Abstract
Background
Antibiotics require more prudent prescribing, dispensing and administration than other medicines because these medicines are at a greater risk of antimicrobial resistance (AMR). Studying the current medicine use practices and factors affecting the prescribing of an antibiotic would help decision makers to draft policies that would enable a more rational use of medicines.
Methods
A prospective, descriptive, and cross-sectional study was conducted to assess the current prescribing practices including antibiotics use in six community pharmacies in Asmara. A total of 600 encounters were reviewed using the WHO core prescribing indicators between May 5 and May 12, 2019 using stratified random sampling technique. Descriptive statistics and logistic regression were employed using IBM SPSS® (version 22).
Results
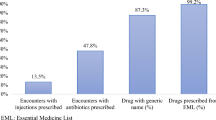
The average number of medicines per prescription was 1.76 and 83.14% of the medicines were prescribed using generic names while 98.39% of the medicines were from the National Essential Medicines List (NEML). The percentage of prescriptions containing antibiotics was 53%. The number of encounters containing injections was 7.8%. Patient age, gender and number of medicines prescribed were significantly associated with antibiotic prescribing at bivariate and multivariable models. Subjects under the age of 15 were approximately three times more likely to be prescribed antibiotic compared to subjects whose age is 65 and above (Adjusted Odds Ratio (AOR): 2.93, 95%CI: 1.71–5). Similarly, males were more likely to be prescribed antibiotic than females (AOR: 1.57, 95%CI: 1.10–2.24). Subjects to whom three to four medicines prescribed were two times more likely to be prescribed an antibiotic compared to those who were to be prescribed one to two medicines per encounter (AOR: 2.17, 95%CI: 1.35–3.5). A one-unit increase in the number of medicines increased the odds of antibiotic prescribing increased by 2.02 units (COR: 2.02; 95%CI: 1.62–2.52).
Conclusions
This study found that the percentage of antibiotics being prescribed at the community pharmacies in Asmara was 53% which deviated significantly from the WHO recommended values (20–26.8%). Furthermore, the percentage of encounters with an injection was 7.8% lower than the WHO value of 13.4–24.0%. Patients’ age, gender and number of medicines were significantly associated with antibiotic prescribing.
Similar content being viewed by others
Background
The World Health Organization (WHO) defines rational use of medicines as giving the right medicine, for the right patient, at the right dose, for the right duration and at the right (lowest) cost to them and their community [1]. Different studies have shown that as much as half of prescribed medicines are inappropriately prescribed or dispensed. Moreover the association between an increased misuse of antibiotics and emergence and spread of resistant microorganisms has been confirmed by many studies [2,3,4,5,6,7,8]. Inappropriate prescribing of antibiotics, i.e. prescribing an antibiotic when no antibiotic is needed or giving the wrong antibiotic at inappropriate dose for an inappropriate duration, has been pointed out as one of the factors leading to the misuse of antibiotics. Globally, the inappropriate use of antibiotics in primary care and hospital settings is a major contributing factor to the spread of antimicrobial resistance (AMR). WHO has classified AMR as a public health threat of growing concern in need of immediate attention [9,10,11]. Then it is not surprising that currently more than 700, 000 deaths per year are in some way attributed to AMR and this is projected to reach 10 million lives and cost 100 trillion USD by 2050 [12].
Judicious use of antibiotics in the ambulatory setting contributes to the rational use of antibiotics as a whole because antibiotic consumption in the community setting is an important part of the general antibiotic consumption. Outpatient pharmacies are good starting places where information regarding antibiotic use in the community could be gleaned from. According to the Center for Diseases Control (CDC), 30% of all antibiotics prescribed in outpatient clinics in the United States are unnecessary. Even when antibiotics are needed, states the study, prescribers are often inclined to prescribe medicines that may be less effective and carry more risk over the “first-line” medicines recommended by essential medicines list (EML) or national guidelines [13].
Low income countries (LIC) suffer from an increased burden of infectious diseases, shortage of medicines and competent healthcare professionals. Prescribers when faced with shortage of medicines are more likely to prescribe irrationally. AMR rates are higher in LIC as compared to high income countries (HIC) and irrational medicine use is higher in developing countries than the developed world [3, 14, 15].
