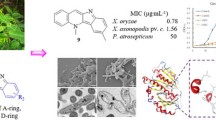
Abstract
Natural products decursin and decursinol angelate were recently reported as benign fungicides for controlling rice blast. Inspired by the structural similarity of the cumarin compounds and gained hint from the skeletal motifs, we designed and prepared synthetic compounds to increase the natural product efficacy and evaluated their antifungal activities against various plant disease pathogens in vitro. Synthetically prepared compound 4 and 5 indeed suppressed the mycelial growth of B. cinerea, F. oxysporum, P. italicum, and R. quercus-mongolicae. Additionally, compound 5 effectively prevents the growth of C. coccodes and C. parasitica. Furthermore, both 4 and 5 possess better inhibitory activities on spore germination of F. oxysporum and M. oryzae than the natural product decursin and commercial pesticide Iprodione. These results suggest that the effect of the lead compound for plant disease protection can be improved by tuning the structure of the original natural product and decursinol chloroacrylates 4 and 5 are candidates for the control of F. oxysporum and M. oryzae.
Similar content being viewed by others
Introduction
Plant diseases have damaged the quantity and the quality of crop production, causing massive economic problems and further threatening global food safety. Plant disease symptoms vary in type and severity, and can even lead to the death of animals that feed on. For example, rice blast disease caused by M. Oryzae now spreads in 85 rice-cultivating countries. It causes leaf, neck rot, panicle, node blast, and collar rot, and an annual loss in global rice production is about 10 to 30% [1]. Also, B. cinerea, which causes soft rot and gray mold, has a wide host range of more than 200, including grapefruit and vegetables, resulting in significant economic losses. Although several chemical reagents are available to prevent this pathogen, the genomic plasticity of B. cinereal makes the control difficult [2]. To date, chemical fungicides are one of the most widely utilized means of controlling plant diseases worldwide. However, long-term use of them can increase the resistance of pathogens and cause fatal damage to aquatic ecosystems [3, 4]. Therefore, it is urgent to develop new fungicides that are fatal to fungi but harmless to the environment and animals.
Since the discovery of penicillin, there has been continuous research on developing drugs and crop protecting reagents through chemical modification and simplification of natural bioactive compounds. Natural substances have biocompatibility, high selectivity, and low environmental impact so that similar effects can be expected on their derivatives [5]. For instance, Canagliflozin, sold under the name Invokana to treat type 2 diabetes, is a derivative of phlorizin, a natural substance found in the root bark of unripe apples. The drug, which acts as an inhibitor of SGLT2, has developed into analogues with increased selectivity, such as dapagliflozin and canagliflozin [6]. Natural products Strobilurin A and B were first isolated from S. tenacellus in 1977 and found that they have inhibitory activity against various fungi. Although strobilurins rapidly inhibit spore germination fungi and do not cause harm to terrestrial animals, they result in critical damage to aquatic animals. In the past 20 years, numerous synthetic strobilurin fungicides such as pyraclostrobin, fluoxastrobin and orysastrobin have been developed and used [7].
Decursin 1 is one of the coumarin compounds in Angelica gigas, which is used as a medicinal agent in Oriental Medicine. In 1966, the substance was first isolated, and its biochemical activity has since been studied [8]. Decursin has (1) inhibitory effects on several cancer cell lines, including breast cancer, and (2) potential as the medicine of inflammatory diseases caused by macrophages [9, 10]. On the other side, the antimicrobial effects of decursin have rarely been studied in the field of crop protection. Interestingly, Kim and coworkers disclosed decursin and its constitutional isomer decursinol angelate (B) inhibit spore germination and mycelial growth of M. oryzae, proving decursin useful in plant disease control, especially rice blast [11, 12].
Decursin and decursinol angelate have the same molecular formula but different substitution patterns at the unsaturated ester part (Scheme 1). The skeletal difference is subtle, but the inhibition rate was noticeably different [12]. We envisioned that changing substituents in the unsaturated ester part could increase or decrease the inhibition rate and eventually develop a benign candidate for rice blast. With the question in mind, we designed several unsaturated ester tales, focusing on the possible modification sites (Scheme 2). Based on the rationale, derivatives 2–6 of decursin were synthesized from decursinol by Steglich esterification conditions and were tested their activities on various plant pathogens (Table 1).
