Abstract
Background
Treatment of latent tuberculosis infection (LTBI) is effective in preventing progression to TB disease. This study aimed to synthesize available evidence on the efficacy, adherence, and safety of LTBI treatment in order to assist policymakers to design appropriate national treatment policies and treatment protocols.
Method
The PRISMA-NMA was used to review and report this research. Randomized controlled trials which compared the efficacy and safety of LTBI treatments were included. A systematic literature search was done to identify relevant articles from online databases PubMed/ MEDLINE, Embase, and Cochrane Center for Clinical Trial database (CENTRAL). The network meta-analysis was done using R- studio Version 1.4.1103.
Result
In this review, 42 studies were included, which enrolled 46,022 people who had recent contact with patients with active tuberculosis, evidence radiological of previous tuberculosis, tuberculin test equal or greater than 5 mm, radiographs that indicated inactive fibrotic or calcified parenchymal and/or lymph node lesions, had conversion to positive results on a tuberculin skin test, participants living with HIV, chronic Silicosis, immigrants, prisoners, old people, and pregnant women who were at risk for latent TB were included.
The incidence of TB among people living with HIV who have taken 3RH as TPT was lower, followed by 48%,followed by 6H (41%). However, 3HP has also the potential to reduce the incidence of TB by 36% among HIV negative patients who had TB contact history. Patients’ adherence to TPT was higher among patients who have taken 4R (RR 1.38 95% CI 1.0,1.89) followed by 3RH (34%). The proportion of subjects who permanently discontinued a study drug because of an adverse event were three times higher in the 3RH treatment group. Furthermore, the risk of grade 3 and 4 liver toxicity was significantly higher in 9H followed by 1HP, and 6H.
Conclusion
From this review, it can be concluded 3RH and 6H has a significant impact on the reduction of TB incidence among PLWH and 3HP among HIV negative people who had TB contact history. However, combinations of rifampicin either with isoniazid were significantly associated with adverse events which resulted in permanent discontinuation among adult patients. Furthermore, grade 3 and 4 liver toxicity was more common in patents who have taken 9H, 1HP, and 6H. This may support the current recommended TPT regimen of 3HP, 3RH, and 6H.
Similar content being viewed by others
Introduction
Tuberculosis (TB) remains the leading cause of morbidity and mortality from a single infectious disease [1], with one-fourth of the global population, approximately 2 billion persons, estimated to be infected with TB [2, 3]. The occurrence of latent tuberculosis infection (LTBI)—a state of persistent immune response to stimulation by Mycobacterium tuberculosis antigens with no evidence of clinically manifest active TB – is impeding the effort to prevent and control TB [2, 4-6]. Treatment of LTBI is effective in preventing progression to TB disease while approximately 5%–10% of persons with LTBI progress to active TB disease if untreated [7-9]. The probability of progression to active TB disease is higher in specific risk groups including people living with HIV, receiving dialysis, preparing for an organ or hematological transplant, prisoners, health workers, immigrants, homeless, silicosis, diabetes, and drug addicted [10-14].
WHO recommends TB preventive treatment (TPT) a key approach to end TB. The current TPT treatment consists of the preferred three rifamycin-based preferred regimens: 3 months of once-weekly isoniazid plus rifapentine, 4 months of daily rifampin, or 3 months of daily isoniazid plus rifampin [3, 4]. Some studies reported that rifamycin-based regimens are effective and safe for treatment of LTBI, with higher treatment completion rates [15-20]. Some other studies reported that regimens isoniazid monotherapy, daily for 6–9 months, is efficacious but with a higher risk of toxicity and a lower treatment completion rates compared to rifamycin-based regimens with shorter treatment durations [21, 22]. Studies also demonstrated that 3 months of once-weekly isoniazid plus rifapentine is non-inferior to other regimens, but with slightly higher adverse events [21-25]. A recent study also showed a beneficial effect of one month daily isoniazid and rifapentine combination-therapy [26]. There are concerns about pragmatic and long-term aspects of TPT, including adherence, potential emergence of drug resistance, and cost-effectiveness in resource-constrained settings [27]. Several systematic reviews as well as network meta-analyses have yet been documented about treatment of LTBI [8, 28-30]; however, majority focused on effectiveness of different regimens that did not provide information about the indirect relative (comparision of different TPTs with one another indirectly) safety of the TPT regimens. Also, these studies have provided separate information about the efficacy of TPT among people living with HIV, immigrants, children, and people who had Tb cotact history. We belive that there is a need for an updated and more comprencive evidence regarding the efficacy and safety of TPTs for different population groups.
We, therefore, conducted this systematic review and network meta-analysis of randomized controlled trials (RCTs) using the frequentist model to provide an up-to-date summary and analysis of previously published studies that have evaluated LTBI regimens and made informative comparisons of their relative efficacy and adverse event profiles.
Methods
The protocol for this systematic review and network meta-analysis has been registered at the International Prospective Register of Systematic Reviews (PROSPERO) database, ID: CRD42022334163 [31]. The PRISMA statement extension for systematic reviews incorporating network meta-analysis (PRISMA-NMA) was used to review and report this research [32].
Eligibility Criteria
-
○ The PICOS format [33] was used to identify eligible studies.
Participants
-
○ People who had recent contact with patients with active tuberculosis, evidence radiological of previous tuberculosis, tuberculin test equal or greater than 5 mm, radiographs that indicated inactive fibrotic or calcified parenchymal and/or lymph node lesions, had conversion to positive results on a tuberculin skin test, participants living with HIV, chronic Silicosis, immigrants, prisoners, old people, and pregnant women who were at risk for latent TB were included.
-
○ Tuberculosis (TB) contacts are people who have close contact with patients with infectious TB.
Interventions
-
12 months 600 mg rifamycin plus 300 mg isoniazid (12RH).
-
3 months 600 mg rifamycin plus 300 mg isoniazid (3RH).
-
3 months of once-weekly 900 mg isoniazid plus 900 mg rifapentine (3HP).
-
18 months daily 300 mg isoniazid (18H).
-
72 months daily 300 mg isoniazid (72H).
-
4 months daily 600 mg rifamycin plus 300 mg isoniazid (4RH).
-
6 months daily 300 mg isoniazid plus 800 mg ethambutol (6EH).
