Abstract
Background
Disinfectant towelettes are increasingly being used as a means to prevent transmission of clinically important pathogens which could lead to healthcare-associated infections (HAIs). However, the efficacy of disinfectant towelette products when tested under realistic use conditions is understudied. A test model was designed to replicate realistic wiping conditions. The objective of this study was to determine the impact of varied contact time on disinfectant towelette efficacy under these conditions.
Methods
Five product types were tested against Staphylococcus aureus (ATCC 6538) and Pseudomonas aeruginosa (ATCC 15,442) at five contact times (30 s, one min, two min, three min, and 10 min) on hard, non-porous laminate templates to determine the impact of contact time on disinfectant towelette efficacy when tested under realistic use.
Results
Product type had a significant impact on the efficacy of disinfectant towelettes when tested under conditions reflective of realistic use. The effect of contact time was limited and no differences in efficacy were seen at a contact time of one min compared with the other contact times tested. Only one disinfectant towelette product achieved a mean 5-log reduction under the tested conditions.
Conclusion
Efficacy of disinfectant towelettes was primarily impacted by product type when applied in a model designed to replicate realistic use in which only a limited effect of contact time was observed. There is a need for further investigation into which factors have the greatest impact on disinfectant towelette efficacy when applied in clinical settings.
Highlights
Contact time had limited impact on the efficacy of disinfectant towelettes.
Product type significantly impacted the efficacy of disinfectant towelettes.
Disinfectant towelettes did not achieve expected log reductions under conditions of realistic use.
Similar content being viewed by others
Introduction
In the United States, an estimated 3.2% of hospital patients are affected by healthcare-associated infections (HAIs) [1]. These infections are caused by several pathogens such as Staphylococcus aureus and Pseudomonas aeruginosa, both of which ranked among the top four most frequently reported HAI pathogens from 2015 to 2017 [2]. Research suggests that over half of HAIs may be preventable [3]. Cleaning and disinfection of environmental surfaces are often recommended as strategies for controlling HAIs [4] as there is evidence that these measures are associated with lower transmission and infection frequency [5,6,7]. Improvements in disinfection are of particular interest, as they have been noted to reduce HAI [8] and several studies have indicated that the use of disinfectants results in better outcomes than cleaning alone [9, 10].
In recent years, disinfectant towelette product use has increased [11, 12] as these products provide numerous benefits over traditional methods of disinfection, as reviewed by Boyce, (2021) [13]. Disinfectant towelette products have been demonstrated to out-perform other methods of disinfection [14] and lead to increases in compliance with cleaning protocols [15]. Despite their benefits, experts are concerned that the methods currently used to test products do not accurately reflect the way these products are typically used [16, 17]. There is a need for further research on the performance of disinfectant towelette products under conditions encountered under realistic use, as reviewed by Boyce, 2021 [13] and Song et al., 2019 [11]. This includes the need for further study of realistic contact times [13].
The contact times for many disinfectant products have been criticized as being unrealistic [12]. Berendt et al., (2011) [18] observed wiping to occur for only 1–2 s for a given object. A contact time of one min has been utilized previously to reflect a realistic contact time [19] and has been considered by experts to be adequate for disinfection with liquid disinfectant products [20]. While other studies have examined the impact of contact time on the efficacy of disinfectant towelettes [21, 22], few have studied this variable under conditions reflective of realistic use. Tarka et al., (2019) [23] examined the efficacy of several disinfectant towelette products across a range of contact times; however, the products studied all had contact times of one min or less, and the contact times examined were at or beyond the label contact time for all products studied.
The objective of this study was to determine the efficacy of disinfectant towelette products when applied for a practical, relevant contact time of one min compared to contact times of both shorter and longer duration, and when done so under conditions designed to reflect realistic use. We tested five disinfectant towelette products against S. aureus and P. aeruginosa below, at, and beyond label-defined contact time using an experimental model designed to reflect realistic use. We hypothesized that contact time would significantly affect the efficacy of the disinfectant towelette products tested, and that disinfectant towelette products with longer label contact times would not achieve the desired efficacy at lower contact times (i.e., one min) that are more representative of those seen with realistic use.
