Abstract
Background
The study was performed to investigate the efficacy and safety of preoperative dexamethasone (DXM) in preventing postoperative pulmonary complications (PPCs) after minimally invasive esophagectomy (MIE).
Methods
Patients who underwent total MIE with two-field lymph node dissection from February 2018 to February 2023 were included in this study. Patients who were given either 5 mg or 10 mg DXM as preoperative prophylactic medication before induction of general anesthesia were assigned to the DXM group, while patients who did not receive DXM were assigned to the control group. Preoperative evaluations, intraoperative data, and occurrence of postoperative complications were analyzed. The primary outcome was the incidence of PPCs occurring by day 7 after surgery.
Results
In total, 659 patients were included in the study; 453 patients received preoperative DXM, while 206 patients did not. Propensity score-matched analysis created a matched cohort of 366 patients, with 183 patients each in the DXM and control groups. A total of 24.6% of patients in the DXM group and 30.6% of patients in the control group had PPCs (P = 0.198). The incidence of respiratory failure was significantly lower in the DXM group than in the control group (1.1% vs 5.5%, P = 0.019). Fewer patients were re-intubated during their hospital stay in the DXM group than in the control group (1.1% vs 5.5%, P = 0.019).
Conclusions
Preoperative DXM before induction of anesthesia did not reduce overall PPC development after MIE. Nevertheless, the occurrence of early respiratory failure and the incidence of re-intubation during hospitalization were decreased.
Trial registration
Chinese Clinical Trial Registry (No. ChiCTR2300071674; Date of registration, 22/05/2023)
Similar content being viewed by others
Background
Major surgery is often associated with an increased inflammatory response, which may be related to the development of postoperative complications. Esophagectomy, one of the most invasive surgical procedures, is associated with high morbidity and mortality, especially with respect to pulmonary complications (Geller et al. 2019). A recent study showed that lung injury arising from the inflammatory response to surgical stress and oxidative stress is associated with pulmonary morbidity after esophagectomy (Shinozaki et al. 2021). To improve the postoperative clinical course, preoperative glucocorticoids have been introduced to attenuate the release of proinflammatory mediators such as IL-6, IL-8, and tumor necrosis factor (TNF) (Shinozaki et al. 2021; Takeda et al. 2003; Matsutani et al. 1998; Shimada et al. 2000). A few studies have investigated the protective effect of glucocorticoids in patients undergoing open esophagectomy (Gao et al. 2014; Nakamura et al. 2008). It is suggested that the benefits of cytokine response-modulating therapy may become blunted in the presence of major complications. Considering that minimally invasive esophagectomy (MIE) is associated with less severe surgical trauma and fewer postoperative complications, investigating the prophylactic effect of glucocorticoids on postoperative pulmonary complications (PPCs) following MIE might produce informative results.
The long-term glucocorticoid dexamethasone (DXM) is one of the most frequently used preanesthetic drugs because of its antiemetic and analgesic effects (Corcoran et al. 2022; Parthasarathy et al. 2018). In theory, the relatively long half-life of DXM may have a prolonged anti-inflammatory effect following surgery. However, whether prophylactic DXM before induction of anesthesia alleviates surgical stress and reduces postoperative complications (especially pulmonary complications) following MIE has never been exclusively studied. The aim of this study was to investigate the efficacy and safety of prophylactic DXM on PPC development after MIE.