-
1 month daily 300 mg isoniazid plus 600 mg rifapentine (1HP).
-
2 months daily 600 mg rifamycin (2R).
-
4 months of once-weekly 900 mg isoniazid plus 600 mg rifapentine (4HP).
-
2 months of twice-weekly 600 mg isoniazid plus 600 mg rifapentine (2HP TW).
-
3 months of once-weekly 900 mg isoniazid plus 900 mg rifapentine (3HP).
-
4 months daily 600 mg rifamycin (4R).
-
3 months daily 600 mg rifamycin (3R).
-
6 months daily 300 mg isoniazid (6H)
-
9 months daily 300 mg isoniazid (9H)
-
12 months daily 300 mg isoniazid (12H)
-
18 months daily 300 mg isoniazid (18H)
-
24 months daily 300 mg isoniazid (24H)
-
72 months daily 300 mg isoniazid (72H)
Comparator
-
3 months of once-weekly 900 mg isoniazid plus 900 mg rifapentine (3HP), or
-
Placebo
Outcome measures
Primary outcomes
-
Treatment efficacy, thus the overall incidence of TB among all kinds of participants, PLWHIV, and HIV negative participants who have taken TPT.
Secondary outcomes
-
○ Adverse event including serious adverse event was assessed.
-
○ Adherence to medications
-
○ The incidence of TB in patients living with chronic silicosis.
Studies
RCTs published from 1993–2022, involving participants of any age group which compared the efficacy, safety, or adherence of LTBI regimens and exploratory analysis of data from RCTs. Those studies conducted and published before 1993 were either not freely available for access or not aligned with the current WHO INH recommended dose (300 mg/day). Regimens containing PZA were not considered among those of primary interest due to their poor toxicity profile. Two reviwers assessed the titles and abstracts from the primary search independently. Those seemingly meeting inclusion criteria were further assessed by review of full texts by the same two reviewers. Disagreements were resolved by consensus.
Electronic searches
A systematic literature search was done to identify relevant articles from online databases PubMed/ MEDLINE, Embase, and Cochrane Center for Clinical Trial database (CENTRAL). To search and assess ongoing or unpublished trials, ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform, and the US Food and Drug Administration (FDA) were searched. The search was done according to guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions [33].
The search strategies in PubMed for the MeSH terms and text words were “Tuberculosis” [MeSH Terms]) OR “Latent Tuberculosis Prevention” [MeSH]) AND “Tuberculosis Prevention therapy” [MeSH]) AND “isoniazid” [MeSH]) AND “rifapentine” [MeSH]) AND “rifamycin” [MeSH]) AND “ethambutol” [MeSH]).
Study selection, data collection, and data analysis
We used the Cochrane Handbook for Systematic Reviews of Interventions [34], the R- studio Version 1.4.1103, and the EndNote X7 for data management and analysis. Two authors independently reviewed the results and disagreements resolved through discussion. When clarification was necessary, the trial authors were contacted.
Data extraction and management
The title and abstract were produced from the electronic search and independently screened by two authors based on RCTs that were LTBI. The information collected were trial characteristics including methods, participants, interventions, and outcomes as well as data on dose and drug ratios of the combinations. Relevant information such as title, name of the journal, year of publication, author’s first name, country, type of participant, age, sex, randomization, post treatment follow up time, methody of study drug adminstartion (directly observed therapy (DOT) or self administration), publication status, study design, study setting, follow-up period, sample size, funding source, baseline characteristics of study subjects, adherence, TB incidence, adverse events, and serious adverse events were extracted from each article using a structured data extraction format adapted from Cochrane. The number of participants randomized and the number analyzed in each treatment group for each outcome were also captured. Two authors independently extracted the data and cross-checked. For dichotomous outcomes, the number of participants experiencing the event and the number of participants in each treatment group were documented.
Assessment of risk of bias in included studies
The risk of bias for each trial was evaluated by two review authors independently using the Cochrane Collaboration's tool for assessing the ‘Risk of bias’ [33].
Meta-analysis and network meta-analysis
The network meta-analysis was done using R- studio Version 1.4.1103. The network meta-analysis were performed using the frequentist model for each treatment comparison, using the Netmeta package. To indentify which treatment has the highest effects, the netrank function implemented in {netmeta} used. It allowed us to generate a ranking of treatments, indicated which treatment was more or less likely to produce the largest benefits. This frequentist method uses P-scores to rank treatments, which measure the certainty that one treatment is better than another treatment, averaged over all competing treatments.
Geometry of network
Network geometry used nodes to represent different LTBI treatments and edges to represent the head-to-head comparisons between network nodes. The nodes' size and edge thickness were represented sample sizes of intervention and numbers of included trials, respectively. The network nodes were categorized as follows: 1. 6H, 2. 9H, 3. 12H, 4. 24H, 5. 3HP, 6. 3RHZ, 7. 4HP TW, 8. 2RZ, 9. 2R, 10. 3R, 11. 4R 12. 2HP TW, 13. 1HP, 14. 6EH, 15. 4RH, 16. 72 H, 17. 18H, 18. 3RH, 19, 12RH, and 20. Placebo.
Description of network diagram
Imagine that we have extracted data from some randomized controlled trial i, which compared the effect of treatment A to another condition B. Our graph has two core components. The first one are two circles (so-called nodes), which represent the two conditions A and B in trial i. The second component is the line connecting these two nodes. This line is called an edge. The edge represents how A and B relate to each other. Also, imagine that we have also obtained data from another study j. This trial also used the control condition B. But instead of administering A, this study used another treatment C. In study j, treatment C was also compared to B (Fig. 1).
It is clearly visible that the graph now contains two effect size estimates: θ^i,A,B, comparing A to B, and θ^j,C,B, the comparison between C and B. Since both of these effect sizes were directly observed in “real” trials, we call such information direct evidence. Therefore, we denote these effect sizes with θ^directB,A and θ^direct B,C. The B condition (our control group) is directly connected to all other nodes. It takes only one “step” in the graph to get from B to the two other nodes A and C: B → A, B → C. In contrast, A and C only have one direct connection, and they both connect to B: A → B and C → B (Fig. 1).