There are many factors which could affect whether a patient is prescribed an antibiotic or not. Gender and age could determine the extent of antibiotic prescribing. A study done in the UK reported that females received 43% more antibiotics than males, and adult females were more likely to receive an antibiotic than either children or elderly [16].
WHO in collaboration with the International Network of Rational Medicine Use (INRDU) has developed a group of indicators to assess the use of antibiotics in health facilities. The three main indicators are prescribing indicators, facility indicators and patient care indicators. These set of indicators could be used as benchmarks to enable the introduction of antibiotic stewardship programs (ASPs) in different healthcare settings [2, 3, 15]. Many studies have reported using the WHO indicators [17,18,19,20,21,22,23,24,25]. Studying prescribing patterns of medicines in general and antibiotic in particular aids in identifying irrational prescribing behaviors to make therapy more rational and cost effective [26].
Eritrea, a small developing country in the horn of Africa, is faced with the challenges of irrational medicine use and implementing rational use of medicines. A recent study conducted in the inpatient setting of a tertiary Eritrean hospital reported a high rate of antibiotic use [27]. Thus studying the prescribing behaviors would help in paving a way towards the introduction of ASP in Eritrean hospitals. Many hospitals in the developed world have introduced ASPs to combat the irrational use of antibiotics.
The introduction of ASP requires the study of antibiotics prescribing behavior in inpatient and outpatient settings as a benchmarking tool before its implementation. Given the notable difference in socio-economic standing among different countries, the prescribing indicators of developing countries deviate significantly from the WHO reference targets [2]. It is our hope that this study would serve as a starter for a much broader study of antibiotics and the introduction of ASPs in Eritrean hospitals.
To the best of our knowledge there is no such published research conducted in Eritrea and thus the status of prescribing behavior is unknown. The aim of the study was therefore to assess the current prescribing behavior using WHO core medicine prescribing indicators and to determine the association of patient gender, age and total number of medicines prescribed with antibiotic prescribing.
Methods
Study design
A descriptive and cross-sectional study with a quantitative approach was conducted prospectively to assess prescribing behavior and factors associated with antibiotic prescribing.
Study setting
Eritrea has a total of 49 community pharmacies and 39 drug shops. Out of the 49 community pharmacies, 13 are governmentally owned. Six of the governmentally owned community pharmacies are found in Asmara. Two of them are found inside the national referral hospitals: Orotta and Halibet. Besides, the majority of patients who cannot find their medicines inside the Out-patient department (OPD) pharmacies of hospitals get their medicines from these six governmentally owned community pharmacies located in Asmara because of the better availability and affordability of medicines in these pharmacies. The study was carried out in six governmentally owned community pharmacies in Asmara, the capital city of Eritrea with a population of 427, 183 according to a 2017 report [28]. A “prescriber” in the Eritrean context and this article includes Medical Doctors, Nurses, Associate Nurses, Health Assistants and other lower health cadres could prescribe based on the setting and scarcity of other health professionals.
Sample size and sampling technique
According to WHO, at least 600 encounters should be included to study current prescribing practices of a facility [29]. A stratified random sampling was used. The six community pharmacies were considered as strata. Within stratum, systematic random sampling was used. The 600 prescriptions were then proportionally allocated. Expected total prescriptions from the six community pharmacies in 6 working days are 4560. By dividing it into 600, every 8th prescription was selected to reach the required sample size.
Data collection
Data was collected from prescriptions prospectively between May 5 and May 12, 2019 by three pharmacists. All the necessary data required to measure the prescription indicators were then entered into a WHO standard prescription indicator collection form. To avoid ambiguities in the definition of an “antibiotic”, a previously set definition by WHO [29] was employed. By complying with WHO methods and guidelines, data reliability was assured.
