Abstract
Background
The study aimed to elucidate the species taxonomy, clinical manifestations, virulence gene profiles and antimicrobial susceptibilities of Aeromonas strains isolated from life-threatening bacteremia in southeastern China.
Methods
Clinical samples of Aeromonas causing bacteremia were isolated from a teaching hospital in Wenzhou from 2013 to 2018 and a retrospective cohort study was performed. Aeromonas strains were identified at species level by housekeeping gene gyrB. Virulence and drug resistance-associated genes were screened by polymerase chain reaction (PCR) and antimicrobial susceptibility testing (AST) was performed by the VITEK 2 Compact system.
Results
A total of 58 Aeromonas isolated from patients with bacteremia were collected during 6 years (2013–2018). 58 isolates were identified to five different species, where Aeromonas dhakensis appeared to be the predominant species (26/58), followed by Aeromonas veronii (13/58), Aeromonas caviae (10/58), Aeromonas hydrophila (7/58) and Aeromonas jandaei (2/58). 16 of 58 patients had poor prognosis. Poor prognosis was significantly associated with liver cirrhosis and inappropriate empirical antimicrobials therapy. The progression of bacteremia caused by Aeromonas was extremely fast, especially in A. dhakensis infections. Virulence genes aer, lip, hlyA, alt, ast, and act, were detected at ratios of 24.1% (14/58), 62.1% (36/58), 65.5% (38/58), 58.6% (34/58), 15.5% (9/58) and 65.5% (38/58), respectively. Antimicrobial susceptibility testing exhibited that 9 out of 58 isolates were identified as multi-drug resistant (MDR) organism. The blaTEM gene was identified in all 9 MDR isolates. blaSHV, blaAQU-1, blaMOX, blaCepH, blaCphA and aac(6′)-Ib-cr were detected in 4 isolates, 2 isolates, 1 isolate, 3 isolates, 8 isolates, and 3 isolates, respectively. The majority of Aeromonas strains maintained susceptible to 3rd generation cephalosporins, aminoglycosides, fluoroquinolones and furantoin.
Conclusions
The prevalence and dangerousness of Aeromonas infections, especially A. dhakensis, are underestimated in clinic. Continuous monitoring is essential to keep track of MDR Aeromonas due to the increasing prevalence recently and a more effective measure is required to control the spread of resistance determinants.
Similar content being viewed by others
Backgroud
Aeromonas species are Gram-negative and rod-shaped bacteria, which are ubiquitous in aquatic environment, foodstuffs, and soil. Aeromonas are responsible for a variety of human infectious diseases, such as gastroenteritis, wound infections, hepatobiliary infections, necrotizing fasciitis and septicemia [1]. Humans carry Aeromonas species in their gastrointestinal tract. The carrying rate of Aeromonas in the feces of healthy people ranges from 0 to 4% [2]. Many infections caused by Aeromonas are self-limiting. However, in patients who have severe underlying diseases or immunocompromised individuals, invasiveness infections can be urgent and rapid-developing [3].
The Aeromonas taxonomy is complex. Nowadays, accurate laboratory identification is still a great challenge. Conventional biochemical tests, 16S rRNA sequencing and matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) analysis are unreliable in identifying Aeromonans at the species level. For example, Aeromonas dhakensis (formerly known as Aeromonas aquariorum) is often misidentified as Aeromonas hydrophila by traditional biochemical methods [4]. Accurate identification can be achieved by housekeeping genes sequencing, including rpoD and gyrB, or multilocus phylogenetic analysis (MLPA) [1].
Virulence factors produced by Aeromonas species are multifactorial, including adhesins, cytotoxins, hemolysins, lipases, proteases, the capacity to form biofilms, the use of specific metabolic pathways, and mediate virulence factor expression through quorum sensing [5]. The reported mortality rate among patients with Aeromonas bacteremia ranges from 24 to 63% [3]. A. dhakensis has been found prevalent in human infections and probably more lethal than other Aeromonas species in recent years. The pathogenicity of Aeromonas seems to be varied among different species levels. Moreover, along with the overuse of antimicrobials in agriculture, fish farming and clinical settings, increasing resistance has been noted in Aeromonas [6]. The antibiotic susceptibility varies with the geographical area and the species of Aeromonas tested [2]. Appropriate antimicrobials treatment is necessary to control the development of infections.
