Abstract
Background
Aneurysmal subarachnoid hemorrhage (aSAH) is a devastating disease that leads to important morbidity and mortality in a young patient population. Anemia following aSAH is common and may be exacerbated by the treatments instituted by clinicians as part of standard care. The role and optimal thresholds for red blood cell (RBC) transfusion in this patient population remains unknown.
Methods/design
We will conduct a systematic review of the literature using MEDLINE, EMBASE, and EBM Reviews (including Cochrane Central databases) using a comprehensive search strategy for observational and interventional studies of RBC transfusion in aSAH. Our primary objective is to evaluate the association of RBC transfusion with mortality in aSAH patients. Secondary objectives include a) determining associations between RBC transfusion and poor neurologic outcome, b) defining an optimal RBC transfusion threshold in aSAH patients, and c) describing complications associated with RBC transfusion in aSAH patients. We plan a descriptive reporting of all included citations including study characteristics, methodological quality, and reported outcomes. Clinical and statistical heterogeneity observed between studies will be described. If appropriate, meta-analyses of suitable studies and interpretation of their results will be performed. Effect measures will be converted to obtain relative risks and odds ratios (RR and ORs) with 95% confidence intervals and pooled according to study design (randomized trials and observational studies respectively) using a random effects model.
Discussion
This review will summarize the existing observational and trial evidence regarding RBC transfusion in aSAH patients. The analytical plan has made considerations for different study designs, both observational and interventional in nature, and will summarize the best available evidence to inform the end user and policy and guideline producers and to highlight areas in need of further study.
Systematic review registration
PROSPERO CRD42014014806
Similar content being viewed by others
Background
Aneurysmal subarachnoid hemorrhage (aSAH) has an estimated incidence rate of 10 per 100,000 patient years in the general population [1,2] and is a common neurologic cause for intensive care unit (ICU) admission [3]. It affects a relatively young population [4] and thus accounts for significant potential years of meaningful life lost [5] given the important morbidity and mortality the disease imports. Mortality remains high affecting almost 50% of the patients afflicted with aSAH [1,4,6-8].
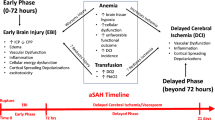
The period that follows the acute hemorrhage is fraught with multiple possible complications. Anemia is common, affecting more than 50% of aSAH patients [9,10], and is independently associated with poor outcome after SAH [11,12], regardless of SAH severity [13]. Although anemia is defined by the World Health Organization as a hemoglobin of less than 130 g/L in men and 120 g/L in women [14], anemia is typically considered clinically relevant in SAH populations when moderate (or ≤100 g/L) in both [9,10]. Anemia develops a mean of 3.5 days from admission [9], which coincides with the typical onset of another important complication, vasospasm.
Vasospasm is seen in 50% to 75% of patients with aSAH [4,15] leading to ischemic injury or delayed ischemic neurologic deficits (DINDs) from reduced cerebral perfusion. Standard of care management of vasospasm includes hyperdynamic therapy [4], which traditionally included hypertension, hypervolemia and hemodilution (the so-called ‘HHH therapy’). By its very nature, it may lead to or exacerbate anemia. Interestingly, there appears to be stronger data supporting hypertension as being the most beneficial aspect of hyperdynamic therapy in maintaining adequate cerebral perfusion [16] (and hence oxygen delivery) while hemodilution, and its effects on hemoglobin concentration, is considered to be potentially harmful [17].
Recent techniques of micropipetting and monitoring of brain tissue partial pressure of oxygen bring evidence to suggest brain tissue at risk from ischemia benefit from improved oxygen delivery [18,19]. Observational work has shown that brain tissue partial pressure of oxygen is higher with higher hemoglobin concentrations [19] and increases with red cell transfusion [20]. However, transfusion in critically ill patients remains a hotly debated issue. The landmark TRICC trial drastically changed how critically ill patients are managed with respect to RBC transfusion with their finding that a restrictive transfusion strategy (Hb trigger of ≤70 g/L) was as safe as a liberal strategy (Hb trigger ≤100 g/L) and trended towards better outcome [21]. This study, however, is not necessarily generalizable to an aSAH population, as very few patients with neurologic diagnoses were included. Observational work in aSAH has shown that both anemia and red cell transfusion are each risk factors for poor outcome [12,22]. Further, evidence is conflicting as to which of anemia or transfusion carries the bigger risk, or if a risk exists at all [12,23-26]. A recent systematic review examined transfusion thresholds among all neurocritical care patients but was limited to those studies that compared at least two different thresholds [27]. As such, only six studies met inclusion for this review, only one of which included aSAH patients. Our knowledge synthesis will examine the current clinical evidence around anemia and RBC transfusion thresholds and the effect of transfusion on death, neurologic outcomes, and transfusion adverse events among critically ill patients with aSAH.