Data processing and statistical analysis
The collected data were double entered on CSPro Software (version 7.0) and exported to Statistical Package for Social Sciences (IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.) for statistical analysis. Descriptive summaries of the socio-demographic variables were computed using mean, standard deviation (SD) or median interquartile range (IQR) as appropriate. The association between antibiotic prescribing and its predicting factors was explored using logistic regression. Odds ratio with 95% confidence interval was reported in all logistic regression analyses. All analyses were considered significant when p < 0.05.
Results
A total of 600 prescriptions were analyzed, corresponding to 600 patients, of which 60.3% were female and 38.8% male with a median age of 25 (IQR: 42) (Table 1).
A total of 1055 medicines were prescribed. The average number of medicines per prescription was 1.76 (Standard Deviation (SD): 0.85). Most of the medicines were prescribed by generic name (83.14%). Almost all the prescribed medicines (98.39%) were on the Eritrean essential medicines list. Antibiotics were prescribed in 331 encounters (53%). Forty seven prescriptions had injections amounting to 7.8% of encounters (Table 2).
Of the total number of medicines prescribed, 349 (33.1%) were antibiotics. The most commonly prescribed antibiotics were: amoxicillin (42.1%), ciprofloxacin (20.1%), co-trimoxazole (9.5%) and amoxicillin-clavulanate (4.9%). Medicines most commonly co-prescribed with an antibiotic were: paracetamol (n = 106/326), Oral Rehydration Salt (ORS) (n = 31/326), omeprazole (n = 22/326) and ibuprofen (n = 21/326).
The WHO has developed a classification system called AWaRe which stands for Access, Watch and Reserve [30]. According to this classification antibiotics have been classified into three groups based on whether they need to be generally accessed (Access Group) or carefully watched (Watch) group or only reserved for special situations (Reserve Group). The following table shows the percentage of individual antibiotics and their category (Table 3).
Predictors of antibiotic prescribing
Age (p < 0.001), gender (p = 0.002) and number of medicines prescribed (p = 0.025) were significantly associated with antibiotic prescribing at the bivariate level (Table 4).
The full model containing all predictors was statistically significant, χ2 (5, N = 600) =50.71, p < 0.001 indicating that the model was able to distinguish between respondents who had prescribed to antibiotics and did not. Moreover, good fit was observed as per the Hosmer-Lemeshow goodness fit test (χ2 = 3.91, df = 7, p = 0.789). The model as a whole explained between 8.5% (Cox and Snell R-squared) and 11.4% (Nagelkerke R-squared) of the variance in antibiotic prescription and correctly classified 63.0% of those who had antibiotic prescription. Sensitivity of the model, percentage of the group with antibiotic prescription that had accurately identified by the model was 58.3%. Moreover, the specificity was 64.2%.
By adjusting potential confounders through multivariate logistic regression, age (p < 0.001), gender (p = 0.013) and number of medicines prescribed (p = 0.014) were still significantly associated with antibiotic prescribing. Subjects under the age of 15 were approximately three times more likely to be prescribed antibiotic compared to subjects whose age is 65 and above (AOR: 2.93, CI: 1.71–5). Similarly, males were more likely to be prescribed antibiotic than females (AOR: 1.57, CI: 1.10–2.24). Subjects to whom three to four medicines prescribed were two times more likely to be prescribed antibiotic compared to those who were to be prescribed one to two medicines per encounter (AOR: 2.17, CI: 1.35–3.5) (Table 5).
There is a significant increase in antibiotic prescribing with an increase in the number of medicines prescribed (p < 0.001). As the number of medicines prescribed increases by one unit, the odds of antibiotic prescribing increased by 2.02 units (COR: 2.02; 95%CI: 1.62–2.52).
Discussion
Prescribing indicators
In our study the average number of medicines per encounter was 1.76, consistent with the WHO optimal value of 1.6–1.8; which will be henceforward referred to as the standard (Table 2). However, when compared with other studies, it was much lower than 2.8 reported from India [1], 2.34 from Ethiopia [17], and 2.4 from Saudi Arabia [22]. This variation in the number of medicines prescribed per patient could be due to the difference in the study settings and prescribing practices among various medical specialties.