Methods
Study population
All consecutive patients diagnosed with thoracic esophageal cancer who underwent total MIE with two-field lymph node dissection at a single academic institution (Peking University Cancer Hospital) from February 2018 to February 2023 were included in the study. Before the study’s commencement, approval was obtained from the hospital’s ethics committee (No. 2023YJZ36). The study was registered in the Chinese Clinical Trial Registry (No. ChiCTR2300071674) and was based on a previous retrospective study (Chinese Clinical Trial Registry, ChiCTR2300071571). The requirement for informed consent was waived because of the retrospective study design. In addition, patients who met one or more of the following criteria were excluded from the study: (1) esophageal cancer recurrence, (2) treatment with corticosteroids before surgery, (3) treatment with additional intraoperative corticosteroids except for prophylactic DXM, (4) American Society of Anesthesiologists (ASA) classification score greater than III, (5) COVID-19 infection within 1 month prior to surgery or during hospitalization, (6) change of operation method during surgery (e.g., unresectable tumor, conversion to an open procedure), and (7) incomplete data. All operations were performed by one of three senior esophageal cancer surgeons from the same ward, and general anesthesia was administered by the same team of thoracic anesthesiologists. Postoperative treatment was managed by clinicians on the same general ward or in the intensive care unit (ICU). All patients’ electronic medical records were reviewed for baseline characteristics, operative information, and postoperative variables during the hospital stay. Preoperative evaluation included a complete medical history, physical examination, and relevant laboratory tests and investigations. Pulmonary history was defined as a history of chronic obstructive pulmonary disease, bronchiectasis, asthma, pulmonary bullae, pulmonary infection within 1 month, and/or pulmonary tuberculosis. Intraoperative data included operative records and anesthesia records. Postoperative follow-up consisted of the duration of ICU stay/hospital stay, postoperative laboratory and imaging data, and the occurrence of PPCs and non-pulmonary complications.
Anesthesia and surgical technique
All patients underwent general anesthesia with one-lung ventilation or two-lung ventilation under artificial pneumothorax. Epidural anesthesia was not routinely used for patients undergoing total MIE in our institution. Regional anesthesia such as paravertebral block, transversus abdominis plane block, and intercostal nerve block were applied at the attending anesthesiologists’ discretion. A glucocorticoid (5 mg or 10 mg DXM) was administered as a preoperative prophylactic medication before induction of general anesthesia at the anesthesiologists’ discretion. Intubation was completed with either a double-lumen endotracheal tube, a single-lumen endotracheal tube, or the combination of a single-lumen tube and a bronchial blocker after intravenous induction of general anesthesia. A protective lung ventilation strategy was adopted; i.e., a tidal volume of 4 to 6 mL/kg and a positive end-expiratory pressure of 2 to 5 cm H2O. General anesthesia was maintained with a combination of inhaled and intravenous anesthesia. Patient-controlled intravenous analgesia with opioids was used for postoperative analgesia for all patients. The MIE procedure included two-field lymph node dissection with anastomosis in the neck, which consisted of right transthoracic subtotal esophagectomy and dissection of mediastinal and abdominal lymph nodes. The thoracic procedures were performed by thoracoscopic surgery in the left lateral-prone position. The abdominal procedures were performed by total laparoscopic surgery. After surgery, all patients were transferred to the post-anesthesia care unit and then either back to the general ward after full recovery or to the ICU if continued mechanical ventilation was necessary or severe comorbidities were present.
Variables and data extraction
Data on PPCs were collected during the hospital stay. The primary outcome was the incidence of PPCs occurring by postoperative day (POD) 7, assuming that PPCs related to intraoperative management would occur early. Late PPCs may be associated with factors other than the initial surgery, such as aspiration or wound infection (Howells et al. 2016). PPCs were defined as grade ≥ II complications according to the Clavien-Dindo classification of respiratory system complications (Table 1) (Dindo et al. 2004). Major pulmonary complications occurring by POD 7 were analyzed separately, including pneumonia (defined as the presence of a new or progressive radiographic infiltrate plus at least two of three clinical features including fever of > 38 °C, leukocytosis or leukopenia, and purulent secretions), pleural effusion requiring an additional drainage procedure, atelectasis requiring bronchoscopic treatment, pneumothorax requiring thoracocentesis, respiratory failure (defined as postoperative PaO2 of < 60 mmHg or arterial oxyhemoglobin saturation measured with pulse oximetry of < 90% despite oxygen therapy, or the need for non-invasive mechanical ventilation), acute respiratory distress syndrome, acute aspiration, and tracheobronchial injury. The secondary outcome was non-pulmonary complications occurring during the hospital stay, which included anastomotic leakage, wound infection, cardiac complications (including new-onset arrhythmia, myocardial ischemia or infarction, and heart failure), chylothorax, and recurrent laryngeal nerve injury. The length of the hospital stay was also analyzed.