Materials and methods
Five disinfectant towelette products (Table 1) were tested against S. aureus (ATCC 6538) and P. aeruginosa (ATCC 15,442) at five contact times (30 s, one min, two min, three min, and 10 min). Three biological replicates were conducted for each disinfectant, contact time, and bacteria permutation. Experimental procedures were adapted from EPA MB-33-00 [24].
Preparation of the test suspension
Test suspensions with soil load were prepared according to EPA MB-33-00 [24]. Briefly, 10 mL of tryptic soy broth (TSB; BD, New Jersey, USA) were inoculated with 100 µL of thawed bacterial stock and incubated at 36 ± 1 °C for 16–24 h with 200 rpm shaking. Soil components were added to the bacterial cultures to produce a test suspension containing 0.25% (w/v) bovine serum albumin (Thermo Fisher, Waltham, MA), 0.35% (w/v) yeast extract (Thermo Fisher, Waltham, MA), and 0.08% mucin (MilliporeSigma, Burlington, MA).
Preparation of the wiping templates
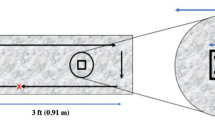
Wiping templates were defined as 2 ft x 2 ft squares on Formica® laminate sheets (Midwest Manufacturing, Eau Claire, WI) (Fig. 1). Templates were disinfected prior to inoculation by applying 10% bleach, followed by 0.3% hydrogen peroxide. The board was then rinsed three times with sterile ultrapure water followed by a final application of 70% ethanol. Each template was inoculated with five 10 µL droplets of test suspension in the designated inoculation area (Fig. 1). This volume yielded an average recovery of 1.2 × 107 CFU for S. aureus and 2.8 × 107 CFU for P. aeruginosa upon drying. The templates were left undisturbed under ambient conditions until the inoculum was fully dried (approximately 1–2 h).
A wiping template (left) was defined as 2 ft x 2 ft in area. A 2.5 cm x 2.5 cm square was traced to serve as the inoculation area. Within this square, five droplets of 10 µL inoculum were dispensed in an “X” formation. A sampling area 10 cm in diameter was defined around the inoculation area. The sampling area was swabbed with a neutralizing buffer saturated swab to collect residual test inoculum. This design was adapted from EPA MB-33-00 [24]
Preparation of the disinfectant wipes
Four of the five test disinfectants (HP1, QAC1, QAC2, and QAC3; Table 1) were purchased as ready-to-use pre-saturated wipes. Prior to use, canisters were inverted several times to distribute the disinfectant throughout the canister and lids were wiped with 70% ethanol to minimize contamination during handling. Prior to testing, the first three wipes were removed from the canister to ensure that the wipes used for testing contained an even distribution of liquid disinfectant. QAC4 was purchased as a concentrate and diluted 1:256 in synthetic hard water and applied to EasyWipes (Diversey Holdings LTD, Fort Mill, SC). Synthetic hard water was prepared using the guidance for AOAC Hard Water as described in EPA MB-30-02 [25]. EasyWipes were cut to approximately 6” x 6.8”, a size comparable to that of the ready-to-use wipes used in the study. The dry wipes were impregnated on the day of use using a liquid-to-towelette ratio of 4.9 mL diluted disinfectant per wipe based on the suggested ratio provided on the EasyWipes canister.