However, there is an indirect connection between A and C. This connection exists because B serves as the link, or bridge, between the two conditions: A → B → C. As a result, there is indirect evidence for the relationship between A and C, which can be derived from the structure of the network (Fig. 1).
Using information from the directly observed edges, we can calculate the effect of the indirectly observed comparison between A and C. We denote this non-observed, indirect effect size with θ ^ indirect A,C. Furthermore, we can see that the edges in the plot have a different thickness. The degree of thickness represents how often we find a specific comparison in our network Fig. 1.
Assessment of heterogeneity
Heterogeneity among the included trials was assessed by inspecting the forest plots and the Cochrane Q and I2 statistic was used to measure heterogeneity among the trials in each analysis, the Chi2 test with a P < 0.10 to indicate statistical significance was used.
To further determine evaluate inconsistency in our network model, Net heat plots was done. The gray boxes signify how important a treatment comparison is for the estimation of another treatment comparison. The bigger the box, the more important the comparison. The colored backgrounds signify the amount of inconsistency of the design in a row that can be attributed to the design in a column. Field colors can range from a deep red (which indicates strong inconsistency) to blue (which indicates that evidence from this design supports evidence in the row).
Another method to check for consistency in our network is net splitting. This method splits our network estimates into the contribution of direct and indirect evidence, which allows us to control for inconsistency in the estimates of individual comparisons in our network. When a difference is p < 0.05, there is a significant disagreement (inconsistency) between the direct and indirect estimate.
Result
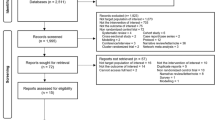
The search resulted in a total of 320 studies, of which 55 full-text eligible studies were evaluated further and 37 of them fulfilled the inclusion criteria and included in the network meta-analysis and qualitative analysis (Fig. 2).
Characteristics of included studies
In this review, 37 studies were included, which enrolled 46,022 participants living with HIV, chronic Silicosis, had contact history with TB infected person, immigrants, prisoners, old people, and pregnant women who were at risk for latent TB were included in Table 1.
Methodological quality and risk of bias
Our summary shows that majority of the studies were either open label (high risk for bias) or unclear risk for bias. The rest of the domains were low risk for bias. The’Risk of bias assessments are summarized in Fig. 3.
Overall TB incidence
In this analysis, 29 studies and 15 treatments were included. The test random effect model for heterogeneity (within designs) and inconsistency (between designs) were not statically significant (0.154 and 0.482). The Q value for a full design-by-treatment interaction random effects model also shows that there is no inconsistency (Between designs; Q = 8.79, P-value = 0.8445, tau = 0.2714, tau2 = 0.0736). The network diagram shows that majority (nine) studies compared placebo with 6 months isoniazid Fig. 4.
Consistently, the forest plot showed that there are other high-performing treatment regimens beyond the 6 years of continuous isoniazid therapy. The result also showed that some of the confidence intervals are overlapping which makes a clear-cut decision less easy. While 72H, 3HP, 3RH, and 6H significantly reduce the risk of active TB infection by 61%,47%, and 40% respectively compared to placebo (Fig. 5).
Publication Bias
The funnel plot for incidence of TB was quite symmetrical and this was corroborated by Egger’s test, which was not significant (p = 0.2680).
Consistency
To further investigate consistency, Net-split has been done and the result shows that there is no significant disagreement between the direct and indirect evidence. Furthermore, the result form the Net-heat plot also shows that the overall consistency of our model is low.
Incidence of TB among patients living with HIV
In this analysis 17 studies and 11 treatments were included. The heterogeneity/inconsistency in our network model is very low, with t tau^2 = 0.1592; tau = 0.3990; I^2 = 51.8%. Furthermore, the test for inconsistency (between design) was also low (Q = 1.58, P = 0.9541). The network diagram shows that majority (seven) studies compared placebo with 6 months isoniazid Fig. 6.
The forest plot shows that 3RH, and 6H have shown a significant effect on reducing the incidence of TB among patients living with HIV who have received TPT (RR 0.52 95% CI 0.29–0.91 and RR 0.59 95% CI 0.39–88, respectively) Fig. 7. However, the Net rank shows that 6 months Ethambutol plus Isoniazid have a lowest P-score, which seems this combination therapy is not the best option.
Consistency
To further investigate consistency, Net-split has been done and the result shows that there is no significant disagreement between the direct and indirect evidence. Furthermore, the result form the Net-heat plot also shows that the overall consistency of our model is low.
Incidence of TB among HIV-negative patients who had TB contact history
In this analysis 3 studies and 5 treatments were included. The forest plot shows that 3HP has a significant benefit over other TB prevention therapies in reducing the incidence of TB among HIV negative patients who had TB contact history Fig. 8. The network diagram shows that more studies compared 24 H and placebo Fig. 9.
Adherence of patients to TPT
In this study 14 studies and 6 treatments were involved. The result shows within study heterogeneity was not statistically significant (tau2 = 0.0042; tau = 0.0652; I2 = 66%). The adherence of TPT was good among patients who have been treated with 4R followed by 3RH Fig. 10. However, patients who were treated with 6H had the least adherence rate compared to patients who were treated with other treatments Fig. 10. The network diagram show that more studies compared 3HP with 9H Fig. 11.
Adverse events
The included studies [11, 23, 24] have reported peripheral neuropathy as an adverse event and it was more common among patients who have been treated with 6H, 9H, and 3RH. Also, few patients who have been treated with 3R and 4R were also experienced peripheral neuropathy [54]. Furthermore, neutropenia and anemia were also common among patients who have been treated with 9H and 1HP [26]. The proportion of patients who have been experienced headache was higher in 3HP and 3RH [12, 18]. One multi-center study [56] conducted on pregnant women was reported that 12 women experienced fetal loss less that 20 weeks (4 from 3HP and 8 from 9H arms) and two congenital anomalies from 9H arm.
AE led to treatment discontinuation
In these 11 studies and 7 treatments were included. The forest plot shows that the proportion of subjects who permanently discontinued a study drug because of adverse event were higher in three months daily combination of rifampin and isoniazid (3RH) Fig. 12. However, it was low in patients who have been treated with four months daily rifampin. The network graph shows that most studies compared 3HP with 9H Fig. 13.