The prevalence and dangerousness of Aeromonas infections seems to be underestimated, as they vary among different geographic regions and types of infections [7], but fundamental reports are still insufficient in many countries. Wenzhou, a coastal city located in southeast China with subtropical climate, is prone to Aeromonas infection due to the humid subtropical climate. Incidences of bacteremia due to Aeromonas have been increasingly observed in Wenzhou with high morbidity and mortality in clinic. The present study aimed to investigate the clinical manifestations of bacteremia due to Aeromonas species over a 6-year period in a teaching hospital in southeastern China, and to assess the risk factors associated with mortality. Virulence gene determinants and antimicrobial susceptibility were also analyzed for the sake of advancing the understanding of Aeromonas causing bacteremia and establishing appropriate therapy strategy.
Methods
Bacterial strains and identification
This study was conducted at the First Affiliated Hospital of Wenzhou Medical University, a 4100-bed teaching hospital located in southeast China. A total of 58 isolates were obtained from patients with positive blood cultures for Aeromonas species between January 2013 and December 2018. The isolates were primarily identified using the MALDI-TOF MS (BioMérieux, Marcy I’ Etoile, France). Strains were further identified by housekeeping gene sequencing (gyrB). Strains used in this study were stored in 20% glycerol at − 80 °C.
Data collection and definition
Retrospective cohort study was performed. The medical records of all patients with Aeromonas bacteremia were retrospectively reviewed and the following information was collected: demographics, symptoms and signs, monomicrobial or polymicrobial infection, antimicrobials susceptibility pattern and drugs application, source of infection, co-morbidities, and patient outcomes. Patient with the first positive blood culture collected within 48 h after admission were defined as community-acquired infection. Nosocomial infection was defined as the bacteremia episode detected at least 48 h after admission. Prognosis poor was defined as the death of a patient or the patient discharged from hospital due to continuously deteriorating conditions with a clinical course suggestive of persistently active infection. Prognosis well was defined as bacteremia associated symptoms improved without recurrence within 30 days [8, 9]. Antimicrobials treatments given before the antimicrobials susceptibility testing results became available were defined as empirical therapy. Inappropriate antimicrobials treatments were defined as the usage of those drugs which demonstrated ineffective against the causative isolates in vitro.
Detection of genetic determinants related to virulence and drug resistance
The identified strains were recovered by streaking on nutrient agar plate and incubating for 24 h at 35 ℃. Total DNAs of Aeromonas isolated from bacteremia were obtained with an AxyPrep Bacterial Genomic DNA Miniprep kit (Axygen Scientific, Union City, CA, USA) and were used as polymerase chain reaction (PCR) templates for subsequent gene detection. Six virulence associated genes were selected as virulence markers, including aerolysin (aerA), heat-stable cytotonic enterotoxin (ast), heat-labile cytotonic enterotoxin (alt), cytotoxic enterotoxin (act), hemolysin (hlyA), and phospholipase (lip). The presence of the β-lactamase genes (blaTEM, blaSHV, blaCTX-M-1, blaAQU-1, blaMOX, blaCepH, blaIMP, blaVIM and blaCphA), and plasmid-mediated quinolone resistance genes (qnrA, qnrB, qnrD and aac(6′)-Ib-cr) was also analyzed. Primer sequences for the amplification were as previously described [8, 10,11,12]. The positive PCR amplicons were sequenced by Shanghai MajorbioBioPharm Technology Co. (Shanghai, China). The sequences were blasted using BLAST at NCBI website (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Antimicrobial susceptibility testing (AST)
The antimicrobial susceptibility patterns of all isolates to a panel of antimicrobials were determined using the VITEK 2 Compact System, including ampicillin (AMP), ampicillin/sulbactam (SAM), ceftriaxone (CRO), ceftazidime (CAZ), cefotetan (CTT), cefazolin (CZO), cefepime (FEP), piperacillin/tazobactam (TZP), aztreonam (ATM), imipenem (IPM), levofloxacin (LEV), ciprofloxacin (CIP), Trimethoprim/sulfamethoxazole (SXT), amikacin (AMK), gentamicin (GEN), tobramycin (TOB) and furantoin (NIT). The breakpoints were interpreted according to the Clinical and Laboratory Standards Institute (CLSI) 2018 guidelines.