Disinfectant testing
A disinfectant wipe was applied to the upper left-hand corner of the wiping template and moved across the template manually using four horizontal passes (Fig. 2A). This design allowed for partial depletion of the disinfectant towelette prior to wiping over the inoculation zone. Contact time was initiated at the end of the final pass. All wiping procedures were performed by the same individual to minimize variability. After the defined contact time had elapsed for each product, a PUR-Blue™ large tip neutralizing surface swab (World Bioproducts LLC, Woodinville, WA) was passed over the sampling area three times to collect the sample (Fig. 2B). The swab was vortexed for 30 s [26] prior to serial dilution of the neutralizing buffer in phosphate buffered saline. Dilutions were filtered over 0.2 μm polyethersulfone filters (Pall Corporation, Port Washington, NY) using an EZ-Fit™ Manifold filtration system (MilliporeSigma, Burlington, MA). Filters were plated onto tryptic soy agar (TSA; BD, Franklin Lakes, NJ) and incubated at 36 ± 1 °C for 24–48 h prior to counting. A non-inoculated template served as a negative control and two inoculated, untreated templates served as positive controls. Negative controls were swabbed with macrofoam swabs (World Bioproducts LLC, Woodinville, WA) saturated with sterile phosphate buffered saline and processed as described above.
A. Wiping pattern on the board. Templates were wiped in four horizontal passes. Contact time for each product was set to record post-wiping. B. The sampling area was swabbed three times in its entirety; the first pass was done horizontally, the second pass vertically, and the third pass at a diagonal starting in the upper left and ending in the lower right of the sampling area
Terminal cleaning
After all templates were sampled, a terminal cleaning protocol was performed to remove residual soils prior to further testing. A 10% bleach solution was applied for a minimum of 10 min followed by 0.3% hydrogen peroxide. A multi-purpose cleaning spray (Babyganics, Westbury, NY) was applied and wiped dry with paper towels. The board was then rinsed once with ultrapure water followed by a final application of 70% ethanol.
Data analysis
Dilutions yielding colony counts of 20–200 CFU were used for calculating bacterial recoveries. If two or more dilutions for a given test yielded a count within this range, the dilution with the higher total CFU was used. When no colonies were present across any dilutions filtered, a count of 1 CFU was used for calculations at the lowest dilution filtered. All colony counts from untreated and treated templates were log-transformed. Bacterial log reduction was calculated by subtracting the log density of a treated template from the average log density of the two control templates for a given replicate. Three biological replicates were performed for each combination of disinfectant and organism.
Statistical analyses were conducted in SAS software, Version 9 of the SAS system for Linux. Copyright© 2012–2018 SAS Institute Inc. SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc., Cary, NC, USA. A general linear model was performed to determine the overall effects of contact time and product type on disinfectant efficacy for each organism studied. A Tukey’s t-test post-hoc analysis was conducted to determine differences among individual contact times and products for a given organism. Figures were prepared using Microsoft Office Suite (Microsoft Office 365) and GraphPad Prism version 9.4.1 for Mac OS X, GraphPad Software, San Diego, CA, USA.
Results
Overall, we found that the efficacy of the disinfectant towelettes was significantly affected by product type, but the effect of contact time was limited. The mean log10 reductions for each product type at all tested contact times i.e., 30 s, one min, two min, three min, and 10 min for P. aeruginosa and S. aureus are shown in Figs. 3 and 4, respectively.
Contact time has limited effect on bacterial log reduction
For tests against P. aeruginosa, a contact time of 10 min resulted in significantly higher log reductions when compared with a contact time of 30 s (Fig. 5; p = 0.0129). No significant differences among individual contact times were observed for S. aureus(Fig. 6). No significant difference in bacterial log reduction was observed with a contact time of one min compared with any other contact time tested for either organism.