Nausea and vomiting
In this analysis 12 studies and 9 treatments were included. The test for a full design-by-treatment interaction random effects mode shows that there is no inconsistency between designs (Q = 7.66 and P-value = 0.053). Nausea and vomiting were more common with patient who have been treated with 3 months weekly combination of isoniazid and rifapentine (3HP) (RR 5.91 95% CI 2.30–15.20), followed by two months twice weekly combination of isoniazid and rifapentine (2HP TW) Fig. 14. However, it was less common among patients who were treated with 4 months daily refampin and nine months daily isoniazid. The network diagram shows that most studies compared 3HP with two months twice weekly HP (2HP TW) Fig. 15.
Skin rash
In this analysis 9 studies and 7 treatments were included. The risk of skin rash has no significant difference between the included treatments Figs. 16 and 17.
Flu like symptom
In this analysis 3 studies and 4 treatments were included. There was no inconsistency between designs. Flu like symptom was more common in patients who was were treated with all the included treatments Fig. 18 and majority of the studies compared 3HP with two months twice weekly combination of HP Fig. 19.
Hypersensitivity reaction
In this analysis two studies and three treatments were included. There was no inconsistency between designs. Among other adverse events attributed to a study drug, the proportion of subjects with possible hypersensitivity was higher in the 3 months daily combination of rifampin and isoniazid (3RH) Fig. 20 and most of the studies compared 3HP with 3RH Fig. 21.
Grade III and IV liver toxicity
In this analysis 17 studies and 9 treatments were included. The test for inconsistency using a full design-by-treatment interaction random effects model shows that there is no inconsistency between designs (Q = 2.41, tau = 0, tau2 = 0, I2 = 0%, P = value = 0.66). Liver enzyme elevation after TPT initiation have been noticed in many patients. However, the proportion of subjects with hepatotoxicity that was attributed to a study drug was higher in the 9 months daily isoniazid, followed by 1HP, and 6H. On the contrary, it was less common among patients who have been treated with 3 months daily rifampin Fig. 22. The network diagram shows that majority of the studies were compared 9H with 3HP and 1HP Fig. 23.
Serious adverse events
In this analysis 14 studies and 11 treatments were included. The test for heterogeneity between designs shows moderate inconsistency (I2 = 69.9%). However, a test for a full design-by-treatment interaction random effects model shows that there is no inconsistency between designs (Q = 0.3, and P-value = 0.58). Some patients from all treatment groups experienced SAEs, however, the risk of serious adverse events has no statistical difference between the included studies. But, the Net-rank result shows that the frequency of SAES were more common in patients who have been treated with 3HP and 9H. However, it was less common in patients who have been treated with 12 months daily combination of rifampin and isoniazid Fig. 24. The forest plot also shows that there was no significant difference on the risk of SAEs between the treatments and network graph shows that more studies compared 9H with 3HP and 1HP Fig. 25. Furthermore, grade three bilirubin level elevation was higher in 3RH and 12H [15, 25, 31].
Discussion
Ttreatment of individuals with LTBI is considered a fundamental strategy for the control of TB. Prevention of TB by treating individuals with LTBI is a cost-effective intervention when directed at those with the greatest likelihood of TB, such as recently infected cases, individuals with untreated residual lesions or immuno suppression, children, and recent immigrants from highly endemic regions [59]. Globally, tuberculosis is the principal cause of death in up to one third of people dying who have HIV infection [60-62]. Patients with human immunodeficiency virus (HIV) infection and latent tuberculosis are at substantial risk for the development of active tuberculosis [59]. Many people living with HIV are still dying from TB, despite the availability of ART and measures to control opportunistic infections such as TB are especially important. The prevention of tuberculosis in people with HIV infection has both clinical and public health importance [59].
In this study, the incidence of TB among people living with HIV who have taken 3RH as TPT was lower followed by 6H. However, 3HP shows a significant reduction on the incidence of TB among HIV negative patients who had TB contact history. In terms of preventing TB among PLWHIV, while initiating TPT, it is necessary to consider potential harm such as hepatotoxicity and development of drug resistance, and acceptability of the selected regiment by the patient [3, 30]. A previous network meta-analysis has reported that all regimens of interest except 9H showed significant benefits in preventing active TB compared to placebo [29]. On the contrary, another previous network meta-analysis reported that 6 to 12 months of isoniazid were no more efficacious in preventing microbiologically confirmed TB than rifamycin-containing regimens [30]. But, a previous meta-analysis also reported that prolonged regimens (prolonged H and 6H) were more effective in preventing TB [63-65]. However, a 12-dose regimen of once-weekly isoniazid and rifapentine has been shown to be noninferior to 9 months of daily isoniazid in a large and well conducted clinical trial [66]. It has also showed a significant benefits in preventing the development active TB among patients who had a household TB contact history, immigrant population, and old people [16, 19, 67]. Perhaps, those studies which assessed the efficacy of prolonged isoniazid regimens, followed the patients for a short period of time after treatment; thus, the incidence of TB cases detected are only those patients who developed TB while on treatment or shortly after. Yet, difference on the post-treatment follow up time could also have an impact on the incidence of TB among patients. Other factors including the setting where the patients were living (e.g., places where TB incidence were higher), tuberculin skin test positive (ie, ≥ 5 mm induration) at enrolment, high fat intake with TPT regimens, low CD4+ cell count, drug addiction, and high ART coverage could also be a confounding factors [3, 30, 65, 68-70].
In order to enhance the potential benefit of TPT and achieve the anticipated efficacy level, patients’ adherence to TPT needs to be higher. The efficacy, adherence, and safety of TPT depends in treatment regimen selection [59]. In this study, patients’ adherence to TPT was higher among patients who have taken 4R followed by 3RH. According to the result from previous network meta-analysis, even if the definitions of regimen completion varied across studies, regimens of 3–4 months were associated with a greater likelihood of adequate completion [29]. Consistently, a previous study [21] reported that, compared with the 9H regimen, the 3HP regimen had a higher completion rate with lower hepatotoxicity and well-tolerated adverse drug reaction [18]. Most of the previous studies associated higher compliance rate with shorter duration of regimen, drug tolerability, self-limiting adverse drug reactions (ADRs), directly observed administration, and dosing schedule, these factors could affect the completion rate [18, 71]. Other factors such as difference in population group (e.g., prisoners and other marginalized communities), precarious social and economic situation, the diagnosis resulting from screening, which suggests a lack of patient motivation, as there was no known TB focus could also have an impact on the completion rate. In immigrant population, precarious employment, economic difficulties, lack of family support and language and cultural barriers all make patient follow-up more difficult. As this was a healthy population that had immigrated with the intention of working, worrying about their health was not a priority.