Out of the 600 encounters studied, 53% encounters contained antibiotics (standard 20–26%). This is slightly higher than the 46% for the Africa Region [2] and lower than what other studies have reported from Ethiopia 82.5% [31] and 58.1% [32]. This high percentage of antibiotics being prescribed may be due various reasons. First increased prevalence of infectious diseases in the developing countries results in increased amounts of antibiotics being prescribed, second the high level of routine empirical treatments seen in resource-poor countries. A third reason could be patient pressure on prescribers. A very recent study [33] conducted in Asmara found the rate of self-medication using antibiotics to be 45.1%. This result might show that since patients are highly involved in self-medication using antibiotics, they are more likely to ask prescribers directly or indirectly to prescribe antibiotics. Fourth, allowing other healthcare professionals e.g. health assistants, associate nurses, nurses, health technicians and others to prescribe. In Eritrea one physician serves 18,041 patients, to cope with this shortage of physicians, low level health cadres are licensed to prescribe medicines [34]. As the level of expertise of the prescriber decreases the odds of irrational medicine prescribing is more likely to increase.
We found that in 7.8% of all the encounters at least one injection was included (Table 2). This figure is lower than the 13.4–24.1% WHO standard. Since administration of an injection is associated with risks and injections need trained health care professional for administration, prescribers are advised to prescribe non-parenteral routes of administration whenever possible. Other studies have reported higher rates of injections being prescribed in Ethiopia 11.2% [31], 26.5% [35] and 38% [32]. These studies from Ethiopia were conducted in hospital pharmacies which could somehow account for the increased percentage of injections being prescribed.
Prescribing using generic name permits dispensers to substitute therapeutic equivalents when one particular brand is not available and generic medicines are cheaper which ensures better availability in the market. Generic name prescribing was 83.1%, lower than the recommended 100% (Table 2). Better generic prescribing studies have been reported from Ethiopia [31] at 97%, Iran 95% [3], and Ghana 93% [36]. This could be due to the old prescribing habits of prescribers, familiarity with brand names. Based on our results, generic name prescribing is something which needs to be addressed in Asmara healthcare settings. Educational activities targeting prescribers should be held to promote generic name prescribing and medical students should be encouraged to use generic prescribing.
Almost all (98.83%) of the medicines were prescribed from the EML (Table 2), this is similar with a study done in Ethiopia which reported 98.7% [32]; 80.3% [23]. However, our result was much higher than the 28.5% reported by in India [37]. Eritrea follows a centralized procurement system which adheres to the EML; this could in part explain the high percentage of the medicines being prescribed within the EML.
In our study 71.9% (251/349) of the antibiotics were on the Access group while 22.1% (77/349) were in the Watch group and no single antibiotic (0%) was from the Reserve group. And 6% (21/349) of the antibiotics were ungrouped in these three classes. From the Watch group, Ciprofloxacin accounts for 90.1% of the total medicines used in the category. Therefore the use of Ciprofloxacin in the community needs to be carefully monitored.
Predictors of antibiotic prescribing
This study found significant association of antibiotic prescription with patients’ age, gender, and number of medicines (Table 4). Being under the age of 15 was significantly related with antibiotic prescription. Similar studies from Bangladesh [38], Yemen [39], Cameroon [40] found that patients under the age of 15 received the highest proportion of antibiotics when compared with the other patient groups.
Our study found that males were more likely to receive an antibiotic than females, similar to a study in Bangladesh [38] and in Ethiopia [41]. This is in sharp contrast with a study done by Smith et al. [16], Anong et al. [40] and Streit et al. [42], where they reported that female gender was more significantly associated with antibiotic prescribing. This study showed that prescribing 3 to 4 medicines per encounter was significantly associated with antibiotic prescribing (Table 5). An increase in one unit of medicine increased the odds ratio of using an antibiotic by 2.02, this was similar to a study from Zambia which reported that a one-unit increase increased the odds by 2.7 (P < 0.001; OR = 2.68, 95%CI 2.20–3.25) [43].