Efficacy Achieved by Disinfectant Towelette Products Against P. aeruginosa by Contact Time. Least Squares Means with 95% Confidence Intervals log10 reduction of P. aeruginosa achieved at each contact time tested across all products. Letters are Tukey groupings for disinfectant towelette efficacy at different contact times against S. aureus. Bars with the same letters are not statistically different
Efficacy Achieved by Disinfectant Towelette Products Against S. aureus by Contact Time. Least Squares Means with 95% Confidence Intervals log10 reduction of S. aureus achieved at each contact time tested across all products. Letters are Tukey groupings for disinfectant towelette efficacy at different contact times against S. aureus. Bars with the same letters are not statistically different
Product type significantly affects bacterial log reduction
In testing against P. aeruginosa, HP1 and QAC3 yielded greater log reductions than QAC1 (Fig. 7; p < 0.0001 for both), QAC2 (Fig. 7; p < 0.0001 for both), and QAC4 (Fig. 7; p < 0.0001 for both). QAC4 yielded significantly higher log reductions compared with QAC1 (Fig. 7; p = 0.0287). In testing against S. aureus, HP1 yielded a higher overall log reduction compared to all other products tested (Fig. 8; p < 0.0001 for QACs 1–4). Testing with QAC4 resulted in greater log reduction of S. aureus than with QAC1 (Fig. 8; p < 0.0001) and QAC2 (Fig. 8; p = 0.0005). QAC3 also yielded significantly greater log reductions than did QAC1 (Fig. 8; p < 0.0001) and QAC2 (Fig. 8; p = 0.0019).
Efficacy Achieved by Disinfectant Towelette Products Against P.aeruginosa by Product Type. Least Squares Means with 95% Confidence Intervals log10 reduction of P. aeruginosa achieved by each product type across all contact times tested. Letters are Tukey groupings for disinfectant towelette efficacy against P. aeruginosa. Bars with the same letters are not statistically different
Efficacy Achieved by Disinfectant Towelette Products Against S.aureus by Product Type. Least Squares Means with 95% Confidence Intervals log10 reduction of S. aureus achieved by each product type across all contact times tested. Letters are Tukey groupings for disinfectant towelette efficacy against S. aureus. Bars with the same letters are not statistically different
Discussion
The objective of this study was to determine the impact of contact time on the efficacy of disinfectant towelette products against two clinically relevant bacteria i.e., S. aureus and P. aeruginosa, under conditions designed to reflect realistic use. The results from this study indicate that the impact of contact time on the efficacy of disinfectant towelettes is limited under these conditions. When evaluating the effect of contact time on disinfectant efficacy overall, contact time did not have a significant effect on disinfectant towelette efficacy in testing against S. aureus when comparing the individual contact times tested. For P. aeruginosa, the only significant difference seen among specific contact times was for that of 30 s and 10 min, which were the two most extreme contact times tested in this study. Further, no significant differences were observed between a contact time of one min and any of the other contact times tested when examining the overall effect of contact time.
The effect of contact time was evaluated as the performance of all disinfectant towelette products against the test organism at a given timepoint. The aim of this study was to determine if a practical contact time of 1 min could generally achieve sufficient disinfection, not to evaluate the performance of individual disinfectant towelette products. Therefore, we did not evaluate the effect of contact time for each individual disinfectant towelette product.
We hypothesized that a contact time of one min would not be sufficient for products with a label contact time of longer duration. Previous research [27] demonstrated that disinfectants were significantly less effective at contact times shorter than label contact time; however, the method used for testing efficacy was different than the one used in the present study as Hong et al., (2017) [27] examined liquid disinfectants. West et al., (2018) [28] also observed a significant effect of contact time when testing liquid disinfectant. The present findings suggest that the effect of contact time is different when examining disinfectant towelette products using a method designed to reflect realistic use. Research examining the efficacy of disinfectant towelette products reports similar findings to those seen in the study presented here. West et al., (2019) [22] observed that the type of disinfectant towelette product tested against S. aureus significantly impacted efficacy while contact time did not. Brown et al., (2020) [21] found that the type of disinfectant used had a greater effect on disinfectant efficacy than did contact time. The discrepancy may be related to the type of disinfectant product studied, i.e., liquid disinfectants vs. disinfectant towelettes, as experts have already defined contact time differently for liquid disinfectants versus disinfectant towelette and spray products due to differences in their testing methods [29]. These findings suggest that the choice of disinfectant towelette product for disinfection may matter more than the contact time over which it is applied. Further investigation into the relative importance of different factors of disinfectant efficacy among the varied types of disinfectants is warranted.