Statistical analysis
IBM SPSS Statistics for Windows, version 26.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis. Categorical variables are reported as numbers (percentage) and were analyzed with Pearson’s chi-squared test or Fisher’s exact test. Continuous variables were reported as mean ± standard deviation or median (interquartile range). Normally distributed data are analyzed with the independent-samples t-test, and non-normally distributed data are analyzed with the Mann–Whitney U test. To adjust for unbalanced covariates between the patients who received DXM preoperatively (DXM group) and patients who did not (control group), propensity score-matched analysis was used. Baseline characteristics and preoperative/intraoperative data were analyzed, including age, sex, height, weight, body mass index (BMI), ASA classification, comorbidities, hemoglobin concentration, albumin concentration, preoperative treatment, tumor location, tumor pathology, and anesthesia technique. Variables with a P value of < 0.1 in the univariate analysis between the two groups and perioperative risk factors reported to be associated with PPCs (e.g., age, BMI, pulmonary comorbidity, former smoking status, ventilation technique, duration of surgery, intraoperative fluid intake, and operative blood loss (Kinugasa et al. 2004; Zingg et al. 2011; Uchihara et al. 2018; Ohi et al. 2019; Law et al. 2004; Xing et al. 2015) were included for propensity calculation and matching using a logistic regression model. By setting the matching ratio at 1:1 using the nearest-neighbor method and the caliper width at 0.2 of the standard deviation, two adequately balanced groups (DXM group and control group) were generated for further analysis. A standardized mean difference of < 0.1 between the two groups was considered adequately balanced in matching. Statistical significance was set at P < 0.05.
Results
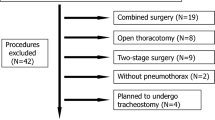
During the study period, 699 patients were scheduled for total MIE with two-field lymph node dissection. A total of 659 patients qualified for enrollment and were included in the study. Among those individuals, 453 patients received prophylactic DXM and 206 patients did not. Prior to matching, there were significant variations in the current smoking status, ASA classification, tumor location, intraoperative fluid intake, and percentage of patients receiving nerve block between the two groups (Table 2). Therefore, the following risk factors were adjusted to compensate for the propensity score: age, BMI, pulmonary comorbidities, smoking status, ASA classification, tumor location, percentage of patients receiving combined nerve block, intraoperative fluid intake, operative blood loss, and duration of surgery. Propensity score-matching created a matched cohort of 366 patients with 183 patients each in the DXM group and the control group (Fig. 1). After matching, there were no significant differences in demographic characteristics or intraoperative data between the two groups (Table 2).
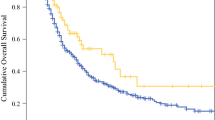
After matching, 45 (24.6%) patients in the DXM group and 56 (30.6%) patients in the control group had PPCs by POD 7 (P = 0.198) (Fig. 2). The incidence of respiratory failure by POD 7 was significantly lower in the DXM group than in the control group (1.1% vs 5.5%, P = 0.019). The incidence of other major PPCs by POD 7 did not differ significantly between the two groups (Table 3). Fewer patients were re-intubated during their hospital stay in the DXM group than in the control group [2 (1.1%) vs 10 (5.5%), P = 0.019].
The incidence of postoperative cardiac complications was significantly lower in the DXM group (2.2%) than in the control group (6.6%, P = 0.041). The occurrences of other major postoperative non-pulmonary complications during the hospital stay were comparable between the two groups (Table 4). The length of hospital stay was 12.0 (10.0–14.0) days in the DXM group and 13.0 (11.0–15.0) days in the control group (P = 0.174).