Phylogenetic and statistical analysis
The positive PCR amplicons (gyrB, virulence determinants, and drug resistance genes) were sequenced by Shanghai MajorbioBioPharm Technology Co. (Shanghai, China). Nucleotide sequences were analyzed and compared using BLAST (http://www.ncbi.nlm.nih.gov/BLAST). A phylogenetic tree was generated using the unrooted neighbor-joining method with the Kimura's 2-parameter method by Mega 5.0 software. Bootstrap values were calculated by 1000 replicates [13]. Statistical analyses were performed using SPSS, version 17.0 (SPSS Inc., Chicago, IL, USA). Pearson’s Chi-square test was used to examine categorical variables and Student’s t test or Mann–Whitney U test was used for continuous variables. Variables with statistical significance in univariate analysis were submitted to multivariate analysis. Risk factors for prognosis of Aeromonas bacteremia were analyzed with multivariate logistic regression models. Odds ratios (OR) were calculated with 95% confidence interval. A P value of < 0.05 was regarded as statistically significant.
Results
Aeromonas diversity
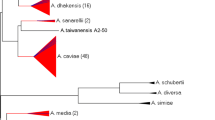
Phylogenetic tree based on housekeeping gene gyrB exhibited that all 58 isolates were divided into 5 different species, with the predominant species being A. dhakensis (26/58). Besides, 13 isolates of Aeromonas veronii, 10 isolates of Aeromonas caviae, 7 isolates of A. hydrophila and 2 isolates of Aeromonas jandaei were identified at the species level (Fig. 1). The MALDI-TOF MS system showed poor coincidence with housekeeping gene sequencing analysis at the species level. The concordance rate between MALDI-TOF and gyrB sequencing was 53.4%. A. dhakensis was incorrectly identified as A. hydrophila by MALDI-TOF MS. Moreover, two A. jandaei strains were misidentified as A. hydrophila or Aeromonas veronii.
Characteristics of investigated patients
During the investigated period, 58 patients were detected with positive blood culture of Aeromonas. 16 patients had poor prognosis (death or therapy failure), where A. dhakensis (12/26) was the most common Aeronomas species, followed by A. veronii (2/13), A. caviae (1/10), A. hydrophila (1/7) and A. jandaei (0/2). The average age of the 58 patients with positive blood culture was 61 ± 16.7 years old and the percentage of male patients was 70% (40/58). Polymicrobial infections were detected in nine cases, which were co-infected with Klebsiella pneumoniae (3 cases), Escherichia coli (3 cases). Proteus vulgaris (1 case), Klebsiella oxytoca (1 case) and Enterobacter cloacae (1 case). Univariate analysis indicated that significant differences were observed within the parameters of liver cirrhosis, inappropriate empirical antimicrobials treatment, thrombocytopenia, the community-acquired infection, and clinical outcomes (Septic shock, admission to ICU and the length of stay in hospital). Multivariate logistic regression analysis showed that poor prognosis was only significantly associated with liver cirrhosis (OR = 7.41, 95% CI, 1.32–41.55, P < 0.05) and inappropriate empirical antimicrobials (OR = 16.91, 95% CI, 3.04–94.22, P < 0.05). The exact characteristics of patients were listed in Table 1. The outcomes of A. dhakensis bacteremia were worsen than other species (P < 0.05).
Distribution of virulence and drug resistance determinants
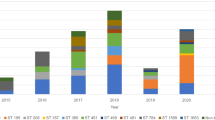
Virulence encoding genes, including aer, lip, hlyA, alt, ast, and act, were detected at ratios of 24.1% (14/58), 62.1% (36/58), 65.5% (38/58), 58.6% (34/58), 15.5% (9/58) and 65.5% (38/58), respectively. Virulence genes profile of 58 Aeromonas isolates was showed in Fig. 2. At least one virulence determinants was found in all 58 isolates. The gene hlyA and act were most prevalent in these isolates. Single virulence gene was detected in 12.1% (7/58) of isolates, and more than two virulence genes were found in remaining strains. There was no significant difference in virulence genes between strains isolated from patients with poor prognosis and those with well prognosis. Additionally, no statistical significance was observed in the prevalence of all the studied virulence genes between isolates separated from community acquired and nosocomial infection. We found 27 different combination patterns (PTs) of six examined genes. The two most prevalent PT (n ≥ 5) were PT1 (lip/hlyA/alt/act, n = 6) and PT2 (lip/alt, n = 5). Only one isolate of A. hydrophila carried all the investigated virulence genes, and the patient was cured after 32 days of hospitalization. Notably, 5 of 6 isolates grouped into PT1 were A. dhakensis, among which 3 lead to poor prognosis. The blaTEM gene was identified in all 9 MDR isolates. blaSHV, blaAQU-1, blaMOX, blaCepH, blaCphA and aac(6′)-Ib-cr were detected in 4 isolates, 2 isolates, 1 isolate, 3 isolates, 8 isolates, and 3 isolates, respectively.