Among the disinfectant towelette products tested, HP1, a wipe impregnated with hydrogen peroxide-based disinfectant, performed the best against both S. aureus and P. aeruginosa. Conversely, QAC1, a wipe impregnated with a quat alcohol-based disinfectant, resulted in the least log reduction of both S. aureus and P. aeruginosa. It was hypothesized that HP1 would perform best as it is the product with the shortest label contact time, and therefore, should be effective at most of the contact times tested in the study as compared to QAC4, which had the longest contact time of the products tested and was not expected be effective at contact times lower than its label contact time. However, QAC1 had the second shortest contact time of the products tested and resulted in the least log reductions of both S. aureus and P. aeruginosa. These findings further support the notion that contact time plays a limited role in disinfectant towelette efficacy.
While others have examined the efficacy of disinfectant towelettes across varied contact times, the current study is novel because the conditions under which the disinfectant towelette products were tested were designed to be more representative of realistic use, such as in a clinical setting. Under these conditions, the majority of the disinfectant towelettes did not achieve the minimum 5-log reduction required under the EPA Product Performance Test [30] Guidelines, even at their label contact times. Only QAC3 was able to achieve an average 5-log reduction under the testing conditions used in this study; even then, this was only achieved against P. aeruginosa, not S. aureus. This discrepancy in performance under the novel testing conditions is not surprising, as this has been seen previously in the literature when testing commercial disinfectant towelette products under conditions more reflective of realistic use [23, 31]. Further, others [32] have found that the method used to test disinfectant efficacy results in significant differences in performance. These results support the notion that current testing methods are not adequately reflective of the performance of these products in real life [16, 17].
The primary limitations of this study relate to variability within the study design. One such limitation is the lack of control of confounding variables associated with a model reflective of realistic use. This variability is acknowledged by others in the field [33]. The testing conditions implemented in this study model differed from the conditions used in EPA MB-33-00 [24], as the disinfectant towelettes were wiped over a larger surface made of a different material. Such factors are relevant to the disinfection process [12]. However, the individual contributions of these variables were not assessed as part of the present study. Thus, it can be hypothesized that the discrepancy in expected performance based on label claims and the actual performance of disinfectant towelette products tested in this study is explained by using testing conditions reflective of realistic use; however, it is difficult to speculate which specific conditions may be responsible for these discrepancies.
Conclusion
Contact time had limited impact on the efficacy of several disinfectant towelette products when applied in a model designed to simulate realistic use of disinfectants such as that seen in clinical settings. Significant differences in efficacy were seen among the different product types tested, and this finding was consistent in testing against both S. aureus and P. aeruginosa. These findings suggest that the disinfectant towelette product used may have a greater impact on disinfectant efficacy than other factors such as contact time, but further research is warranted.
Data availability
All quantitative data generated or analyzed during this study are included in this published article.
Abbreviations
- ATCC:
-
American Type Culture Collection
- CDC:
-
Center for Disease Control
- HAIs:
-
Healthcare-associated infections
- HP:
-
Hydrogen Peroxide
- QAC:
-
Quaternary Ammonium Compounds
- EPA:
-
Environmental Protection Agency
References
Magill SS, O’Leary SJ, Janelle DL, Thompson G, Dumyati J, Nadle LE, et al. Changes in prevalence of health care-associated infections in U.S. hospitals. N Engl J Med. 2018;379:1732–44.
Weiner-Lastinger LM, Abner S, Edwards JR, Kallen AJ, Karlsson M, Magill SS, et al. Antimicrobial-resistant pathogens associated with adult healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network, 2015–2017. Infect Control Hosp Epidemiol. 2020;41:1–18.