Materials and methods (including Safety information)
Unless otherwise noted, all reactions were carried out under Ar in flamed-dried glassware using anhydrous solvents. Anhydrous solvents were prepared by distillation over the indicated drying agents prior to use and were transferred under Ar: THF, Et2O (Mg/anthracene), toluene (Na/K), CH2Cl2, MeOH (Mg); DMF and Et3N were dried by an adsorption solvent purification system based on molecular sieves. Thin layer chromatography (TLC): Macherey–Nagel precoated plates (POLYGRAM®SIL/UV254). Flash chromatography: Merck silica gel 60 (40−63 µm) with technical grade solvents. NMR: Spectra were recorded on Bruker AV VIII 400 or 600 spectrometers in the solvents indicated. The solvent signals were used as references, and the chemical shifts were converted to the TMS scale (CDCl3: δC = 77.0 ppm; residual CHCl3 in CDCl3: δH = 7.26 ppm; CD3OD: δC = 49.0 ppm; residual CHD2OD in CD3OD: δH = 3.31 ppm; CD2Cl2: δC = 54.0 ppm; residual CHDCl2 in CD2Cl2: δH = 5.32 ppm). FT-IR spectra were obtained on Thermo Scientific Nicolet 6700 and reported in frequency of the absorption (cm−1). High resolution mass spectra (HRMS) were recorded on an AB SCIEX Q-TOF 5600 mass spectrometer. Optical rotation (\({[\alpha ]}_{D}^{20}\) and \({[\alpha ]}_{D}^{25}\)): Krüss P8000-T, 10 cm/1 mL cell. Unless otherwise noted, all commercially available compounds (Acros, Aldrich, Alfa Aesar, TCI) were used as received. Melting points were determined on a A. KRÜSS OPTRONIC M3000.
Decursin (compound 1)
N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (0.392 g, 2.00 mmol, 2 equiv) and DMAP (0.0489 g, 0.0400 mmol, 0.4 equiv) were added to a stirred mixture of 3-methyl crotonic acid (0.120 g, 1.20 mmol, 1.2 equiv) and decursinol (0.246 g, 1.00 mmol) in CH2Cl2 (8 mL) at room temperature. After stirring overnight, the reaction was quenched with H2O. After phase separation, the aqueous layer was rinsed with ethyl acetate. The organic extracts were combined, dried over Na2SO4, and concentrated in vacuo. Purification of the crude product by flash chromatography (hexane:EtOAc, 7:3) gave the title compound as a white solid (0.199 g, 60.6%). Rf – 0.80 (50% EtOAc: 50% Hexane); 1H NMR (400 MHz, Chloroform-d) δ 7.57 (d, J = 9.4 Hz, 1H), 7.14 (s, 1H), 6.78 (s, 1H), 6.23 (d, J = 9.4 Hz, 1H), 5.66 (s, 1H), 5.07 (app.t, J = 4.9 Hz, 1H), 3.18 (dd, J = 17.1, 4.8 Hz, 1H), 2.85 (dd, J = 17.1, 4.8 Hz, 1H), 2.13 (s, 3H), 1.87 (s, 3H), 1.37 (s, 3H), 1.35 (s, 3H); 13C NMR (101 MHz, Chloroform-d) δ 165.9, 161.4, 158.6, 156.6, 154.3, 143.3, 128.8, 116.1, 115.7, 113.4, 112.9, 104.8, 76.9, 69.2, 28.0, 27.6, 25.1, 23.3, 20.5; HR-MS (ESI): m/z calcd for C19H21O5+ [M + H]+: 329.1384, found 329.1384
Spectral characteristics were identical to those previously reported [13].
