsRNA_PcNLP6_5′ and dsRNA_PcNLP6_3′ targeting the 5′ and 3′ regions of PcNLP6, respectively (Fig. 1). A total of four dsRNAs were synthesized to evaluate their suppressive effects on P. capsici infection.
Determination of the treatment time of dsRNAs and P. capsici
In our previous study, pepper mottle virus was suppressed the most when dsRNAs were infiltrated 2 days before viral inoculation [12], suggesting that pre-treatment of dsRNAs provides sufficient time for siRNA production and RISC formation to counteract virus infection. Hence, we injected dsRNAs into N. benthamiana leaves 2 days before P. capsici inoculation as well (Additional file 1: Fig. S2a).
We performed qRT-PCR at 3, 6, 12, 24, 27, 30, and 36 h post-inoculation (hpi) of P. capsici (without dsRNAs) to examine the kinetics of PcNLP2 and PcNLP6 expression during the infection stage in N. benthamiana leaves. The highest PcNLP2 and PcNLP6 expressions were observed at 24 hpi (Additional file 1: Fig. S3). Therefore, we measured the size of infected lesions and PcNLP2/PcNLP6 expression in P. capsica-inoculated leaves at 24 hpi using FOBI and qRT-PCR, respectively (Additional file 1: Fig. S2).
P. capsici via suppression dsRNAs targeting PcNLP2 and PcNLP6
dsRNAs targeting PcNLP2, PcNLP6, or mock (50 µg each) were infiltrated into the abaxial side of 3 week-old N. benthamiana leaves, which were inoculated with P. capsici zoospore drops 2 days later. At 24 hpi, infected lesions were significantly suppressed in dsRNA_PcNLP2_5′-treated leaves compared with mock-treated leaves (Fig. 2a, b), but no significant change in lesion size was observed in dsRNA_PcNLP2_3′-treated leaves (Additional file 1: Fig. S4a). On the contrary, dsRNA_PcNLP6_3′-treated leaves showed significantly suppressed lesions (Fig. 3a, b), while dsRNA_PcNLP6_5′-treated leaves did not (Additional file 1: Fig. S4b). These results indicate that dsRNA-mediated gene suppression varies with the region targeted, highlighting the importance of dsRNA design.
Suppression of Phytophthora capsici infection and PcNLP2 expression via PcNLP2-targeting dsRNA. a The left figure shows the scheme of dsRNA treatment and P. capsici infection, and the right figure shows the phenotype result of P. capsici infection after treatment with mock and dsRNA_PcNLP2_5′, respectively, in 3 week-old Nicotiana benthamiana leaves using a fluorescence in vivo imaging system FOBI. Scale bar = 1 cm. b Quantification of P. capsici infection lesion size using ImageJ. c Quantification of PcNLP2 transcript level using quantitative real-time PCR (qRT-PCR). Mock: Treated with dsRNA targeting Renilla luciferase; dsRNA_PcNLP2_5′: Treated with dsRNA_PcNLP2_5′. Data represent mean ± standard error of mean (SEM; N = 3). Significance is determined by Student’s t-test, *P < 0.05 and **P < 0.01
Suppression of P. capsici infection and PcNLP6 expression via PcNLP6-targeting dsRNA. a The left figure shows the scheme of dsRNA treatment and P. capsici infection, and the right figure shows the phenotype result of P. capsici infection after treatment with mock and dsRNA_PcNLP6_3′, respectively, in 3 week-old N. benthamiana leaves using FOBI. Scale bar = 1 cm. b Quantification of P. capsici infection lesion size using ImageJ. c Quantification of PcNLP6 transcript level using qRT-PCR. Mock: Treated with dsRNA targeting Renilla luciferase. dsRNA_PcNLP6_3′: Treated with dsRNA_PcNLP6_3′. Data represent mean ± SEM (N = 3). Significance is determined by Student’s t-test, *P < 0.05 and **P < 0.01
Additionally, we analyzed PcNLP2 and PcNLP6 transcript levels in leaves-treated with dsRNA_PcNLP2_5´ and dsRNA_PcNLP6_3´, respectively. Compared with mock-treated leaves, PcNLP2 and PcNLP6 expression were more than two-fold lower in dsRNA_PcNLP2_5′-treated leaves and dsRNA_PcNLP6_3′-treated leaves, respectively (Figs. 2c and 3c). Therefore, the exogenous application of these designed dsRNAs could significantly suppress P. capsici infection and inhibit PcNLP2 and PcNLP6 expression in N. benthamiana.