Discussion
In the present study, 24.6% of patients in the DXM group and 30.6% of patients in the control group developed PPCs by POD 7. There was a non-significant trend of reduced PPCs in patients treated with preoperative DXM administration. When major PPCs were analyzed separately, the incidence of respiratory failure was significantly lower in patients receiving preoperative DXM. This finding was consistent with several previous studies on other prophylactic glucocorticoids. Recently published Japanese nationwide inpatient data showed an association between prophylactic methylprednisolone use in oncologic esophagectomy and decreased respiratory failure as well as lower in-hospital mortality (Hirano et al. 2022). Likewise, an investigation into the effect of preoperative methylprednisolone on surgery-related complications following esophagectomy revealed that patients who received a prophylactic glucocorticoid had a significantly lower rate of organ system failure, including respiratory failure. In that investigation, patients in the glucocorticoid group had lower plasma levels of IL-6 and IL-8 than patients in the control group, indicating that glucocorticoids might have attenuated surgical stress–induced inflammatory responses by protecting against elevation of proinflammatory cytokine levels (Sato et al. 2002). In particular, the authors suggested that the improvement of respiratory function was related to the shorter duration of mechanical ventilation in patients who received prophylactic glucocorticoids. In the present study, the duration of mechanical ventilation was not analyzed; however, the re-intubation rate within POD 7 was lower in the DXM group (although the difference was not statistically significant), and the overall re-intubation rate during hospitalization was significantly lower in patients who received preoperative DXM. This may have resulted in a shorter duration of mechanical ventilation. Another investigation also revealed that intraoperative methylprednisolone administration was associated with a decreased risk of acute respiratory failure following esophagectomy (Park et al. 2012). Consistent results were reported in other studies, in which methylprednisolone pretreatment was associated with reduced intubation days, a higher postoperative ratio of arterial oxygen partial pressure to fractional inspired oxygen, and fewer postoperative respiratory complications (Takeda et al. 2003; Raimondi et al. 2006; Tsukada et al. 2006). These findings suggest a potential benefit of preoperative corticosteroid use in both open esophagectomy and MIE. On the basis of previous research and the results of the present study, we consider preoperative DXM effective in reducing the occurrence of respiratory failure following MIE. The mechanism underlying this association is unclear. The inflammatory response triggered by major surgery may induce proinflammatory cytokine secretions, endothelial dysfunction, glycocalyx damage, and neutrophil activations, leading to tissue and multisystem organ destruction (Margraf et al. 2020). It is suggested that lung injury induced by the inflammatory response to surgical stress and oxidative stress is associated with pulmonary morbidity after esophagectomy (Shinozaki et al. 2021). Previous studies have shown that glucocorticoids may lower the risk of respiratory failure by inhibiting the inflammatory response to surgical stress through suppression of proinflammatory cytokine release (Takeda et al. 2003; Sato et al. 2002; Raimondi et al. 2006). In one study, for example, the plasma norepinephrine and arginine vasopressin concentrations were significantly lower in patients treated with methylprednisolone than in the control group (Takeda et al. 2003). The postoperative plasma IL-6 concentration was also found to be lowered by preoperative glucocorticoid administration (Raimondi et al. 2006). The laboratory data in another study suggested that corticosteroids may attenuate surgical stress-induced inflammatory responses both directly by suppressing the release of proinflammatory cytokines and by inducing IL-10 synthesis (Sato et al. 2002). However, because of the retrospective design of the present study, data reflecting inflammatory responses could not be collected. Further studies are required to better understand the possible mechanism associated with the protective effect of respiratory function by preoperative DXM.
In addition to a reduced incidence of respiratory failure, we observed fewer postoperative cardiac complications in the DXM group. These findings were similar to previous studies, in which reduced cardiovascular failure or reduced cardiovascular complications were observed in patients treated with glucocorticoids (Gao et al. 2014; Sato et al. 2002; Engelman and Maeyens 2010). Generally, a single dose of glucocorticoid can inhibit the synthesis of proinflammatory mediators such as IL-6, IL-8, and TNF-α in cardiac surgery and other major non-cardiac surgeries such as procedures involving the esophagus and abdomen (Matsutani et al. 1998; Yilmaz et al. 1999; Schulze et al. 1997). Research has shown that higher levels of circulatory cytokines such as IL-6, IL-8, and TNF-α are closely related to impaired hemodynamics and may contribute to postoperative myocardial ischemia and deteriorated clinical outcomes after cardiac surgery (Deng et al. 1996; Hennein et al. 1994). Notably, a single dose of glucocorticoid was associated with a lower incidence of postoperative atrial fibrillation and fewer cases of myocardial injury after cardiac surgery (Liu et al. 2021; Xu et al. 2011; Zhou et al. 2023). However, inconsistent conclusions have been drawn regarding the influence of preoperative glucocorticoids on postoperative cardiac complications (Chaney et al. 2001). According to our results, we speculate that there may be a link between a reduced rate of postoperative cardiac complications and preoperative DXM administration. However, this speculation must be verified by future investigations.