(S)-8,8-Dimethyl-2-oxo-7,8-dihydro-2H,6H-pyrano[3,2-g]chromen-7-yl (E)-2-methylbut-2-enoate (compound 2)
N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (0.636 g, 3.24 mmol, 2 equiv) and DMAP (0.0792 g, 0.648 mmol, 0.4 equiv) were added to a stirred mixture of Tiglic acid (0.195 g, 1.95 mmol, 1.2 equiv) and decursinol (0.400 g, 1.62 mmol) in CH2Cl2 (8 mL) at room temperature. After being stirred at this temperature overnight, the reaction was quenched with H2O. After phase separation, the aqueous layer was rinsed with ethyl acetate. The organic extracts were combined, dried over MgSO4, and concentrated in vacuo. Purification of the crude product by flash chromatography (hexane:EtOAc, 7:3) gave the title compound as transparent oil (0.465 g, 87.5%). Rf – 0.69 (50% EtOAc: 50% Hexane); 1H NMR (400 MHz, Chloroform-d) δ 7.58 (d, J = 9.5 Hz, 1H), 7.15 (s, 1H), 6.84 – 6.78 (m, 2H), 6.23 (d, J = 9.5 Hz, 1H), 5.08 (t, J = 5.0 Hz, 1H), 3.25 – 3.15 (m, 1H), 2.88 (dd, J = 17.2, 5.3 Hz, 1H), 1.82 – 1.78 (m, 3H), 1.76 (m, 3H), 1.39 (s, 3H), 1.37 (s, 3H); 13C NMR (101 MHz, Chloroform-d) δ 167.3, 161.4, 156.6, 154.4, 143.3, 138.6, 128.8, 128.3, 116.0, 113.5, 113.0, 104.8, 76.9, 70.3, 27.9, 25.2, 23.3, 14.6, 12.2; HR-MS (ESI): m/z calcd for C19H21O5+ [M + H]+: 329.1384, found 329.1383.
(S)-8,8-dimethyl-2-oxo-7,8-dihydro-2H,6H-pyrano[3,2-g]chromen-7-yl (E)-pent-2-enoate (compound 3)
A mixture of (S)-(+)-decurinol (0.144 g, 0.583 mmol, 1 equiv), N,N’-Dicyclohexylcarbodiimide (0.181 g, 0.875 mmol, 1.5 equiv), and 4-(dimethylamino)pyridine (0.0285 g, 0.233 mmol, 0.4 equiv) was dissolved in anhydrous dichloromethane. Then, trans-2-pentenoic acid (0.064 ml, 0.641 mmol, 1.1 equiv) was added, and the reaction mixture was stirred at room temperature overnight. The reaction mixture was then filtered through a pad of Celite with CH2Cl2, and the filtrate was concentrated in vacuo. Purification of the crude product by flash chromatography (hexane:EtOAc, 7:3) gave the title compound as a white solid (0.168 g, 88.3%). Rf – 0.68 (50% EtOAc: 50% Hexane); 1H NMR (400 MHz, Chloroform-d) δ 7.58 (d, J = 9.5 Hz, 1H), 7.15 (s, 1H), 7.04 (dt, J = 15.6, 6.3 Hz, 1H), 6.81 (s, 1H), 6.23 (d, J = 9.5 Hz, 1H), 5.80 (dt, J = 15.7, 1.7 Hz, 1H), 5.11 (app.t, J = 4.9 Hz, 1H), 3.20 (dd, J = 18.2, 4.9 Hz, 1H), 2.88 (dd, J = 17.2, 4.8 Hz, 1H), 2.27 – 2.15 (m, 2H), 1.39 (s, 3H), 1.36 (s, 3H), 1.05 (t, J = 7.4 Hz, 3H); HR-MS (ESI): m/z calcd for C19H21O5+ [M + H]+: 329.1384, found 329.1383. Spectral characteristics were identical to those previously reported [14].
(S)-8,8-dimethyl-2-oxo-7,8-dihydro-2H,6H-pyrano[3,2-g]chromen-7-yl (Z)-3-chloroacrylate (compound 4)
A mixture of (S)-(+)-decurinol (0.202 g, 0.82 mmol, 1.0 equiv), N,N’-Dicyclohexylcarbodiimide (0.254 g, 1.23 mmol, 1.5 equiv), and 4-(dimethylamino)pyridine (0.040 g, 0.33 mmol, 0.4 equiv) was dissolved in anhydrous dichloromethane. Then, cis-chloro acrylic acid (0.096 g, 0.90 mmol, 1.1 equiv) was added, and the resulting mixture was stirred at room temperature overnight. The reaction mixture was then filtered through a pad of Celite with CH2Cl2, and the filtrate was concentrated in vacuo. Purification of the crude product by flash chromatography (hexane:EtOAc, 8:2) gave the title compound as a white solid (126.4 mg, 46.0%). %). Rf – 0.52 (50% EtOAc: 50% Hexane); M. p. 97.9–100.6 ℃;\({[\alpha ]}_{D}^{20}\): + 195.8 (c = 0.1, CHCl3); 1H NMR (400 MHz, Chloroform-d) δ 7.58 (d, J = 9.2 Hz, 1H), 7.16 (s, 1H), 6.80 (s, 1H), 6.75 (d, J = 8.3 Hz, 1H), 6.24 (d, J = 9.5 Hz, 1H), 6.18 (d, J = 8.3 Hz, 1H), 5.15 (app.t, J = 4.9 Hz, 1H), 3.24 (ddd, J = 17.1, 4.8, 1.1 Hz, 1H), 2.92 (dd, J = 17.7, 5.0 Hz, 1H), 1.40 (s, 3H), 1.38 (s, 3H); 13C NMR (101 MHz, Chloroform-d) δ 162.8, 161.3, 156.4, 154.3, 143.3, 134.1, 128.8, 120.9, 115.6, 113.5, 113.0, 104.8, 76.5, 70.8, 27.8, 25.1, 23.2; IR(neat): 3100, 3046, 2982, 2921, 2849, 1721, 1625, 1564, 1516 cm−1 HR-MS (ESI): m/z calcd for C17H16ClO5+ [M + H]+: 335.0681, found: 335.0681.