The proportion of subjects who permanently discontinued a study drug because of adverse event were higher in patients who has taken 3RH. The frequency of nausea and vomiting were higher among patients who have taken 3HP, followed by two months twice a week combination of HP. However, a previous study reported that, compared with 9H and 3HP had associated with low hepatotoxicity and well-tolerated adverse drug reaction [18, 72]. Furthermore, the risk of grade 3 and 4 liver toxicity was significantly higher among patients who have taken 12H, followed by 2RZ. The risk of hepatotoxicity could be related to prolonged isonizide therapy [18, 30] and combination of isoniazid either with rifampicin [73], age, female sex, white race, non-Hispanic ethnicity, decreased body mass index, alcohol consumption, and elevated baseline AST [16, 74, 75]. Other factor such as frequent liver monitoring and symptom-driven monitoring for hepatotoxicity, could also affect the result [21, 23, 74]. In addition, Heptitis C Virus (HCV) infection was also associated with hepatotoxicity when controlling for other factor [76]. Consistently, a previous study also reported that the risk of hepatitis in patients receiving pyrazinamide/rifampin for prevention of latent tuberculosis is increased threefold as compared to patients receiving isoniazid [75].
Study limitation
This study has some limitations. Majority of the included studies were conducted in adult patients living with HIV, people who had recent contact with patients with active tuberculosis, evidence radiological of previous tuberculosis, tuberculin test equal or greater than 5 mm, radiographs that indicated inactive fibrotic or calcified parenchymal and/or lymph node lesions, had conversion to positive results on a tuberculin skin test, participants living with HIV, chronic Silicosis, immigrants, prisoners, old people, and pregnant women who were at risk for latent TB. The result of this study might not be a representative of children who either live with HIV or had household TB contact history.
Conclusion
From this review, it can be concluded 3RH and 6H has a significant impact on the reduction of TB incidence among PLWH and 3HP among HIV negative people who had TB contact history. However, combinations of rifampicin either with isoniazid were significantly associated with adverse events which resulted permanent discontinuation among adult patients. Furthermore, Grade 3 and 4 kiver toxicity was more common in patent’s who have taken 9H, 1HP, and 6H. This may support the current recommended TPT regimen of 3HP, 3RH, and 6H.
Availability of data and materials
All relevant data are within the manuscript and its supporting information files.
Abbreviations
- AE:
-
Adverse event
- CENTRAL:
-
Cochrane Central Register of Controlled Trials
- CI:
-
Confidence interval
- CINeMA:
-
Confidence in Network Meta-Analysis
- E:
-
Ethambutol
- EH:
-
Ethambutol Isoniazid combination
- GADE:
-
Grading of Recommendations, Assessment, Development, and Evaluations
- H:
-
Isoniazid
- HP:
-
Isoniazid plus Rifapentine
- LTBI:
-
Latent Tuberculosis Infection
- NMA:
-
Network meta-analysis
- PICO:
-
Population, Intervention, Comparison, and outcome
- PLWHIV:
-
Patient Living with Human Immune Virus
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- R:
-
Rifampicin
- RCTs:
-
Randomized Control Trials
- RH:
-
Rifampicin Isoniazid combination
- SAE:
-
Serious Adverse Event
- TB:
-
Tuberculosis
- TPT:
-
Tuberculosis Prevention Therapy
- WHO:
-
World Health Organization
References
WHO. Global tuberculosis report 2021. Geneva: World Health Organization; 2021. Licence: CC BY-NC-SA 3.0 IGO. 2021.
World Health Organization. Latent tuberculosis infection - Executive Summary. Who; 2018. p. 6.
Sterling TR, Njie G, Zenner D, et al. Guidelines for the Treatment of Latent Tuberculosis Infection: Recommendations from the National Tuberculosis Controllers Association and CDC. MMWR Recomm Rep 2020. 2020;69(No. RR-1):1–11.
Sterling TR, Njie G, Zenner D, Cohn DL, Reves R, Ahmed A, et al. Guidelines for the Treatment of Latent Tuberculosis Infection: Recommendations from the National Tuberculosis Controllers Association and CDC, 2020. MMWR Recomm Rep. 2020;69(1):1–11.
Huaman MA, Sterling TR. Treatment of Latent Tuberculosis Infection—An Update. Clin Chest Med. 2019;40(4):839–48.
Person AK, Pettit AC, Sterling TR. Diagnosis and treatment of latent tuberculosis infection: an update. Curr Respir Care Rep. 2013;2(4):199–207.
Kritski AL, Marques MJ, Rabahi MF, Vieira MA, Werneck-Barroso E, Carvalho CE, et al. Transmission of tuberculosis to close contacts of patients with multidrug-resistant tuberculosis. Am J Respir Crit Care Med. 1996;153(1):331–5.
Zenner D, Beer N, Harris RJ, Lipman MC, Stagg HR, van der Werf MJ. Treatment of Latent Tuberculosis Infection: An Updated Network Meta-analysis. Ann Intern Med. 2017;167(4):248–55.
Stagg HR, Zenner D, Harris RJ, Muñoz L, Lipman MC, Abubakar I. Treatment of latent tuberculosis infection: a network meta-analysis. Ann Intern Med. 2014;161(6):419–28.
WHO. Latent tuberculosis infection. Patient Care. 2004;38:13–4.
Hawken MP, Meme HK, Elliottc LC, Chakaya JM, Morris JS, Githui WA, et al. Isoniazid preventive therapy for tuberculosis in HIV-1-infected adults: Results of a randomized controlled trial. AIDS. 1997;11(7):875–82.
Ruan Q Ling, Huang X Tian, Yang Q Luan, Liu X Feng, Wu J, Pan K Chuan, et al. Efficacy and safety of weekly rifapentine and isoniazid for tuberculosis prevention in Chinese silicosis patients: a randomized controlled trial. Clin Microbiol Infect. 2021;27(4):576–82.