The possible association between glucocorticoid use and surgical site complications such as impaired wound healing is a major concern (Wang et al. 2013; Bootsma et al. 2018). In one study, the incidence of graft dehiscence was higher in patients who received preventive hydrocortisone than in the control group (Jeong et al. 2019). In another study investigating postoperative acute lung injury after esophagectomy, superimposed infections were observed in 30% of the patients treated with corticosteroids, and surgical site complications (including wound dehiscence and anastomotic site leakage) were observed in 27% of the patients (Choi et al. 2019). This indicates the need for special attention to surgical complications related to glucocorticoids. Although the type and dosage of corticosteroids used in this study were not clarified, we speculate that a higher dosage was used because corticosteroids were used to treat acute respiratory distress syndrome. By contrast, whereas several research groups reported no adverse influence of glucocorticoid administration on anastomotic leakage, severe infection, and suture failure, two studies revealed the opposite results, showing protective effects of methylprednisolone pretreatment on surgical anastomotic leakage (Gao et al. 2014; Hirano et al. 2022; Park et al. 2012; Engelman and Maeyens 2010; Weijs et al. 2014). An investigation including a large sample of 8725 participants undergoing non-urgent, non-cardiac surgery demonstrated that 8 mg of intravenous DXM was non-inferior to placebo with respect to the incidence of surgical site infection (Corcoran et al. 2021). Similar to the dosage administered in that study, 5 mg or 10 mg of prophylactic DXM was used in the present study, and comparable occurrences of postoperative complications of anastomotic leakage and wound infection between the two groups were observed. These results suggest that a low dose of DXM pretreatment may not increase the incidence of adverse effects following MIE. However, because of the lack of consensus evidence produced by previous studies, further large-scale studies on the potentially negative impact of prophylactic DXM following MIE are warranted.
The clinical benefits and risks associated with preoperative glucocorticoid use remain unclear because of conflicting study results and lack of thorough investigation (Jeong et al. 2019; Choi et al. 2019; Yano et al. 2005). To our knowledge, although a few studies have investigated the effect of perioperative glucocorticoids on esophagectomy, most of the trials included a small number of participants and are now outdated (Takeda et al. 2003; Matsutani et al. 1998; Sato et al. 2002; Takeda et al. 1997). Studies focusing on the impact of prophylactic glucocorticoids on PPCs following MIE are also limited. The present study is the first to focus exclusively on the impact of preoperative DXM on the development of PPCs following MIE. The strengths of our study are our analysis of recent data (within 5 years) reflecting current practice and our focus on PPC development in patients undergoing MIE. Additionally, although previous studies have investigated hydrocortisone and methylprednisolone, there has been no thorough investigation on prophylactic DXM despite the fact that this glucocorticoid is one of the most frequently used pre-medications before anesthetic induction. Our study fills this gap.