(S)-8,8-dimethyl-2-oxo-7,8-dihydro-2H,6H-pyrano[3,2-g]chromen-7-yl (E)-3-chloroacrylate (compound 5)
To a solution of (S)-( +)-decurinol (0.207 g, 0.84 mmol, 1 equiv), N,N’-Dicyclohexylcarbodiimide (0.347 g, 1.68 mmol, 1.5 equiv), and 4-(dimethylamino)pyridine (0.0410 g, 0.356 mmol, 0.4 equiv) in anhydrous CH2Cl2 was added trans-chloro acrylic acid (0.0984 g, 0.924 mmol, 1.1 equiv). The reaction mixture was stirred at room temperature overnight. The reaction mixture was then filtered through a pad of Celite with CH2Cl2, and the filtrate was concentrated in vacuo. Purification of the crude product by flash chromatography (hexane:EtOAc, 8:2) gave the title compound as a white solid (69.8 g, 24.8%). Rf – 0.69 (50% EtOAc: 50% Hexane); M.p. 150.7–153.3 ℃;\({[\alpha ]}_{D}^{20}\): + 55.0 (c = 0.2, CHCl3); 1H NMR (400 MHz, Chloroform-d) δ 7.58 (d, J = 9.5 Hz, 1H), 7.35 (d, J = 13.5 Hz, 1H), 7.15 (s, 1H), 6.79 (s, 1H), 6.27–6.19 (m, 2H), 5.13 (app.t, J = 4.7 Hz, 1H), 3.21 (dd, J = 17.3, 4.8 Hz, 1H), 2.89 (dd, J = 17.3, 4.6 Hz, 1H), 1.38 (s, 3H), 1.36 (s, 3H); 13C NMR (101 MHz, Chloroform-d) δ 163.5, 161.3, 156.3, 154.4, 143.2, 139.0, 128.8, 124.4, 115.4, 113.6, 113.1, 105.0, 76.5, 70.9, 27.9, 25.0, 23.4; IR(neat): 3020, 3085, 3005, 2927, 2851, 1714, 1622, 1604, 1560 cm−1; HR-MS (ESI) m/z calcd for C17H16ClO5+ [M + H]+: 335.0689, found: 335.0681.
(S)-8,8-dimethyl-2-oxo-7,8-dihydro-2H,6H-pyrano[3,2-g]chromen-7-yl (E)-3-(pyridin-4-yl)acrylate (compound 6)
N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (0.108 g, 0.55 mmol, 1.1 equiv) and DMAP (0.0061 g, 0.05 mmol, 0.1 equiv) were added to a stirred mixture of (E)-3-(pyridin-4-yl)acrylic acid (0.082 g, 0.55 mmol, 1.1 equiv) and decursinol (0.123 g, 0.50 mmol) in CH2Cl2 (5 mL) at room temperature. After being stirring at this temperature for 24 h, the reaction was quenched with H2O. After phase separation, the aqueous layer was rinsed with CH2Cl2. The organic extracts were combined, dried over Na2SO4, and concentrated in vacuo. Purification of the crude product by flash chromatography (hexane:EtOAc, 3:7) gave the title compound as a white solid (0.169 g, 89.6%). Rf – 0.32 (70% EtOAc: 30% Hexane); M.p. 211–215 °C;\({[\alpha ]}_{D}^{20}\)= + 53.9 (c = 0.25, CH2Cl2); 1H NMR (CDCl3, 400 MHz): δ 8.72–8.59 (m, 2H), 7.79–7.53 (m, 2H), 7.36–7.30 (m, 2H), 7.18 (s, 1H), 6.83 (s, 1H), 6.58 (d, J = 16.0 Hz, 1H), 6.24 (d, J = 9.4 Hz, 1H), 5.21 (app.t, J = 4.6 Hz, 1H), 3.26 (ddd, J = 17.2, 4.8, 1.2 Hz, 1H), 3.00–2.89 (m, 1H), 1.44 (s, 3H), 1.39 (s, 3H); 13C NMR (CDCl3, 151 MHz): δ 165.3, 161.3, 156.4, 154.4, 149.6, 143.2, 142.6, 142.5, 128.8, 123.1, 122.4, 115.4, 113.7, 113.2, 105.0, 76.6, 71.0, 28.0, 25.0, 23.6; IR (film): 3067, 2981, 2931, 2852, 1724, 1626, 1562, 1515, 1135 cm−1; HRMS: Calcd for C22H20NO5+ [M + H]+ 378.1336, found 378.1342.