Since the study was a cross-sectional one, the results might not reflect the seasonal variation of medicine use. Furthermore, the findings of the study were based on the capital city, Asmara, so it might not be generalized to the whole country. Further nationwide studies are needed to get the complete picture of medicine use in the country.
Conclusion
Our study revealed that the prescribing indicators in the public community pharmacies in Asmara showed deviation from the WHO standard. The percentage of prescriptions which contained an antibiotic was 53% which is double of the optimal values set by WHO (20–26.8%) and the percentage of encounters with an injection was 7.8% lower than the WHO value of 13.4–24.0%. Average number of medicines prescribed per encounter, adherence to EML and percentage of encounters with injection were in compliance the WHO recommended values. Patient age, gender and number of medicines per encounter showed significant association with antibiotic prescribing.
To promote generic prescribing and comply with the INRDU/WHO recommendations, regular updating and wide dissemination of National Standard Treatment Guideline and should be promoted, extensive continuing education programmes targeting prescribers should be introduced. At the hospital level, the Medicines and Therapeutic Committees (MTCs) should be strengthened and new MTCs set up in hospitals where no such committees exist. Based under the supervision and recommendation of MTCs ways of introducing Antibiotic Stewardship Programmes (ASPs) should be diligently sought.
Availability of data and materials
The data used in this study is available from the corresponding author upon reasonable request.
Abbreviations
- AMR:
-
Antimicrobial Resistance
- AOR:
-
Adjusted Odds Ratio
- ASP:
-
Antibiotic Stewardship Program
- CDC:
-
Center for Disease Control
- COR:
-
Crude Odds Ratio
- EML:
-
Essential Medicines List
- HIC:
-
High Income Country
- INRDU:
-
International Network of Rational Medicine Use
- LIC:
-
Low Income Country
- MTC:
-
Medicines and Therapeutics Committee
- RDU:
-
Rational Medicine Use
- SPSS:
-
Statistical Package for Social Sciences
- WHO:
-
World Health Organization
References
Atif M, Sarwar MR, Azeem M, Umer D, Rauf A, Rasool A, Ahsan M, Scahill S. Assessment of WHO/INRUD core drug use indicators in two tertiary care hospitals of Bahawalpur, Punjab, Pakistan. J Pharm Policy Pract. 2016;9(1):27.
Ofori-Asenso R, Brhlikova P, Pollock AM. Prescribing indicators at primary health care centers within the WHO African region: a systematic analysis (1995–2015). BMC Public Health. 2016;16(1):724.
Ahmadi F, Zarei E. Prescribing patterns of rural family physicians: a study in Kermanshah Province, Iran. BMC Public Health. 2017;17(1):908.
Song P, Li W, Zhou Q. An outpatient antibacterial stewardship intervention during the journey to JCI accreditation. BMC Pharmacol Toxicol. 2014;15(1):8.
Sarwar MR, Saqib A, Iftikhar S, Sadiq T. Antimicrobial use by WHO methodology at primary health care centers: a cross sectional study in Punjab, Pakistan. BMC Infect Dis. 2018;18(1):492.
Wojkowska-Mach J, Godman B, Glassman A, Kurdi A, Pilc A, Rozanska A, Skoczyński S, Wałaszek M, Bochenek T. Antibiotic consumption and antimicrobial resistance in Poland; findings and implications. Antimicrob Resist Infect Control. 2018;7(1):136.
Zhang D, Cui K, Lu W, Bai H, Zhai Y, Hu S, Li H, Dong H, Feng W, Dong Y. Evaluation of carbapenem use in a tertiary hospital: antimicrobial stewardship urgently needed. Antimicrob Resist Infect Control. 2019;8(1):5.
van Limburg M, Sinha B, Lo-Ten-Foe JR, van Gemert-Pijnen JE. Evaluation of early implementations of antibiotic stewardship program initiatives in nine Dutch hospitals. Antimicrob Resist Infect Control. 2014;3(1):33.
World Health Organization. Interventions and strategies to improve the use of antimicrobials in developing countries: a review. Geneva: World Health Organization; 2001.
Akpan M, Ahmad R, Shebl N, Ashiru-Oredope D. A review of quality measures for assessing the impact of antimicrobial stewardship programs in hospitals. Antibiotics. 2016;5(1):5.