This study has several limitations. First, this was a retrospective study in a single center, and the results may have therefore been affected by biases such as variability in standard practices among clinicians. However, all participants underwent propensity score-matched analysis to ensure maximal uniformity in patient characteristics as well as preoperative and intraoperative variables, including anesthesia-related factors; this analysis may have overcome some bias. Second, the anti-inflammatory effects of glucocorticoids were suggested to be dose-dependent. Nevertheless, the DXM group in the present study received a fixed dose of either 5 mg or 10 mg of DXM; the effects under different dosages were not studied separately. Thus, the optimum dosage of prophylactic DXM requires further investigation. Third, although preoperative corticosteroids may have an impact on the perioperative blood glucose concentration, these data were missing for some of the patients because of the retrospective study design and were therefore not included in the analysis. However, we collected data on postoperative wound infection, which is the most concerning postoperative complication in patients with perioperative hyperglycemia, and these data did not differ significantly between the two groups. Fourth, the postoperative pain score and the occurrence of postoperative nausea and vomiting might have influenced our results; however, these data could not be collected because of the retrospective design. Finally, the results of secondary outcomes regarding postoperative non-pulmonary complications should be interpreted with caution; they might be biased because the propensity score-matched analyses were mainly based on variables associated with PPCs. Future investigations using large sample sizes and a prospective design are required for more information, especially regarding the underlying mechanism of the protective effect of preoperative DXM on PPCs.
Conclusion
Preoperative DXM before induction of anesthesia did not reduce overall PPC development. However, the postoperative occurrence of respiratory failure and the incidence of re-intubation during hospitalization were decreased. The optimal dosage of prophylactic DXM should be thoroughly analyzed using prospective randomized controlled trials.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- IL:
-
Interleukin
- TNF:
-
Tumor necrosis factor
- MIE:
-
Minimally invasive esophagectomy
- PPCs:
-
Postoperative pulmonary complications
- DXM:
-
Dexamethasone
- ASA:
-
American Society of Anesthesiologists
- ICU:
-
Intensive care unit
- POD:
-
Postoperative day
- BMI:
-
Body mass index
References
Bootsma BT, Huisman DE, Plat VD, Schoonmade LJ, Stens J, Hubens G, et al. Towards optimal intraoperative conditions in esophageal surgery: A review of literature for the prevention of esophageal anastomotic leakage. Int J Surg. 2018;54(Pt A):113–23.
Chaney MA, Durazo-Arvizu RA, Nikolov MP, Blakeman BP, Bakhos M. Methylprednisolone does not benefit patients undergoing coronary artery bypass grafting and early tracheal extubation. J Thorac Cardiovasc Surg. 2001;121(3):561–9.
Choi H, Cho JH, Kim HK, Choi YS, Kim J, Zo JI, et al. Prevalence and clinical course of postoperative acute lung injury after esophagectomy for esophageal cancer. J Thorac Dis. 2019;11(1):200–5.
Corcoran TB, Myles PS, Forbes AB, Cheng AC, Bach LA, O’Loughlin E, et al. Dexamethasone and Surgical-Site Infection. N Engl J Med. 2021;384(18):1731–41.
Corcoran TB, Martin C, O’Loughlin E, Ho KM, Coutts P, Chan MT, et al. Dexamethasone and clinically significant postoperative nausea and vomiting: a prespecified substudy of the randomised perioperative administration of dexamethasone and infection (PADDI) trial. Br J Anaesth. 2022;129(3):327–35.
Deng MC, Dasch B, Erren M, Möllhoff T, Scheld HH. Impact of left ventricular dysfunction on cytokines, hemodynamics, and outcome in bypass grafting. Ann Thorac Surg. 1996;62(1):184–90.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13.
Engelman E, Maeyens C. Effect of preoperative single-dose corticosteroid administration on postoperative morbidity following esophagectomy. J Gastrointest Surg. 2010;14(5):788–804.
Gao Q, Mok HP, Wang WP, Xiao F, Chen LQ. Effect of perioperative glucocorticoid administration on postoperative complications following esophagectomy: A meta-analysis. Oncol Lett. 2014;7(2):349–56.
Geller AD, Zheng H, Gaissert H, Mathisen D, Muniappan A, Wright C, et al. Relative incremental cost of postoperative complications of esophagectomy. Semin Thorac Cardiovasc Surg. 2019;31(2):290–9.
Hennein HA, Ebba H, Rodriguez JL, Merrick SH, Keith FM, Bronstein MH, et al. Relationship of the proinflammatory cytokines to myocardial ischemia and dysfunction after uncomplicated coronary revascularization. J Thorac Cardiovasc Surg. 1994;108(4):626–35.