Antimicrobial susceptibility profiles
Antimicrobials susceptibility testing exhibited that the majority of the 58 isolates maintained susceptible to aminoglycosides, fluoroquinolones and furantoin (Table 2). Resistance to ceftazidime, cefotetan, ceftriaxone, cefepime, piperacillin/tazobactam, aztreonam were 10.3%, 13.8%, 15.5%, 1.7%, 10.3% and 5.2%, respectively. No significant increase in resistance during six years was observed. 9 out of 58 isolates were identified as multi-drug resistant (MDR) organism, including 4 isolates of A. dhakensis, 3 A. hydrophila, 1 A. veronii, and 1 A. caviae. Among which, six MDR strains were isolated in 2017 and 2018. The first MDR strain was recovered from a 78-year-old woman with community-acquired infection in 2013. 24.1% (14/58) isolates were non-susceptible to imipenem.
Discussion
Aeromonas spp. are of increasing importance for causing multiple of clinical infections, including diarrhea, soft tissue infection, and bacteremia. Aeromonas bacteremia is an urgent, rapid-developing disease with high mortality [14]. Moreover, according to similar clinical manifestations, Aeromonas infections are often misdiagnosed as Vibrio infections before microbiology identification by laboratory, which may lead to improperly use of antimicrobials and ineffective treatment [14]. In this study, four patients were misdiagnosed as Vibrio vulnificus infections before laboratory identification. The symptoms progressed rapidly and these patients were severely inflamed with ecchymosis and blisters in 2 days. Unfortunately, all of them developed into multiple organ dysfunction syndrome (MODS) and resulted in poor prognosis. Coincidentally, they all got infected in community and suffered from liver cirrhosis. Among whom, one patient received ineffective empirical therapy by using imipenem alone. This pathogenic Aeromonas isolate was subsequently confirmed to produce Aeromonas spp. specific “Carbapenem hydrolyzing Aeromonas” metallo-beta-lactamase (CphA) [15] and to be resistant to imipenem in vitro while remaining susceptible to many other antimicrobials, such as the third- cephalosporins, quinolones and aminoglycosides. Aeromonas infections are reported to be prevalent in regions with a high prevalence of chronic hepatitis and warm climate, like Taiwan, which is regarded as one of the endemic areas [16]. However, in mainland China, the incidence of Aeromonas bacteremia in human beings remains to be elucidated. Wenzhou is in the southeastern coastal area with subtropical climate. Increasing prevalence of Aeromonas bacteremia has been found with high morbidity and mortality in the hospital studied.
Aeromonas are not difficult to isolate, but identification at species level is challenging due to its phenotypic heterogeneity. Compared with the use of 16 s rRNA gene, nucleotide sequencing of housekeeping genes, such as gyrB, rpoB and rpoD, can provided a more definitive identification of the genus [17]. Several researches have shown that MALDI-TOF MS could efficiently identify A. dhakensis, which is often clinically misidentified as A. hydrophila by phenotypic methods [4]. Nevertheless, A. dhakensis couldn’t be identified by MALDI-TOF MS in this study, possibly because it hasn’t been included in the commercial database of BioMérienx system. Housekeeping gene gyrB sequencing exhibited that A. dhakensis was the most common Aeromonas species, followed by A. veronii. This is in contrast to the previous reports in which the authors stated that A. hydrophila and A. caviae were the most frequent Aeromonas species causing bacteremia in Taiwan, and A. caviae was the most common pathogen contributing to Aeromonas bacteremia in Japan [18]. Notably, A. dhakensis and A. jandaei were misidentified as A. hydrophila or A. veronii by MALDI-TOF MS. The patients with bacteremia caused by A. dhakensis are reported to have a higher sepsis-related mortality rate than those with other species in recent years, with the application of molecular biological method [19]. Similarly, bacteremia caused by A. dhakensis is more lethal than other species in our research. Notably, the importance of A. dhakensis in human infections might be seriously underrated and should be re-evaluated along with the changing taxonomy, and more accurate epidemiological researches are needed to establish the bacteriology distribution of Aeromonas bacteremia in different regions.