Schreiber PW, Sax H, Wolfensberger A, Clack L, Kuster SP. Swissnoso. The preventable proportion of healthcare-associated infections 2005–2016: systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2018;39:1277–95.
Septimus E, Weinstein RA, Perl TM, Goldmann DA, Yokoe DS. Approaches for preventing healthcare-associated infections: go long or go wide? Infect Control Hosp Epidemiol. 2014;35:797–801.
Otter JA, Yezli S, French GL. The role played by contaminated surfaces in the transmission of nosocomial pathogens. Infect Control Hosp Epidemiol. 2011;32:687–99.
Donskey CJ. Does improving surface cleaning and disinfection reduce health care-associated infections? Am J Infect Control. 2013;41:12–9.
Dancer SJ. The role of environmental cleaning in the control of hospital-acquired infection. J Hosp Infect. 2009;73:378–85.
Rutala WA, Kanamori H, Gergen MF, Knelson LP, Sickbert-Bennett EE, Chen LF, et al. Enhanced disinfection leads to reduction of microbial contamination and a decrease in patient colonization and infection. Infect Control Hosp Epidemiol. 2018;39:1118–21.
Alfa MJ, Lo E, Olson N, MacRae M, Buelow-Smith L. Use of a daily disinfectant cleaner instead of a daily cleaner reduced hospital-acquired infection rates. Am J Infect Control. 2015;43:141–6.
Robertson A, Barrell M, Maillard JY. Combining detergent/disinfectant with microfibre material provides a better control of microbial contaminants on surfaces than the use of water alone. J Hosp Infect. 2019;103:e101–4.
Song X, Vossebein L, Zille A. Efficacy of disinfectant-impregnated wipes used for surface disinfection in hospitals: a review. Antimicrob Resist Infect Control. 2019;8:139.
Sattar SA, Maillard JY. The crucial role of wiping in decontamination of high-touch environmental surfaces: review of current status and directions for the future. Am J Infect Control. 2013;41:97–104.
Boyce JM. A review of wipes used to disinfect hard surfaces in health care facilities. Am J Infect Control. 2021;49:104–14.
Siani H, Wesgate R, Maillard JY. Impact of antimicrobial wipes compared with hypochlorite solution on environmental surface contamination in health care setting: a double-crossover study. Am J Infect Control. 2018;46:1180–7.
Wiemken TL, Curran DR, Pacholski EB, Kelley RR, Abdelfattah RR, Carrico RM, et al. The value of ready-to-use disinfectant wipes: Compliance, employee time, and costs. Am J Infect Control. 2014;42:329–30.
Sattar SA, Bradley C, Kibbee R, Wesgate R, Wilkinson MAC, Sharpe T, et al. Disinfectant wipes are appropriate to control microbial bioburden from surfaces: use of a new ASTM standard test protocol to demonstrate efficacy. J Hosp Infect. 2015;91:319–25.
Williams GJ, Denyer SP, Hosein IK, Hill DW, Maillard JY. The development of a new three-step protocol to determine the efficacy of disinfectant wipes on surfaces contaminated with Staphylococcus aureus. J Hosp Infect. 2007;67:329–35.
Berendt AE, Turnbull L, Spady D, Rennie R, Forgie SE. Three swipes and you’re out: how many swipes are needed to decontaminate plastic with disposable wipes? Am J Infect Control. 2011;39:442–3.
Speight S, Moy A, Macken S, Chitnis R, Hoffman PN, Davies A, et al. Evaluation of the sporicidal activity of different chemical disinfectants used in hospitals against Clostridium difficile. J Hosp Infect. 2011;79:18–22.
Rutala WA, Weber DJ. Disinfection and sterilization in health care facilities: an overview and current issues. Infect Dis Clin N Am. 2021;35:575–607.
Brown E, Dhanireddy K, Teska P, Eifert J, Williams RC, Boyer R. Influence of drying time on prewetted disinfectant towelettes to disinfect glass surfaces. Am J Infect Control. 2020;48:846–8.