Hirano Y, Konishi T, Kaneko H, Itoh H, Matsuda S, Kawakubo H, et al. Impact of prophylactic corticosteroid use on in-hospital mortality and respiratory failure after esophagectomy for esophageal cancer: nationwide inpatient data study in Japan. Ann Surg. 2022:277(6):e1247-e1253.
Howells P, Thickett D, Knox C, Park D, Gao F, Tucker O, et al. The impact of the acute respiratory distress syndrome on outcome after oesophagectomy. Br J Anaesth. 2016;117(3):375–81.
Jeong H, Choi JW, Ahn HJ, Choi YS, Kim JA, Yang M, et al. The effect of preventive use of corticosteroids on postoperative complications after esophagectomy: A retrospective cohort study. Sci Rep. 2019;9(1):11984.
Kinugasa S, Tachibana M, Yoshimura H, Ueda S, Fujii T, Dhar DK, et al. Postoperative pulmonary complications are associated with worse short- and long-term outcomes after extended esophagectomy. J Surg Oncol. 2004;88(2):71–7.
Law S, Wong KH, Kwok KF, Chu KM, Wong J. Predictive factors for postoperative pulmonary complications and mortality after esophagectomy for cancer. Ann Surg. 2004;240(5):791–800.
Liu L, Jing FY, Wang XW, Li LJ, Zhou RQ, Zhang C, et al. Effects of corticosteroids on new-onset atrial fibrillation after cardiac surgery: a meta-analysis of randomized controlled trials. Medicine (Baltimore). 2021;100(11): e25130.
Margraf A, Ludwig N, Zarbock A, Rossaint J. Systemic inflammatory response syndrome after surgery: mechanisms and protection. Anesth Analg. 2020;131(6):1693–707.
Matsutani T, Onda M, Sasajima K, Miyashita M. Glucocorticoid attenuates a decrease of antithrombin III following major surgery. J Surg Res. 1998;79(2):158–63.
Nakamura M, Iwahashi M, Nakamori M, Ishida K, Naka T, Iida T, et al. An analysis of the factors contributing to a reduction in the incidence of pulmonary complications following an esophagectomy for esophageal cancer. Langenbecks Arch Surg. 2008;393(2):127–33.
Ohi M, Toiyama Y, Omura Y, Ichikawa T, Yasuda H, Okugawa Y, et al. Risk factors and measures of pulmonary complications after thoracoscopic esophagectomy for esophageal cancer. Surg Today. 2019;49(2):176–86.
Park SY, Lee HS, Jang HJ, Joo J, Zo JI. Efficacy of intraoperative, single-bolus corticosteroid administration to prevent postoperative acute respiratory failure after oesophageal cancer surgery. Interact Cardiovasc Thorac Surg. 2012;15(4):639–43.
Parthasarathy P, Babu K, Raghavendra Rao RS, Raghuram S. The effect of single-dose intravenous dexamethasone on postoperative pain and postoperative nausea and vomiting in patients undergoing surgery under spinal anesthesia: a double-blind randomized clinical study. Anesth Essays Res. 2018;12(2):313–7.
Raimondi AM, Guimarães HP, Amaral JL, Leal PH. Perioperative glucocorticoid administration for prevention of systemic organ failure in patients undergoing esophageal resection for esophageal carcinoma. Sao Paulo Med J. 2006;124(2):112–5.
Sato N, Koeda K, Ikeda K, Kimura Y, Aoki K, Iwaya T, et al. Randomized study of the benefits of preoperative corticosteroid administration on the postoperative morbidity and cytokine response in patients undergoing surgery for esophageal cancer. Ann Surg. 2002;236(2):184–90.
Schulze S, Andersen J, Overgaard H, Nørgard P, Nielsen HJ, Aasen A, et al. Effect of prednisolone on the systemic response and wound healing after colonic surgery. Arch Surg. 1997;132(2):129–35.
Shimada H, Ochiai T, Okazumi S, Matsubara H, Nabeya Y, Miyazawa Y, et al. Clinical benefits of steroid therapy on surgical stress in patients with esophageal cancer. Surgery. 2000;128(5):791–8.