In our retrospective analysis, the average age of the 58 patients with positive blood culture was 61.1 ± 16.7 years old, suggesting that older people were more susceptible than younger individuals. However, no significant difference was found in age between prognosis the poor group and the prognosis well group (P > 0.05). 40 out of 58 patients were male, which may attribute to that alcoholic cirrhosis was more prevalent in male than female in our study. Similar to previous researches [20], we also found that the majority of patients had a variety of underlying diseases, including liver cirrhosis, diabetes mellitus, under immunosuppressed conditions, leukemia and other kinds of malignancy. Nearly half of patients in this study were diagnosed with liver cirrhosis. In accordance with previous research [14], our study exhibited that Aeromonas bacteremia accounted for significant morbidity and mortality in cirrhotic patients, suggesting that patients with liver cirrhosis are at risk of developing Aeromonas bacteremia. Moreover, initial inappropriate empirical antimicrobial usage was associated with poor outcomes for patients with Aeromonas bacteremia. The prevalence and high mortality rate of Aeromonas bloodstream infections in cirrhotic patients might be a consequence of dysregulated intestinal bacterial translocation and cirrhosis associated immune dysfunction (CAID) [21]. Among the 58 patients with Aeromonas bacteremia in this study, four patients were claimed to be dead in hospital, and 12 had dismal prognosis and then discharged without treatment. Polymicrobial infection didn’t result in worse prognosis than monomicrobial infection (P > 0.05). We found that consumption of sea food, trauma exposed or contact with water contaminated with Aeromonas [15], preexisting liver cirrhosis were the potential risk factors of Aeromonas infections or even lead to more rapid infection progresses. Additionally, length of hospital stays of community-acquired infections with poor prognosis ranged from 1 to 7 days (median 2 days), indicating that community-acquired infections developed more rapidly and lethally. No statistical significance in prognosis was observed between MDR and non-MDR strains. Compared to antimicrobial susceptibility, the pathogenicity of pathogens and the health status of the patients were probably more critical to the prognosis.
The pathogenicity of Aeromonas is multi-factorial, complex and may be associated with different interaction of various virulence factors acting either synergy or alone. The majority of Aeromonas isolates investigated in this study possess more than two virulence genes and seven strains harbor only one single gene. Isolates carrying more virulence genes didn’t mean higher pathogenicity. One patient died of an A. veronii strain, which only possess lipase encoding gene lip, six days after admission to ICU. However, another one infected by A. hydrophila carrying all the studied virulence determinants was cured after 32 days of hospitalization. The most obvious difference between these two patients was that the former suffered from liver cirrhosis. However, it may be explained by different expression level of the genes or interaction with other virulence factors not included in this study. Inconsistent with the previous study [7], no particular pattern of virulence genes was observed in this study.
Except for ceftriaxone (79.3%) and imipenem (75.9%), more than 80% of the isolates were susceptible to all remaining antimicrobials studied. In spite of intrinsical resistance to many antimicrobials, Aeromonas maintained well susceptible to most antimicrobials generally used in clinic. Relatively high carbapenem resistance rate may be due to the carriage of cphA [15]. Considering the rapid symptom progression and Aeromonas spp. specific drug resistance mechanisms, it is important to select the most appropriate antimicrobials usage or surgical intervention to prevent or cure Aeromonas infections as soon as possible, especially in patients with liver cirrhosis. Antimicrobial susceptibility patterns of Aeromonas spp. exhibited best susceptibility to aminoglycosides, suggesting that aminoglycosides might be recommended for empirical therapy of Aeromonas-associated bacteremia. 6 out of 9 MDR strains were isolated in 2017 and 2018, While the trends of antimicrobial susceptibilities among the years were stable without significant changes. Moreover, the resistance of bacteria associated with food animals and environments to antimicrobial agents represents a potential health threat [22]. It raises an alert for the developing of multidrug resistant strains in Aeromonas spp. isolated from clinic.