Expression of defense-related genes in N. benthamiana treated with dsRNAs
We investigated the expression of some well-known plant defense-related genes in response to targeting PcNLP2 and PcNLP6 with dsRNAs. We performed qRT-PCR on three defense-related genes in N. benthamiana: Pathogenesis-related 1 (PR1), a defense factor that responds to pathogen infection; WRKY8, a disease-associated gene that interacts with mitogen-activated protein kinase involved in plant innate immunity; and Harpin-induced 1 (Hin1), a marker gene for hypersensitive response [24,25,26].
Compared with mock-treated leaves, the expression of NbPR1 and NbHin1 decreased by six-fold each and that of NbWRKY8 by two-fold in dsRNA_PcNLP2_5′-treated leaves. Interestingly, dsRNA_PcNLP6_3′ treatment only reduced the expression of NbHin1 by three-fold, while the expressions of NbPR1 and NbWRKY8 were not affected significantly (Fig. 4). These results suggest that suppression of PcNLP2 and PcNLP6 by dsRNAs may play distinct roles in the expression of NbPR1 and NbWRKY8.
Changes in expression of defense-related genes after treatment with dsRNAs targeting PcNLP2 and PcNLP6, respectively. Quantification of the expression of defense-related genes a NbPR1, b NbWRKY8, and c NbHin1 using qRT-PCR. WT: Uninfected wild-type N. benthamiana; Mock: P. capsica-infected N. benthamiana treated with dsRNA targeting Renilla luciferase; dsRNA_PcNLP2_5′: P. capsica-infected N. benthamiana treated with dsRNA_PcNLP2_5′; dsRNA_PcNLP6_3′: P. capsica-infected N. benthamiana treated with dsRNA_PcNLP6_3′. Data represent mean ± SEM (N = 3). Significance is determined by Student’s t-test, **P < 0.01 and ***P < 0.001
Discussion
Chemical fungicides have been traditionally used to control P. capsici [2, 4], but their long-term use and accumulation may induce unknown mutations in the pathogen. dsRNA-mediated RNAi technology is now being widely used to target plant genes, insects, viruses, and fungi [6]. This approach enables us to respond to plant diseases faster than any other technology, especially in an ecosystem where disease-related mutations are frequent. When a specific gene mutation leads to the emergence of a new plant disease that resists existing chemical pesticides, it can be quickly managed by using dsRNAs targeting the specific mutated gene.
To date, no study has demonstrated the suppression of P. capsici via the suppression of PcNLPs. Our study showed that P. capsici was effectively suppressed using dsRNAs targeting the P. capsici effector genes PcNLP2 and PcNLP6. In particular, dsRNAs targeting different mRNA regions in PcNLP2 and PcNLP6 brought about different effects on P. capsici suppression (Figs. 2, 3, and Additional file 1: File. S4). In our previous study [12], differential cleavage tags of target genes were observed in dsRNA-treated samples. Therefore, the different effects of dsRNAs on P. capsici may be because different amounts of siRNAs are produced from distinct dsRNA sequences, which can be confirmed by analyzing the pool of small RNAs in dsRNA-treated samples.
Since NLPs are known to affect plant defense and immune responses [19], we explored the expression of three well-known defense-related genes in response to PcNLPs suppression using qRT-PCR [27]. Interestingly, inhibition of PcNLP2 significantly downregulated NbPR1, NbWRKY8, and, NbHin1, whereas inhibition of PcNLP6 downregulated NbHin1 only (Fig. 4). The expression of Hin1, a hypersensitive response marker gene [24], was expected to change in the same pattern as the size of P. capsica-infected lesions (Figs. 2b, 3b, and 4c). PR genes have been shown to induce systemic acquired resistance, increasing the defensive capacity of plants against necrotizing infections [25]. Silencing of WRKY8, which interacts with mitogen-activated protein kinase, has been shown to increase the burden of pathogen-induced disease [26]. Therefore, we suggest that dsRNA_PcNLP6_3′ is more effective than dsRNA_PcNLP2_5′ in maintaining the expression of NbWRKY8 and NbPR1 to retain the defense capacity of plants against other pathogen-induced diseases. We also suggest that PcNLP2 and PcNLP6 have distinct functions in the plant immune system after P. capsici infection, since their suppression distinctly influenced the expression of NbWRKY8 and NbPR1. In the future, a transcriptome-wide analysis of dsRNA-treated plants would allow us to profile disease-relevant genes, including other defense-related genes, following suppression of PcNLPs by dsRNAs. This approach would help uncover the relationship between PcNLP effector genes of P. capsici and plant defense genes.