West AM, Teska PJ, Oliver HF. There is no additional bactericidal efficacy of Environmental Protection Agency-registered disinfectant towelettes after surface drying or beyond label contact time. Am J Infect Control. 2019;47:27–32.
Tarka P, Chojecka A, Paduch O, Nitsch-Osuch A, Kanecki K, Kierzkowska A. Bactericidal activity of ready-to-use alcohol-based commercial wipes according to EN 16615 carrier standard. Int J Environ Res Public Health. 2019;16:3475.
Office of Pesticide Programs. (2014, March). Standard operating procedure for Quantitative Petri Plate Method (QPM) for determining the effectiveness of antimicrobial towelettes against vegetative bacteria on inanimate, hard, non-porous surfaces (EPA MB-33-00). Environmental Protection Agency. https://www.epa.gov/sites/default/files/2014-12/documents/mb-33-00.pdf.
Office of Pesticide Programs. (2019, August). Standard operating procedure for preparation of hard water and other diluents for determining preparation of antimicrobial products (EPA MB-30-02). Environmental Protection Agency. https://www.epa.gov/sites/default/files/2019-08/documents/mb-30-02.pdf.
West AM, Nkemngong CA, Voorn MG, Wu T, Li X, Teska PJ, et al. Surface area wiped, product type, and target strain impact bactericial efficacy of ready-to-use disinfectant towelettes. Am Resist Infect Control. 2018;7:122.
Hong Y, Teska PJ, Oliver HF. Effects of contact time and concentration on bactericidal efficacy of 3 disinfectants on hard nonporous surfaces. Am J Infect Control. 2017;45:1284–5.
West AM, Teska PJ, Lineback CB, Oliver HF. Strain, disinfectant, concentration, and contact time quantitatively impact disinfectant efficacy. Antimicrob Resist Infect Control. 2018;7:49.
Rutala WA, Weber DJ. Surface disinfection: treatment time (wipes and sprays) versus contact time (liquids). Infect Control Hosp Epidemiol. 2018;39:329–31.
Office of Chemical Safety and Pollution Prevention. (2018, February). Disinfectants for use on environmental surfaces (EPA 712-C-17-004). Environmental Protection Agency. https://www.regulations.gov/document/EPA-HQ-OPPT-2009-0150-0034.
Tyski S, Grzybowska W, Bocian E. Application of EN 16615 (4-Field test) for the evaluation of the antimicrobial activity of the selected commercial and self-made disinfectant wipes. Int J Environ Res Public Health. 2021;18:5932.
Wesgate R, Robertson A, Barrell M, Teska P, Maillard JY. Impact of test protocols and material binding on the efficacy of antimicrobial wipes. J Hosp Infect. 2019;103:e25–32.
Werner S, Naujox K, Rehm ME, Brückner E. Method for assessment of the range efficacy of presoaked single-use wipes for surface disinfection. HygMed 2018;43:E93-9.
Acknowledgements
The authors thank Rachel Silver for reviewing the manuscript and assisting in preparing study materials. We also thank Rowan Wallar for assisting with the preparation of media and reagents.
Funding
This work was supported by Diversey Inc., Fort Mill, SC, USA.
Author information
Authors and Affiliations
Contributions
AMK, GMT, and CMH conducted the wet lab procedures. AMK and HFO analyzed and interpreted the data generated. AMK wrote the manuscript. GMT contributed to editing the manuscript. XL provided industry experience and designed elements of the experimental protocol. PT provided industry experience, designed elements of the experimental protocol, and contributed to the writing and editing of the manuscript. HFO served as the principal investigator for the study and contributor to the writing and editing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kelley, A.M., Voorn, M.G., Tembo, G.M. et al. Contact time has limited impact on the efficacy of disinfectant towelettes when tested under conditions reflective of realistic use. Antimicrob Resist Infect Control 12, 71 (2023). https://doi.org/10.1186/s13756-023-01266-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13756-023-01266-4