Kim HW, Kim JS. Treatment of Latent Tuberculosis Infection and Its Clinical Efficacy. Tuberc Respir Dis (Seoul). 2018;81(1):6–12.
Legese H, Degefa H, Gebrewahd A, Gebremedhin H. Utilization of isoniazid prophylaxis therapy and its associated factors among HIV positive clients taking antiretroviral therapy at Fre Semaetat primary hospital, Hawzien districts, Tigrai, Northern Ethiopia. Trop Dis Travel Med Vaccines. 2020;6(1):11. https://doi.org/10.1186/s40794-020-00106-2.
Johnson JL, Okwera A, Hom DL, Mayanja H, Kityo CM, Nsubuga P, et al. Duration of efficacy of treatment of latent tuberculosis infection in HIV-infected adults. AIDS. 2001;15(16):2137–47.
Gao L, Zhang H, Xin H, Liu J, Pan S, Li X, et al. Short-course regimens of rifapentine plus isoniazid to treat latent tuberculosis infection in older Chinese patients: a randomised controlled study. Eur Respir J. 2018;52(6):1801470.
Moro RN, Scott NA, Vernon A, Tepper NK, Goldberg SV, Schwartzman K, et al. Exposure to latent tuberculosis treatment during pregnancy the PREVENT TB and the iadhere trials. Ann Am Thorac Soc. 2018;15(5):570–80.
Sun H-Y, Huang Y-W, Huang W-C, Chang L-Y, Chan P-C, Chuang Y-C, et al. Twelve-dose weekly rifapentine plus isoniazid for latent tuberculosis infection: A multicentre randomised controlled trial in Taiwan. Tuberculosis. 2018;111:121–6 Available from: https://linkinghub.elsevier.com/retrieve/pii/S147297921830115X .
Jiménez-Fuentes MA, de Souza-Galvao ML, Mila Augé C, SolsonaPeiró J, Altet-Gómez MN. Rifampicin plus isoniazid for the prevention of tuberculosis in an immigrant population. Int J Tuberc lung Dis Off J Int Union against Tuberc Lung Dis. 2013;17(3):326–32.
Menzies D, Long R, Trajman A, Dion MJ, Yang J, Al Jahdali H, et al. Adverse events with 4 months of rifampin therapy or 9 months of isoniazid therapy for latent tuberculosis infection: A randomized trial. Ann Intern Med. 2008;149(10):689–97.
Sterling TR, Villarino ME, Borisov AS, Shang N, Gordin F, Bliven-Sizemore E, et al. Three Months of Rifapentine and Isoniazid for Latent Tuberculosis Infection. N Engl J Med. 2011;365(23):2155–66.
Villarino ME, Scott NA, Weis SE, Weiner M, Conde MB, Jones B, et al. Treatment for preventing tuberculosis in children and adolescents: A randomized clinical trial of a 3-month, 12-dose regimen of a combination of rifapentine and Isoniazid. JAMA Pediatr. 2015;169(3):247–55.
Moro RN, Borisov AS, Saukkonen J, Khan A, Sterling TR, Villarino ME, et al. Factors Associated With Noncompletion of Latent Tuberculosis Infection Treatment: Experience From the PREVENT TB Trial in the United States and Canada. Clin Infect Dis an Off Publ Infect Dis Soc Am. 2016;62(11):1390–400.
Sterling TR, Moro RN, Borisov AS, Phillips E, Shepherd G, Adkinson NF, et al. Flu-like and Other Systemic Drug Reactions among Persons Receiving Weekly Rifapentine Plus Isoniazid or Daily Isoniazid for Treatment of Latent Tuberculosis Infection in the PREVENT Tuberculosis Study. Clin Infect Dis. 2015;61(4):527–35.
Sterling TR, Scott NA, Miro JM, Calvet G, La Rosa A, Infante R, et al. Three months of weekly rifapentine and isoniazid for treatment of Mycobacterium tuberculosis infection in HIV-coinfected persons. AIDS. 2016;30(10):1607–15.
Chaisson RE, Ramchandani R, Swindells S, Ramchandani R, Gupta A, Benson CA, et al. One Month of Rifapentine plus Isoniazid to Prevent HIV-Related Tuberculosis. N Engl J Med. 2019;381(11): e23.
Cuevas LE, Petrucci R, Swaminathan S. Tuberculosis diagnostics for children in high-burden countries: what is available and what is needed. Paediatr Int Child Health. 2012;32(Suppl 2):S30–7.
Njie GJ, Morris SB, Woodruff RY, Moro RN, Vernon AA, Borisov AS. Isoniazid-Rifapentine for Latent Tuberculosis Infection: A Systematic Review and Meta-analysis. Am J Prev Med. 2018;55:244–52 Elsevier Inc.
Pease C, Hutton B, Yazdi F, Wolfe D, Hamel C, Quach P, et al. Efficacy and completion rates of rifapentine and isoniazid (3HP) compared to other treatment regimens for latent tuberculosis infection: a systematic review with network meta-analyses. BMC Infect Dis. 2017;17(1):265. https://doi.org/10.1186/s12879-017-2377-x.
Yanes-Lane M, Ortiz-Brizuela E, Campbell JR, Benedetti A, Churchyard G, Oxlade O, et al. Tuberculosis preventive therapy for people living with HIV: A systematic review and network meta-analysis. PLoS Med. 2021;18(9): e1003738.
Dawit Getachew Assefa, Ahmed Bedru Omer, Eden Dagnachew Zeleke DTD. Efficacy, adherence, and safety of latent Tuberculosis infection treatments. Systematic review and Network meta-analysis of randomized controlled trials . PROSPERO 2022 CRD42022334163.
Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP, Mulrow C, Catalá-López F, Gøtzsche PC, Dickersin K, Boutron I, Altman DGMD. The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-analyses of Health Care Interventions: Checklist and Explanations. Ann Intern Med. 2015;62(11):777–84. Available from: http://www.prisma-statement.org/Extensions/NetworkMetaAnalysis
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ WV (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane Database Syst Rev. 2021; Available from: Available from www.training.cochrane.org/handbook.