World Health Organization. Antimicrobial resistance: global report on surveillance. Geneva: World Health Organization; 2014.
Tadesse BT, Ashley EA, Ongarello S, Havumaki J, Wijegoonewardena M, González IJ, Dittrich S. Antimicrobial resistance in Africa: a systematic review. BMC Infect Dis. 2017;17(1):616.
Control CfD, Prevention. Antibiotic use in the United States, 2017: progress and opportunities. Atlanta: US Department of Health and Human Services; 2017.
Vialle-Valentin C, Lecates R, Zhang F, Desta A, Ross-Degnan D. Predictors of antibiotic use in African communities: evidence from medicines household surveys in five countries. Tropical Med Int Health. 2012;17(2):211–22.
Atif M, Azeem M, Saqib A, Scahill S. Investigation of antimicrobial use at a tertiary care hospital in southern Punjab, Pakistan using WHO methodology. Antimicrob Resist Infect Control. 2017;6(1):41.
Smith DR, Dolk FCK, Smieszek T, Robotham JV, Pouwels KB. Understanding the gender gap in antibiotic prescribing: a cross-sectional analysis of English primary care. BMJ Open. 2018;8(2):e020203.
Sisay M, Mengistu G, Molla B, Amare F, Gabriel T. Evaluation of rational drug use based on World Health Organization core drug use indicators in selected public hospitals of eastern Ethiopia: a cross sectional study. BMC Health Serv Res. 2017;17(1):161.
Aravamuthan A, Arputhavanan M, Subramaniam K. Assessment of current prescribing practices using World Health Organization core drug use and complementary indicators in selected rural community pharmacies in southern India. J Pharm Policy Pract. 2017;10(1):1.
Barghouthi Achalu T, Mensa M. Retrospective drug use pattern of antibi-otics in pediatric ward of Shenan gibe hospital, Oromia region, Ethiopia. J Antibio Res. 2017;1(1):106.
Otoom S, Batieha A, Hadidi H, Hasan M, Al Saudi K. Evaluation of drug use in Jordan using WHO prescribing indicators; 2002.
Bashrahil K. Indicators of rational drug use and health services in Hadramout, Yemen; 2010.
El Mahalli A. WHO/INRUD drug prescribing indicators at primary health care centres in eastern province, Saudi Arabia; 2012.
Vooss AT, Diefenthaeler HS. Evaluation of prescription indicators established by the WHO in Getúlio Vargas-RS. Braz J Pharm Sci. 2011;47(2):385–90.
Hashemi S, Nasrollah A, Rajabi M. Irrational antibiotic prescribing: a local issue or global concern? EXCLI J. 2013;12:384.
Gidebo KD, Summoro TS, Kanche ZZ, Woticha EW. Assessment of drug use patterns in terms of the WHO patient-care and facility indicators at four hospitals in southern Ethiopia: a cross-sectional study. BMC Health Serv Res. 2016;16(1):643.
Shankar RP, Partha P, Shenoy NK, Easow JM, Brahmadathan KN. Prescribing patterns of antibiotics and sensitivity patterns of common microorganisms in the internal medicine ward of a teaching hospital in Western Nepal: a prospective study. Ann Clin Microbiol Antimicrob. 2003;2(1):7.
Amaha ND, Berhe YH, Kaushik A. Assessment of inpatient antibiotic use in Halibet National Referral Hospital using WHO indicators: a retrospective study. BMC Res Notes. 2018;11(1):904.
Tesfamariam S, Anand IS, Kaleab G, Berhane S, Woldai B, Habte E, Russom M. Self-medication with over the counter drugs, prevalence of risky practice and its associated factors in pharmacy outlets of Asmara, Eritrea. BMC Public Health. 2019;19(1):159.
World Health Organization. How to investigate drug use in health facilities: selected drug use indicators. Geneva: World Health Organization; 1993.
World Health Organization Model List of Essential Medicines, 21st List, 2019. Geneva: World Health Organization; 2019.