Shinozaki H, Matsuoka T, Ozawa S. Pharmacological treatment to reduce pulmonary morbidity after esophagectomy. Ann Gastroenterol Surg. 2021;5(5):614–22.
Takeda S, Ogawa R, Nakanishi K, Kim C, Miyashita M, Sasajima K, et al. The effect of preoperative high dose methylprednisolone in attenuating the metabolic response after oesophageal resection. Eur J Surg. 1997;163(7):511–7.
Takeda S, Takeda S, Kim C, Ikezaki H, Nakanishi K, Sakamoto A, et al. Preoperative administration of methylprednisolone attenuates cytokine-induced respiratory failure after esophageal resection. J Nippon Med Sch. 2003;70(1):16–20.
Tsukada K, Miyazaki T, Katoh H, Masuda N, Fukuchi M, Manda R, et al. Effect of perioperative steroid therapy on the postoperative course of patients with oesophageal cancer. Dig Liver Dis. 2006;38(4):240–4.
Uchihara T, Yoshida N, Baba Y, Yagi T, Toihata T, Oda E, et al. Risk factors for pulmonary morbidities after minimally invasive esophagectomy for esophageal cancer. Surg Endosc. 2018;32(6):2852–8.
Wang AS, Armstrong EJ, Armstrong AW. Corticosteroids and wound healing: clinical considerations in the perioperative period. Am J Surg. 2013;206(3):410–7.
Weijs TJ, Dieleman JM, Ruurda JP, Kroese AC, Knape HJ, van Hillegersberg R. The effect of perioperative administration of glucocorticoids on pulmonary complications after transthoracic oesophagectomy: a systematic review and meta-analysis. Eur J Anaesthesiol. 2014;31(12):685–94.
Xing X, Gao Y, Wang H, Qu S, Huang C, Zhang H, et al. Correlation of fluid balance and postoperative pulmonary complications in patients after esophagectomy for cancer. J Thorac Dis. 2015;7(11):1986–93.
Xu B, Strom J, Chen QM. Dexamethasone induces transcriptional activation of Bcl-xL gene and inhibits cardiac injury by myocardial ischemia. Eur J Pharmacol. 2011;668(1–2):194–200.
Yano M, Taniguchi M, Tsujinaka T, Fujiwara Y, Yasuda T, Shiozaki H, et al. Is preoperative methylprednisolone beneficial for patients undergoing esophagectomy? Hepatogastroenterology. 2005;52(62):481–5.
Yilmaz M, Ener S, Akalin H, Sagdic K, Serdar OA, Cengiz M. Effect of low-dose methyl prednisolone on serum cytokine levels following extracorporeal circulation. Perfusion. 1999;14(3):201–6.
Zhou Z, Long Y, He X, Li Y. Effects of different doses of glucocorticoids on postoperative atrial fibrillation: a meta-analysis. BMC Cardiovasc Disord. 2023;23(1):16.
Zingg U, Smithers BM, Gotley DC, Smith G, Aly A, Clough A, et al. Factors associated with postoperative pulmonary morbidity after esophagectomy for cancer. Ann Surg Oncol; 2011;18(5):1460-1468.
Acknowledgements
We thank Liwen Bianji (Edanz) (http://www.liwenbianji.cn) for editing the English text of a draft of this manuscript.
Funding
None.
Author information
Authors and Affiliations
Contributions
XXL designed the study; collected, analyzed, and interpreted the data; and drafted the manuscript. LY interpreted the data and revised the manuscript. JNY collected the data and assisted in drafting the manuscript. MF collected the data and assisted in drafting the manuscript. HYT revised the manuscript and approved the version to be published. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board at Peking University Cancer Hospital (No. 2023YJZ36). The requirement for written informed consent was waived because of the study’s retrospective design.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, X., Yu, L., Yang, J. et al. Efficacy of preoperative single-dose dexamethasone in preventing postoperative pulmonary complications following minimally invasive esophagectomy: a retrospective propensity score-matched study. Perioper Med 13, 46 (2024). https://doi.org/10.1186/s13741-024-00407-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13741-024-00407-6