HigginsJPT T, ChandlerJ, CumpstonM, LiT, PageMJ et al. Cochrane Handbook for Systematic Reviews ofInterventions version 6.0 (updated August 2019). Cochrane. 2019. Available from: www.trainingcochrane.org/handbook.
Pape JW, Jean SS, Ho JL, Hafner A, Johnson WDJ. Effect of isoniazid prophylaxis on incidence of active tuberculosis and progression of HIV infection. Lancet (London, England). 1993;342(8866):268–72.
Cowie RL. Short course chemoprophylaxis with rifampicin, isoniazid and pyrazinamide for tuberculosis evaluated in gold miners with chronic silicosis: a double-blind placebo controlled trial. Tuber lung Dis. 1996;77(3):239–43.
Gordin FM, Matts JP, Miller C, Brown LS, Hafner R, John SL, et al. A controlled trial of isoniazid in persons with anergy and human immunodeficiency virus infection who are at high risk for tuberculosis. Terry Beirn Community Programs for Clinical Research on AIDS. N Engl J Med. 1997;337(5):315–20.
Whalen cc, Johnson JL, Okwera A, Hom DL, Huebner R, Mugyenyi P, et al. A Trial of Three Regimens to Prevent Tuberculosis in Ugandan Adults Infected with the Human Immunodeficiency Virus. N Engl J Med. 1997;337(12):801–8. https://doi.org/10.1056/NEJM199709183371201.
Martínez Alfaro E, Cuadra F, Solera J, Ángel Maciá M, Geijo P, Sánchez Martínez PA, et al. Assessment of two chemoprophylaxis regimens for tuberculosis in HIV-infected patients. Med Clin (Barc). 2000;115(5):161–5. https://doi.org/10.1016/S0025-7753(00)71496-5.
Fitzgerald DW, Desvarieux M, Severe P, Joseph P, Johnson WDJ, Pape JW. Effect of post-treatment isoniazid on prevention of recurrent tuberculosis in HIV-1-infected individuals: a randomised trial. Lancet (London, England). 2000;356(9240):1470–4.
Hong Kong Chest Service/Tuberculosis Research Centre, Madras/British Medical Research Council. A double-blind placebo-controlled clinical trial of three antituberculosis chemoprophylaxis regimens in patients with silicosis in Hong Kong. Hong Kong Chest Service/Tuberculosis Research Centre, Madras/British Medical Research Council. Am Rev Respir Dis. 1992;145(1):36–41.
Quigley MA, Mwinga A, Hosp M, Lisse I, Fuchs D, Porter JDH, et al. Long-term effect of preventive therapy for tuberculosis in a cohort of HIV-infected Zambian adults. AIDS. 2001;15(2):215–22.
Menzies D, Dion MJ, Rabinovitch B, Mannix S, Brassard P, Schwartzman K. Treatment completion and costs of a randomized trial of rifampin for 4 months versus isoniazid for 9 months. Am J Respir Crit Care Med. 2004;170(4):445–9.
Zar HJ, Cotton MF, Strauss S, Karpakis J, Hussey G, Schaaf HS, et al. Effect of isoniazid prophylaxis on mortality and incidence of tuberculosis in children with HIV: randomised controlled trial. BMJ. 2007;334(7585):136.
Paloma Geijo M, Rosa Herranz C, Vaño D, JesúsGarcía Á, García M, Francisco Dimas J. Pauta corta de isoniazida y rifampicina comparada con isoniazida para la infección latente de tuberculosis. Ensayo clínico aleatorizado. Enferm Infecc Microbiol Clin. 2007;25(5):300–4.
Mohammed A, Myer L, Ehrlich R, Wood R, Cilliers F, Maartens G. Randomised controlled trial of isoniazid preventive therapy in South African adults with advanced HIV disease. Int J Tuberc Lung Dis. 2007;11(10):1114–20.
Rivero A, Lopez-Cortes L, Castillo R, Verdejo J, García M, Martínez-Marcos F, et al. Ensayo clínico aleatorizado para evaluar tres pautas cortas de tratamiento de la infección latente tuberculosa en pacientes infectados por el VIH. Enferm Infecc Microbiol Clin. 2007;25:305–10.
Spyridis NP, Spyridis PG, Gelesme A, Sypsa V, Valianatou M, Metsou F, et al. The effectiveness of a 9-month regimen of isoniazid alone versus 3- and 4-month regimens of isoniazid plus rifampin for treatment of latent tuberculosis infection in children: results of an 11-year randomized study. Clin Infect Dis. 2007;45(6):715–22.
Trajman A, Long R, Zylberberg D, Dion MJ, Al-Otaibi B, Menzies D. Factors associated with treatment adherence in a randomised trial of latent tuberculosis infection treatment. Int J Tuberc Lung Dis. 2010;14(5):551–9.
Madhi SA, Nachman S, Violari A, Kim S, Cotton MF, Bobat R, et al. Primary Isoniazid Prophylaxis against Tuberculosis in HIV-Exposed Children. N Engl J Med. 2011;365(1):21–31. https://doi.org/10.1056/NEJMoa1011214.
Martinson NA, Barnes GL, Moulton LH, Msandiwa R, Hausler H, Ram M, et al. New regimens to prevent tuberculosis in adults with HIV infection. N Engl J Med. 2011;365(1):11–20.
Chan P-C, Yang C-H, Chang L-Y, Wang K-F, Lu B-Y, Lu C-Y, et al. Latent tuberculosis infection treatment for prison inmates: a randomised controlled trial. Int J Tuberc lung Dis. 2012;16(5):633–8.
Swaminathan S, Menon PA, Gopalan N, Perumal V, Santhanakrishnan RK, Ramachandran R, et al. Efficacy of a six-month versus a 36-month regimen for prevention of tuberculosis in HIV-infected persons in India: a randomized clinical trial. PLoS ONE. 2012;7(12):e47400.
White MC, Tulsky JP, Lee JRJ, Chen L, Goldenson J, Spetz J, et al. Isoniazid vs. rifampin for latent tuberculosis infection in jail inmates: Toxicity and adherence. J Correct Heal Care. 2012;18(2):131–42.
Gray DM, Workman LJ, Lombard CJ, Jennings T, Innes S, Grobbelaar CJ, et al. Isoniazid preventive therapy in HIV-infected children on antiretroviral therapy: a pilot study. Int J Tuberc Lung Dis. 2014;18(3):322–7.