Conclusions
Considering the high morbidity and mortality, people should attach great importance to bacteremia caused by Aeromonas spp., especially in those immunocompromised patients with severe underlying diseases. Identification of Aeromonas at the species level is important for predicting clinical severity and outcome. The increasing emergence of MDR strains in recent years requires more attention and monitoring.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- AMK:
-
Amikacin
- AMP:
-
Ampicillin
- AST:
-
Antimicrobial Susceptibility Testing
- ATM:
-
Aztreonam
- CAID:
-
Cirrhosis Associated Immune Dysfunction
- CAZ:
-
Ceftazidime
- CLSI:
-
The Clinical and Laboratory Standards Institute
- CIP:
-
Ciprofloxacin
- CRO:
-
Ceftriaxone
- CTT:
-
Cefotetan
- CZO:
-
Cefazolin
- FEP:
-
Cefepime
- GEN:
-
Gentamicin
- ICU:
-
Intensive Care Unit
- IPM:
-
Imipenem
- LEV:
-
Levofloxacin
- MALDI-TOF MS:
-
Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry
- MDR:
-
Multi-Drug Resistant
- MLPA:
-
Multilocus Phylogenetic Analysis
- NIT:
-
Furantoin
- OR:
-
Odds ratios
- PCR:
-
Polymerase Chain Reaction
- SAM:
-
Ampicillin/Sulbactam
- SXT:
-
Trimethoprim/sulfamethoxazole
- TOB:
-
Tobramycin
- TZP:
-
Piperacillin/Tazobactam
References
Goncalves Pessoa RB, de Oliveira WF, Marques DSC, Dos Santos Correia MT, de Carvalho E, Coelho L. The genus Aeromonas: a general approach. Microb Pathog. 2019;130:81–94.
Anandan S, Gopi R, Devanga Ragupathi NK, Muthuirulandi Sethuvel DP, Gunasekaran P, Walia K, Veeraraghavan B. First report of blaOXA-181-mediated carbapenem resistance in Aeromonas caviae in association with pKP3-A: threat for rapid dissemination. J Glob Antimicrob Resist. 2017;10:310–4.
Chen PL, Wu CJ, Tsai PJ, Tang HJ, Chuang YC, Lee NY, Lee CC, Li CW, Li MC, Chen CC, Tsai HW, Ou CC, Chen CS, Ko WC. Virulence diversity among bacteremic Aeromonas isolates: ex vivo, animal, and clinical evidences. PLoS ONE. 2014;9:e111213.
Chen PL, Lamy B, Ko WC. Aeromonas dhakensis, an Increasingly Recognized Human Pathogen. Front Microbiol. 2016;7:793.
Fernandez-Bravo A,Figueras MJ. An Update on the Genus Aeromonas: Taxonomy, Epidemiology, and Pathogenicity. Microorganisms. 2020;8.
Wickramanayake M, Dahanayake PS, Hossain S,Heo GJ. Antimicrobial resistance of pathogenic Aeromonas spp. isolated from marketed Pacific abalone (Haliotis discus hannai) in Korea. J Appl Microbiol. 2020;128:606–617.
Castelo-Branco Dde S, Guedes GM, Brilhante RS, Rocha MF, Sidrim JJ, Moreira JL, Cordeiro Rde A, Sales JA, Riello GB, de Alencar LP, Paiva Mde A, Vasconcelos DC, de Menezes IS, de Ponte YB, Sampaio CM, Monteiro AJ,Bandeira Tde J. Virulence and antimicrobial susceptibility of clinical and environmental strains of Aeromonas spp. from northeastern Brazil. Can J Microbiol. 2015;61:597–601.
Wu CJ, Chen PL, Hsueh PR, Chang MC, Tsai PJ, Shih HI, Wang HC, Chou PH, Ko WC. Clinical implications of species identification in monomicrobial Aeromonas bacteremia. PLoS ONE. 2015;10:e0117821.
Lay CJ, Zhuang HJ, Ho YH, Tsai YS, Wang LS, Tsai CC. Different clinical characteristics between polymicrobial and monomicrobial Aeromonas bacteremia–a study of 216 cases. Intern Med. 2010;49:2415–21.
Li F, Wang W, Zhu Z, Chen A, Du P, Wang R, Chen H, Hu Y, Li J, Kan B, Wang D. Distribution, virulence-associated genes and antimicrobial resistance of Aeromonas isolates from diarrheal patients and water. China J Infect. 2015;70:600–8.