Experimental procedures
Fungal strains and media
Phytopathogenic fungi and oomycetes (Table 1) were used to test the antifungal activity of decursin and its derivatives. These strains were maintained on potato dextrose agar [15]. All fungal and oomycete strains were cryogenically stored in 20% glycerol at – 80 ℃ before use.
In vitro antifungal activity of decursin and its derivatives
The antifungal activity of decursin and its derivatives was evaluated by a serial broth dilution method as described previously [16]. Phytophathogenic fungi and oomycetes listed in Table 1 were used in this study. Decursin and its derivatives was dissolved in dimethylsulfoxide (DMSO) at a concentration of 20 mg/m1 as a stock solution, which was used to determine the minimal inhibitory concentration (MIC) value against mycelial growth. Decursin and its derivatives were treated in a range of 0.048–200 μg/ml and the final concentration of DMSO was 1% v/v, and potato dextrose broth treated with DMSO was used as a control. All plates were incubated for 4–5 days at 25 ℃, and MIC values were measured. The experiment was repeated three times in triplicate against each fungal and oomycete pathogen [16].
Spore germination assay
For spore germination assay, F. oxysporum strains were cultured in 5 ml of carboxymethylcellulose medium (CMC) for five days at 25 °C on a rotary shaker (200 rpm) (Leslie and Summerell, 2006). To obtain M. oryzae spores, M. oryzae strains were incubated on PDA at 25 °C for 10 days. The percentage of growth inhibition (mean ± standard deviation) was calculated from mean values as:
where A: mycelial growth in control and B: mycelial growth in sample.
Results/discussion
Chemistry
Decursin derivatives could be synthesized by Steglich esterification using carbodiimide reagents such as 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride(EDCI) reagent or N,N'-Dicyclohexylcarbodiimide (DCC) reagent, respectively. However, due to its low toxicity and easily removable byproducts, we preferred EDCI when preparing most substrates. In the case of decursinol chloroacrylates, the use of EDCI reagent did not give the desired products at all, whereas DCC reagent provided desired products in moderate yield (compound 4 and 5). Chloroacrylic acid is thought to be incompatible with EDCI∙HCl because a spot of the acid disappeared on TLC plates, and no ester product was formed. When EDCI∙HCl was used to synthesize derivative 3, isolation of the product from impurities was tedious during the purification process. On the contrary, compound 6 with an aromatic ring was obtained in higher yields using EDCI∙HCl.
In vitro antifungal activity of decursin and its derivatives against various plant pathogens
We investigated in vitro antimicrobial activity of decursin and its derivatives against various plant pathogenic fungi and oomycetes (Table 1). Most tested strains showed relatively strong resistance against decursin and derivatives except M. oryzae (MIC, 25 μg/ml) (Table 1). Whereas most derivatives of decursin showed similar antimicrobial activity against plant pathogens, 4 or 5 efficiently inhibited mycelial growth of some fungal strains (Botrytis cinerea, F. oxysporum, Penicillium italicum, and Raffaelea quercus-mongolicae). Among tested compounds, only 5 strongly inhibited the mycelial growth of Colletotrichum coccodes and Cryphonectria parasitica. In contrast, MIC values of 4 were 100 μg/m1 and 50 μg/m1 against P. italicum and R. quercus-mongolicae, respectively; MIC of 5 was over 200 μg/m1 (Table 1). We also compared the antifungal activity of the derivatives with commercial fungicide Iprodione and Azoxystrobin to gauge any possible commercial value. With respect to Magnaporthe oryzae, compound 4 and 5 are more effective than Iprodione (MIC, 12.5, 3.125, and 25 μg/ml, respectively), at least in the in vitro assay. The presence of halogen atoms within compounds 4 and 5 could contribute to the higher inhibitory effects as halogen atoms can change the electron density of molecules and provide a site for possible hydrogen bonding.