In conclusion, this study demonstrated the successful suppression of P. capsici using dsRNAs. In addition, we suggest that PcNLP2 and PcNLP6 have distinct relationships with plant defense-related genes. Based on these results, RNAi applications using dsRNAs can be used to replace chemical pesticides as well as to screen the function of a gene of interest.
Availability of data and materials
Not applicable.
Abbreviations
- RNAi:
-
RNA interference
- dsRNA:
-
Double-stranded RNA
- siRNA:
-
Small interfering RNA
- RISC:
-
RNA-induced silencing complex
- NLP:
-
Necrosis and ethylene-inducing peptide 1-like protein
- qRT-PCR:
-
Quantitative real-time polymerase chain reaction
- hpi:
-
Hours post-inoculation
References
Rehrig WZ, Ashrafi H, Hill T, Prince J, Van Deynze A (2014) CaDMR1 cosegregates with QTL Pc5.1 for resistance to Phytophthora capsici in pepper (Capsicum annuum). Plant Genome. https://doi.org/10.3835/plantgenome2014.03.0011
Barchenger DW, Lamour KH, Bosland PW (2018) Challenges and strategies for breeding resistance in Capsicum annuum to the multifarious pathogen Phytophthora capsici. Front Plant Sci 9:628
Lamour KH, Stam R, Jupe J, Huitema E (2012) The oomycete broad-host-range pathogen Phytophthora capsici. Mol Plant Pathol 13:329–337
Sanogo S, Ji P (2012) Integrated management of Phytophthora capsicion solanaceous and cucurbitaceous crops: current status, gaps in knowledge and research needs. Can J Plant Pathol 34:479–492
Jackson KL, Yin J, Csinos AS, Ji P (2010) Fungicidal activity of fluopicolide for suppression of Phytophthora capsici on squash. Crop Prot 29:1421–1427
Dalakouras A, Wassenegger M, Dadami E, Ganopoulos I, Pappas ML, Papadopoulou K (2020) Genetically modified organism-free RNA interference: exogenous application of RNA molecules in plants. Plant Physiol 182:38–50
Kamthan A, Chaudhuri A, Kamthan M, Datta A (2015) Small RNAs in plants: recent development and application for crop improvement. Front Plant Sci 6:208
Das PR, Sherif SM (2020) Application of exogenous dsRNAs-induced RNAi in agriculture: challenges and triumphs. Front Plant Sci 11:946
Dalakouras A, Wassenegger M, McMillan JN, Cardoza V, Maegele I, Dadami E, Runne M, Krczal G, Wassenegger M (2016) Induction of silencing in plants by high-pressure spraying of in vitro-synthesized small RNAs. Front Plant Sci 7:1327
Mitter N, Worrall EA, Robinson KE, Li P, Jain RG, Taochy C, Fletcher SJ, Carroll BJ, Lu GQ, Xu ZP (2017) Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nat Plants 3:16207
Dubrovina AS, Kiselev KV (2019) Exogenous RNAs for gene regulation and plant resistance. Int J Mol Sci. https://doi.org/10.3390/ijms20092282
Yoon J, Fang M, Lee D, Park M, Kim K-H, Shin C (2021) Double-stranded RNA confers resistance to pepper mottle virus in Nicotiana benthamiana. Appl Biol Chem 64(1):1–8
Park M, Um TY, Jang G, Choi YD, Shin C (2022) Targeted gene suppression through double-stranded RNA application using easy-to-use methods in arabidopsis thaliana. Appl Biol Chem 65(1):1–8
Kweon Y, Fang M, Shin S-Y, Lee D, Kim K-H, Shin C (2022) Sequence optimization and multiple gene targeting improves the inhibitory efficacy of exogenous double-stranded RNA against pepper mottle virus in Nicotiana benthamiana. Appl Biol Chem 65(1):1–9
Chisholm ST, Coaker G, Day B, Staskawicz BJ (2006) Host-microbe interactions: shaping the evolution of the plant immune response. Cell 124:803–814
Jones JD, Dangl JL (2006) The plant immune system. Nature 444:323–329
Park JH, Shin C (2015) The role of plant small RNAs in NB-LRR regulation. Brief Funct Genomics 14:268–274
Snelders NC, Kettles GJ, Rudd JJ, Thomma B (2018) Plant pathogen effector proteins as manipulators of host microbiomes? Mol Plant Pathol 19:257–259
Qutob D, Kemmerling B, Brunner F, Kufner I, Engelhardt S, Gust AA, Luberacki B, Seitz HU, Stahl D, Rauhut T et al (2006) Phytotoxicity and innate immune responses induced by Nep1-like proteins. Plant Cell 18:3721–3744
Dong S, Kong G, Qutob D, Yu X, Tang J, Kang J, Dai T, Wang H, Gijzen M, Wang Y (2012) The NLP toxin family in Phytophthora sojae includes rapidly evolving groups that lack necrosis-inducing activity. Mol Plant Microbe Interact 25(869):909