Segatore B, Piccirilli A, Setacci D, Cicolani B, Di Sabatino A, Miccoli FP, Perilli M,Amicosante G. First Identification of beta-Lactamases in Antibiotic-Resistant Escherichia coli, Citrobacter freundii, and Aeromonas spp. Isolated in Stream Macroinvertebrates in a Central Italian Region. Microb Drug Resist. 2020;26:976–981.
Nowrotek M, Jalowiecki L,Plaza G. Fluoroquinolone Resistance and Virulence Properties Among Wastewater Aeromonas caviae Isolates. Microb Drug Resist. 2020.
Guo SL, Yang QH, Feng JJ, Duan LH,Zhao JP. Phylogenetic analysis of the pathogenic genus Aeromonas spp. isolated from diseased eels in China. Microb Pathog. 2016;101:12–23.
Syue LS, Chen PL, Wu CJ, Lee NY, Lee CC, Li CW, Li MC, Tang HJ, Hsueh PR, Ko WC. Monomicrobial Aeromonas and Vibrio bacteremia in cirrhotic adults in southern Taiwan: Similarities and differences. J Microbiol Immunol Infect. 2016;49:509–15.
Wu CJ, Ko WC, Lee NY, Su SL, Li CW, Li MC, Chen YW, Su YC, Shu CY, Lin YT, Chen PL. Aeromonas isolates from fish and patients in Tainan City. Taiwan: Genotypic and Phenotypic Characteristics. Appl Environ Microbiol; 2019. p. 85.
Huang TY, Peng KT, Hsu WH, Hung CH, Chuang FY, Tsai YH. Independent predictors of mortality for aeromonas necrotizing fasciitis of limbs: an 18-year retrospective study. Sci Rep. 2020;10:7716.
Wu CJ, Wang HC, Chen PL, Chang MC, Sunny Sun H, Chou PH, Ko WC. AQU-1, a chromosomal class C beta-lactamase, among clinical Aeromonas dhakensis isolates: distribution and clinical significance. Int J Antimicrob Agents. 2013;42:456–61.
Tang HJ, Lai CC, Lin HL, Chao CM. Clinical manifestations of bacteremia caused by Aeromonas species in southern Taiwan. PLoS ONE. 2014;9:e91642.
Chen YW, Yeh WH, Tang HJ, Chen JW, Shu HY, Su YC, Wang ST, Kuo CJ, Chuang YC, Chen CC, Ko WC, Chen CS, Chen PL. UvrY is required for the full virulence of Aeromonas dhakensis. Virulence. 2020;11:502–20.
Kitagawa H, Ohge H, Yu L, Kayama S, Hara T, Kashiyama S, Kajihara T, Hisatsune J, Sueda T, Sugai M. Aeromonas dhakensis is not a rare cause of Aeromonas bacteremia in Hiroshima. Japan J Infect Chemother. 2020;26:316–20.
Bartoletti M, Giannella M, Lewis RE, Viale P. Bloodstream infections in patients with liver cirrhosis. Virulence. 2016;7:309–19.
Meng S, Wang YL, Liu C, Yang J, Yuan M, Bai XN, Jin D, Liang JR, Cui ZG, Li J. genetic diversity, antimicrobial resistance, and virulence genes of aeromonas isolates from clinical patients, tap water systems, and food. Biomed Environ Sci. 2020;33:385–95.
Acknowledgements
Not applicable.
Funding
This work was supported by research grants from the National Natural Science Foundation of China (no. 81802069), and the Planned Science and Technology Project of Wenzhou (no. Y20180191). The funder had no role in the design of the study and the collection, analysis, and interpretation of data and the writing of the manuscript.
Author information
Authors and Affiliations
Contributions
YS, YJZ and WYX carried out experiments. YS, YJZ, RCF and WQ analyzed the data. YS wrote the manuscript. HKH and CQX performed the results analysis and CZ directed the drawing. JMC, LJC and TLZ designed the study and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The need for ethics approval and consent is deemed unnecessary in this research according to the Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sun, Y., Zhao, Y., Xu, W. et al. Taxonomy, virulence determinants and antimicrobial susceptibility of Aeromonas spp. isolated from bacteremia in southeastern China. Antimicrob Resist Infect Control 10, 43 (2021). https://doi.org/10.1186/s13756-021-00911-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13756-021-00911-0