Decursin and the synthetic derivatives would be degraded into alcohols and carboxylic acids by enzymatic hydrolysis. We tested decursinol and cis-3-chloroacrylic acid to identify active components and found both are inactive. These results indicate that the head and tale component should be linked together to show the antifungal activities.
Spore germination and viability assay
The effects of decursin, 4, and 5 on the spore germination of F. oxysporum and M. oryzae were further investigated (Table 2). Decursin slightly inhibited germination of F. oxysporum spores regardless of tested concentrations. However, compounds 4 or 5 significantly decreased germination rates of F. oxysporum spores. In accordance with MIC values, most spores of M. oryzae failed to germinate when of 4 or 5 were supplemented over 50 μg/m1.
To evaluate the viability of germinated spores, we performed FDA and PI double-staining assay. In living cells, the FDA changes from non-fluorescent FDA to the green fluorescent metabolite fluorescein. Contrastively, the nucleus staining dye PI cannot pass through a viable cell membrane. As shown in Fig. 1, spores of F. oxysporum and M. oryzae successfully germinated with strong green fluorescence (Fig. 1). When decursin was treated, however, most of F. oxysporum spores germinated, but germinated spores showed abnormal and short length of germ tube. Moreover, red fluorescence in mycelial cells were easily observed, indicating that some cells of germinated spores are inviable. In treatment with 4 or 5, most mycelial cells died 24 h after germination (Fig. 1A). In M. oryzae, most spores failed to germinate became inviable after treatment of 4 and 5 (Fig. 1B).
Inhibitory effects of decursin and compound 4 and 5 against spore germination of A F. oxysporum and B M. oryzae. [Spores were stained with FDA and PI for fluorescence-based live-dead assays. The concentration of decursin, 4, and 5 was 200 mg/m1. Photographs were taken 24 h after spore germination. Scale bar = 40 μm]
Mycelial growth inhibition test
We further investigated the inhibitory effects of decursin, 4, and 5 on vegetative growth of F. oxysporum and M. oryzae (Table 3 and Fig. 2). As expected, decursin, 4, and 5 significantly reduced mycelial growth of both fungal strains and 5 showed more potent inhibitory activity than 4. Intriguingly, decursin more effectively inhibited mycelial growth of F. oxysporum than 4 or 5 (Table 3 and Fig. 2). In contrast, spore germination and initial mycelial growth were much highly inhibited by 4 or 5 compared to decursin.
In summary, a series of decursin-like compounds were synthesized through steglich esterification and tested for antifungal activities. In the bioassay, decursinol chloroacrylates selectively inhibited the mycelial growth of several fungi, while other derivatives showed no antifungal effects or similar effects to decursin. In particular, the chloroacrylates showed improved spore germination inhibition of F. oxysporum and M. oryzae. As the chloroacrylates are easily degraded in environments, and their decomposed byproducts are found to be inactive, they would be promising candidates for the control of F. oxysporum and M. oryzae. This work suggests that the effect of the lead compound in the development of fungicides for plant disease protection can be improved by modifying the structure of the original natural product. Further studies are underway in our laboratory to evaluate in vivo antifungal activities of the chloroacrylates and will be disclosed in due course.