Moro RN, Scott NA, Vernon A, Tepper NK, Goldberg SV, Schwartzman K, et al. Exposure to Latent Tuberculosis Treatment during Pregnancy. The PREVENT TB and the iAdhere Trials. Ann Am Thorac Soc. 2018;15(5):570–80.
Sun HY, Huang YW, Huang WC, Chang LY, Chan PC, Chuang YC, et al. Twelve-dose weekly rifapentine plus isoniazid for latent tuberculosis infection: A multicentre randomised controlled trial in Taiwan. Tuberculosis. 2018;111(March):121–6.
Surey J, Stagg HR, Yates TA, Lipman M, White PJ, Charlett A, et al. An open label, randomised controlled trial of rifapentine versus rifampicin based short course regimens for the treatment of latent tuberculosis in England: the HALT LTBI pilot study. BMC Infect Dis. 2021;21(1):90.
WHO. Guidelines on the management of latent tuberculosis infection. Geneva: World Health Organization; 2015. Available from: https://www.ncbi.nlm.nih.gov/books/NBK293818/.
Sterling TR, Njie G, Zenner D, Cohn DL, Reves R, Ahmed A, et al. Guidelines for the treatment of latent tuberculosis infection: Recommendations from the national tuberculosis controllers association and CDC, 2020. MMWR Recomm Reports. 2020;69(1):1–11.
WHO consolidated guidelines on tuberculosis. Module 1: prevention – tuberculosis preventive treatment. Geneva: World Health Organization; 2020. Licence: CC BY-NC-SA 3.0 IGO. Available at: https://apps.who.int/iris/.
Cohen A, Mathiasen VD, Schön T, Wejse C. The global prevalence of latent tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2019;54(3):1900655.
Getahun H, Matteelli A, Abubakar I, Aziz MA, Baddeley A, Barreira D, et al. Management of latent Mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. Eur Respir J. 2015;46(6):1563–76.
Den Boon S, Matteelli A, Ford N, Getahun H. Continuous isoniazid for the treatment of latent tuberculosis infection in people living with HIV. AIDS. 2016;30(5):797–801 Available from: https://journals.lww.com/aidsonline/Fulltext/2016/03130/Continuous_isoniazid_for_the_treatment_of_latent.18.aspx .
Akolo C VJ. Cochrane Database of Systematic Reviews Treatment of latent tuberculosis infection in HIV infected persons (Review) Treatment of latent tuberculosis infection in HIV infected persons (Review). Cochrane Database Syst Rev Art. 2010;(1). Available from: www.cochranelibrary.com
Parekh MJ, Schluger NW. Treatment of latent tuberculosis infection. Ther Adv Respir Dis. 2013;7(6):351–6.
Schechter M, Zajdenverg R, Falco G, Barnes GL, Faulhaber JC, Coberly JS, et al. Weekly Rifapentine/Isoniazid or Daily Rifampin/Pyrazinamide for Latent Tuberculosis in Household Contacts. Am J Respir Crit Care Med. 2006;173(8):922–6. https://doi.org/10.1164/rccm.200512-1953OC.
Weiner M, Burman W, Vernon A, Benator D, Peloquin CA, Khan A, et al. Low isoniazid concentrations and outcome of tuberculosis treatment with once-weekly isoniazid and rifapentine. Am J Respir Crit Care Med. 2003;167(10):1341–7.
Guelar A, Gatell JM, Verdejo J, Podzamczer D, Lozano L, Aznar E, et al. A prospective study of the risk of tuberculosis among HIV-infected patients. AIDS. 1993;7(10):1345–9.
Samandari T, Agizew TB, Nyirenda S, Tedla Z, Sibanda T, Shang N, et al. 6-month versus 36-month isoniazid preventive treatment for tuberculosis in adults with HIV infection in Botswana: a randomised, double-blind, placebo-controlled trial. Lancet (London, England). 2011;377(9777):1588–98.
Croda J. Short-course isoniazid plus rifapentine therapy for latent tuberculosis in Brazil. Rev Soc Bras Med Trop. 2015;48:505–6.
Pease C, Hutton B, Yazdi F, Wolfe D, Hamel C, Barbeau P, et al. A systematic review of adverse events of rifapentine and isoniazid compared to other treatments for latent tuberculosis infection. Pharmacoepidemiol Drug Saf. 2018;27(6):557–66.
Leung CC, Rieder HL, Lange C, Yew WW. Series “update on tuberculosis”: Treatment of latent infection with Mycobacterium tuberculosis: Update 2010. Eur Respir J. 2011;37(3):690–711.
Bliven-Sizemore EE, Sterling TR, Shang N, Benator D, Schwartzman K, Reves R, et al. Three months of weekly rifapentine plus isoniazid is less hepatotoxic than nine months of daily isoniazid for LTBI. Int J Tuberc lung Dis Off J Int Union against Tuberc Lung Dis. 2015;19(9):1039–44. i–v.
McNeill L, Allen M, Estrada C, Cook P. Pyrazinamide and rifampin vs isoniazid for the treatment of latent tuberculosis: improved completion rates but more hepatotoxicity. Chest. 2003;123(1):102–6.
Saukkonen JJ, Cohn DL, Jasmer RM, Schenker S, Jereb JA, Nolan CM, et al. An official ATS statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med. 2006;174(8):935–52.
Acknowledgements
We would like to express our gratitude to everyone who have participated in conducting this review.
Funding
This review wasn’t funded by any organization.
Author information
Authors and Affiliations
Contributions
DGA developed the protocol. For this review, DGA reviewed the reference list and extracted data. DGA conducted the analyses, constructed summary of findings tables, and evaluated the quality of evidence using the GRADE approach. AB, EDZ, DTD, WM, NM, TTW, NFB, VDK, MGA, and TM were responsible for the quality assessment and review of the study. All authors reviewed and edited the manuscript. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
We declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Assefa, D.G., Bedru, A., Zeleke, E.D. et al. Efficacy and safety of different regimens in the treatment of patients with latent tuberculosis infection: a systematic review and network meta-analysis of randomized controlled trials. Arch Public Health 81, 82 (2023). https://doi.org/10.1186/s13690-023-01098-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13690-023-01098-z