Feng BZ, Zhu X-p, Fu L, Lv R, Storey DB, Tooley PW, Zhang XG (2014) Characterization of necrosis-inducing NLP proteins in Phytophthora capsici. BMC Plant Biol 14:126–126
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH image to imageJ: 25 years of image analysis. Nat Methods 9:671–675
Liu D, Shi L, Han C, Yu J, Li D, Zhang Y (2012) Validation of reference genes for gene expression studies in virus-infected Nicotiana benthamiana using quantitative real-time PCR. PLoS ONE 7:e46451
Pontier D, Gan S, Amasino RM, Roby D, Lam E (1999) Markers for hypersensitive response and senescence show distinct patterns of expression. Plant Mol Biol 39:1243–1255
Van Loon LC, Van Strien EA (1999) The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol Mol Plant Pathol 55:85–97
Ishihama N, Yamada R, Yoshioka M, Katou S, Yoshioka H (2011) Phosphorylation of the Nicotiana benthamiana WRKY8 transcription factor by MAPK functions in the defense response. Plant Cell 23:1153–1170
Lee HY, Mang H, Choi E, Seo YE, Kim MS, Oh S, Kim SB, Choi D (2021) Genome-wide functional analysis of hot pepper immune receptors reveals an autonomous NLR clade in seed plants. New Phytol 229:532–547
Acknowledgements
We are grateful for helpful discussions with members of the Shin laboratory. We also thank Dr. Doil Choi’s laboratory, especially Dr. Hye-Young Lee, for their materials and helpful comments.
Funding
This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2021R1A5A1032428 and No. 2022R1A2C1011032). This was also supported by a grant from the New breeding technologies development Program (Project No. PJ01652102), Rural Development Administration, Republic of Korea.
Author information
Authors and Affiliations
Contributions
CS conceived the project. MP, YK, DL performed experiments. MP, YK, and CS wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: Table S1. Primer sequences used in dsRNA synthesis. Table S2: Primer sequences used in qRT-PCR. Figure S1. The scheme of double-stranded RNA (dsRNA) synthesis. Figure S2. Diagram of the dsRNA infiltration, Phytophthora capsici infection and screening of the phenotype. (a) Diagram of the dsRNA infiltration and P. capsici infection in Nicotiana benthamiana leaves. Two days after dsRNA syringe infiltration, P. capsici infection was performed. For the P. capsici infection, detached leaf assay was performed. (b) Diagram of the phenotype screening. The infection of the P. capsici was confirmed using a fluorescence in vivo imaging system FOBI. Blue light with yellow filter was used for the screening of the lesion size. After screening the P. capsici infection, quantification of the mRNA expression level was performed using quantitative real-time PCR (qRT-PCR). Figure S3. Transcript expression levels of effector PcNLP2 and PcNLP6. (a) Transcript expression level of PcNLP2 using qRT-PCR. (b) Transcript expression level of PcNLP6 using qRT-PCR. Relative expression levels were calculated by the ΔΔCt method. Data represent mean ± standard error of mean (SEM; N = 3). Significance is determined by Student’s t-test, ***P < 0.001. Figure S4. Phenotype of P. capsici infection in N. benthamiana leaves treated with dsRNA_PcNLP2_3′ and dsRNA_PcNLP6_5′. (a) The left figure shows the scheme of dsRNA treatment and P. capsici infection, and the right figure shows the phenotype result of P. capsici infection after treatment with mock and dsRNA_PcNLP2_3′, respectively, in 3 week-old N. benthamiana leaves using FOBI. Scale bar = 1 cm. (b) The left figure shows the scheme of dsRNA treatment and P. capsici infection, and the right figure shows the phenotype result of P. capsici infection after treatment with mock and dsRNA_PcNLP6_5′, respectively, in 3 week-old N. benthamiana leaves using FOBI. Scale bar = 1 cm.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Park, M., Kweon, Y., Lee, D. et al. Suppression of Phytophthora capsici using double-stranded RNAs targeting NLP effector genes in Nicotiana benthamiana. Appl Biol Chem 66, 5 (2023). https://doi.org/10.1186/s13765-023-00768-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13765-023-00768-4