Bilal AI, Osman ED, Mulugeta A. Assessment of medicines use pattern using World Health Organization’s prescribing, patient care and health facility indicators in selected health facilities in eastern Ethiopia. BMC Health Serv Res. 2016;16(1):144.
Desalegn AA. Assessment of drug use pattern using WHO prescribing indicators at Hawassa University teaching and referral hospital, South Ethiopia: a cross-sectional study. BMC Health Serv Res. 2013;13(1):170.
Ateshim Y, Bereket B, Major F, Emun Y, Woldai B, Pasha I, Habte E, Russom M. Prevalence of self-medication with antibiotics and associated factors in the community of Asmara, Eritrea: a descriptive cross sectional survey. BMC Public Health. 2019;19(1):726.
Mulugeta Russom DT, Elias M, Teklai M, Bahta I, Ahmed H, Tewolde M, Abdulmumini U, Gebregiorgis S, Beyene E. Adverse drug reactions among patients admitted to Eritrean hospitals: prevalence causes and risk factors. Int J Pharmacovigilance. 2017;2(1):1–7.
Gashaw T, Sisay M, Mengistu G, Amare F. Investigation of prescribing behavior at outpatient settings of governmental hospitals in eastern Ethiopia: an overall evaluation beyond World Health Organization core prescribing indicators. J Pharm Policy Pract. 2018;11(1):26.
Sumaila A-N, Tabong PT-N. Rational prescribing of antibiotics in children under 5 years with upper respiratory tract infections in Kintampo municipal Hospital in Brong Ahafo Region of Ghana. BMC Res Notes. 2018;11(1):443.
Durga P, Abhinav P, Varun Raj K, Kishore P. Evaluation of prescribing patterns using WHO indicators at out patient Department of a Private Hospital in Warangal. IOSR J Pharm Biol Sci. 2017;12(3):1–4.
Laizu J, Parvin R, Sultana N, Ahmed M, Sharmin R, Sharmin ZR, et al. Prescribing Practice of Antibiotics for Outpatients in Bangladesh: Rationality Analysis. Am J Pharmacol. 2018;1(1):1008.
Alshakka M, Said K, Babakri M, Ansari M, Aldhubhani A, Hassali MA, Ibrahim MIM. A study on antibiotics prescribing pattern at outpatient department in four hospitals in Aden-Yemen. J Pharm Pract Community Med. 2016;2(3):88–93.
Anong DN, Akoachere J-FK. Prescribing patterns and associated factors of antibiotic prescription in primary health care facilities of Kumbo east and Kumbo west health districts, North West Cameroon. PloS one. 2018;13(3):e0193353.
Gube A, Gonfa R, Tadesse T. Evaluation of antibiotic use in medical Ward of Fitche District hospital, north Showa zone, Oromia region, Ethiopia. Adv Pharmacoepidemiol Drug Saf. 2017;6(217):2167–1052 1000217.
Streit S, Frey P, Singer S, Bollag U, Meli DN. Clinical and haematological predictors of antibiotic prescribing for acute cough in adults in Swiss practices–an observational study. BMC Fam Pract. 2015;16(1):15.
Michelo VLC. Factors associated with irrational drug use at a district Hospital in Zambia: patient record-based observations. Med J Zam. 2015;42(1):25–30.
Acknowledgements
The authors would like to thank the community pharmacy heads who cooperated with the data collection process.
Funding
The study received no funding.
Author information
Authors and Affiliations
Contributions
NDA conceived the idea. DGW and NDA designed the study. DGW, NDA and NA collected the data. EHT did the statistical analysis. NDA, DGW and NA wrote the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study received ethical approval from the Ministry of Health, Research Ethics and Protocol Review Committee.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Amaha, N.D., Weldemariam, D.G., Abdu, N. et al. Prescribing practices using WHO prescribing indicators and factors associated with antibiotic prescribing in six community pharmacies in Asmara, Eritrea: a cross-sectional study. Antimicrob Resist Infect Control 8, 163 (2019). https://doi.org/10.1186/s13756-019-0620-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13756-019-0620-5