Availability of data and materials
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- DCC:
-
N,N′-Dicyclohexylcarbodiimide
- EDCI·HCl:
-
1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide Hydrochloride
- DMAP:
-
4-Dimethylaminopyridine
References
Kato H (2001) Rice blast disease. Pestic Outlook 12(1):23–25
Williamson B, Tudzynski B, Tudzynski P, Van Kan JA (2007) Botrytis cinerea: the cause of grey mould disease. Mol Plant Pathol 8(5):561–580
Yang G-Z, Zhu J-K, Yin X-D, Yan Y-F, Wang Y-L, Shang X-F, Liu Y-Q, Zhao Z-M, Peng J-W, Liu H (2019) Design, synthesis, and antifungal evaluation of novel quinoline derivatives inspired from natural quinine alkaloids. J Agri Food Chem 67(41):11340–11353
Zubrod JP, Bundschuh M, Arts G, Brühl CA, Imfeld G, Knäbel A, Payraudeau S, Rasmussen JJ, Rohr J, Scharmüller A, Smalling K, Stehle S, Schulz R, Schäfer RB (2019) Fungicides: an overlooked pesticide class? Environ Sci Technol 53(7):3347–3365
Hao Y, Wang K, Wang Z, Liu Y, Ma D, Wang Q (2020) Lutonin A and its derivatives as novel antiviral and antiphytopathogenic fungus agents. J Agri Food Chem 68:8764–8773
Chao ED, Henry RR (2010) SGLT2 inhibition-a novel strategy for diabetes treatment. Nat Rev Drug Discov 9(7):551–559
Musso L, Fabbrini A, Dallavalle S (2020) Natural compound-derived cytochrome bc1 complex inhibitors as antifungal agents. Molecules 25(19):4582
Lü J, Kim SH, Jiang C, Lee H, Guo J (2007) Oriental herbs as a source of novel anti-androgen and prostate cancer chemopreventive agents. Acta Pharmacol Sin 28(9):1365–1372
Yim D, Singh RP, Agarwal C, Lee S, Chi H, Agarwal R (2005) A novel anticancer agent, decursin, induces G1 arrest and apoptosis in human prostate carcinoma cells. Can Res 65(3):1035–1044
Jiang C, Guo J, Wang Z, Xiao B, Lee HJ, Lee EO, Kim S-H, Lu J (2007) Decursin and decursinol angelate inhibit estrogen-stimulated and estrogen-independent growth and survival of breast cancer cells. Breast Cancer Res 9(6):1–12
Ryu S-Y, Kim Y-S, Kim H-T, Kim S-K, Choi G-J, Kim J-S, Lee S-W, Heor J-H, Cho K-Y, Kim J-C (2001) Antifungal activities of coumarins isolated from Angelica gigas and Angelica dahurica against plant pathogenic fungi. Korean J Pesticide Sci 5(3):26–35
Yoon M-Y, Kim YS, Ryu SY, Choi GJ, Choi YH, Jang KS, Cha B, Han S-S, Kim J-C (2011) In vitro and in vivo antifungal activities of decursin and decursinol angelate isolated from Angelica gigas against Magnaporthe oryzae, the causal agent of rice blast. Pestic Biochem Physiol 101(2):118–124
Lee S, Shin DS, Kim JS, Oh KB, Kang SS (2003) Antibacterial coumarins from Angelica gigas roots. Arch Pharmacal Res 26(6):449–452
Lee K, Lee JH, Boovanahalli SK, Choi Y, Choo SJ, Yoo ID, Kim DH, Yun MY, Lee GW, Song GY (2010) Synthesis of (S)-(+)-decursin and its analogues as potent inhibitors of melanin formation in B16 murine melanoma cells. Eur J Med Chem 45(12):5567–5575
Leslie JF, Summerell BA (2006) The Fusarium laboratory manual. Blackwell Publishing, Ames, I.A., USA
Nguyen HTT, Choi S, Kim S, Lee J-H, Park AR, Yu NH, Yoon H, Bae C-H, Yeo JH, Choi GJ, Son H, Kim J-C (2020) The Hsp90 inhibitor, monorden, is a promising lead compound for the development of novel fungicides. Front Plant Sci 11:371
Acknowledgements
We acknowledge the National Instrumentation Center for Environmental Management at Seoul National University for access to analytical equipment.
Funding
This work was supported by Research Resettlement Fund for the new faculty of Seoul National University (to Y. Kwon). Also, Rural Development Administration Republic of Korea supported this research (Project No. PJ016243022021 to H. Son).
Author information
Authors and Affiliations
Contributions
All authors equally contributed to the study conception and design. Y-JS and HJ synthesized and characterized materials, and JS carried out the biological assays. HS and YK prepared the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
All authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shin, YJ., Shin, J., Jang, H. et al. Decursinol chloroacrylates useful as fungicides. Appl Biol Chem 65, 53 (2022). https://doi.org/10.1186/s13765-022-00720-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13765-022-00720-y