Objectives
The primary objective of this review is to evaluate the association of RBC transfusion with mortality in aSAH patients. Mortality will be assessed as reported at hospital discharge, 30 days, and 3, 6, and 12 months. Secondary objectives include 1) determining associations between RBC transfusion/liberal transfusion strategy and poor neurologic outcome (as measured by any of modified Rankin Scale (mRS) ≥4, Glasgow Outcome Scale (GOS) ≤3, or extended Glasgow Outcome Scale (eGOS) ≤4), 2) defining an optimal RBC transfusion threshold in aSAH patients, and 3) describing transfusion adverse events associated with RBC transfusion in aSAH patients.
Methods/design
This review will be conducted in accordance with The Cochrane Collaboration [28] principles for Systematic Reviews and reported following the PRISMA guidelines [29].
Search strategy
Our search strategy will be conducted using MEDline + MEDline In-Process & Other Non-indexed Citations, EMBASE Classic + Embase, and EBM Reviews (including Cochrane Central databases) from inception to the moment of review (see Appendix). EMBASE also includes the abstract publications from major international conferences including the International Stroke Conference, Neurocritical Care Society Meeting, Society of Critical Care Medicine, and the International Symposium on Intensive Care and Emergency Medicine. A comprehensive search strategy will be constructed and implemented by a health information specialist with systematic review experience, in collaboration with the research team. MeSH terms will be used to capture each of the principal elements of the research question. To be as inclusive as possible, the strategy will be restricted to focusing on population (aSAH patients) and intervention/exposure (RBC transfusion) and will not be limited by outcome studied. So as to not overlook any possible study for inclusion, our search strategy will include anemia as a search term, separate from transfusion. Our study intervention of RBC transfusion will be targeted by applying inclusion and exclusion criteria as described below. Upon completion, identified citations will be exported to a citation manager (Mendeley Desktop, Mendeley Ltd., v. 1.12.2) for study selection. Manual review of the reference lists of all included studies and previous systematic reviews will be conducted. A final grey literature search will be conducted using ‘Google Scholar’ as well as a review of the trial register (clinicaltrials.gov) for any ongoing and unpublished studies. No language restriction will be utilized in any of the searches. Duplicate citations will be removed. The search strategies will be kept up to date to the time of the end of the review.
Study screening and inclusion
Both observational (cohort, cross-sectional) and interventional studies will be considered. We will include all retrospective and prospective studies with the goal of detailing all of the available evidence regarding RBC transfusion in aSAH patients. We aim not only to consider comparative studies of different transfusion thresholds as has been done in the past [27] but also to include studies that compare exposed from non-exposed patients.
An iterative process for study selection will be followed using the criteria set out in Table 1. Specifically, we will include all interventional or observational studies that report on an adult (age ≥18 years) hospitalized aSAH population (population), examine RBC transfusion (intervention) and compare either two or more different thresholds or to a non-transfused group (comparator), and report on any clinical outcome (outcome). We will exclude any study which reports exclusively on a pediatric population (age <18 years), non-human studies, and any duplicate or sub-study of previously published (and included) cohorts. All records will first be screened by title and abstract. All citations clearly not relevant to the review (for example, wrong population, pre-clinical study, narrative review) will be excluded. This process will be performed in duplicate by two independent reviewers. Any citation in which an abstract is not available and where suitability for inclusion is questioned will proceed to the next stage. All citations not excluded in the first screen will have full articles retrieved for a second review, in duplicate by independent reviewers, and the selection criteria applied. Any differences in classification between the two independent reviewers will be reviewed and consensus decision made. A third independent senior reviewer will be used in any instance in which consensus is not reached.
Our study free-form question is: In adult patients (age ≥18 years) with acute aSAH, is RBC transfusion or a liberal RBC transfusion strategy associated with increased all-cause mortality? We will deem the intervention arm (for interventional studies) or exposure (for observational studies) to be the liberal transfusion strategy or any RBC transfusion respectively administered during the initial hospitalization for aSAH. We will compare this to a restrictive transfusion strategy or non-transfused patients with aSAH. Our primary outcome is all-cause mortality. We will examine hospital mortality and mortality at 30 days as well as 3, 6, and 12 months. We will also examine the following secondary outcomes: 1) poor neurologic recovery (modified Rankin Scale ≥4, GOS ≤3, eGOS ≤4) based on the last follow-up time point, at hospital discharge, and at 3, 6, and 12 months; 2) hospital and ICU length of stay; 3) optimal RBC transfusion threshold; 4) transfusion adverse events; and 5) vasospasm and cerebral infarct incidence.
Data extraction
A data extraction form will be prepared a priori and piloted prior to duplicate extraction by two independent reviewers. Data extraction will include:
-
Study characteristics, design, and methods: title, authors, journal/source, year and language of publication, country, type of study, study period, total number of patients, case ascertainment and/or inclusion/exclusion criteria, randomization, allocation concealment, and blinding methods (where applicable)
-
Sample characteristics: age, sex, admission diagnosis, aSAH grade, aneurysm size and location, comorbidities, and baseline hemoglobin
-
Interventions and co-interventions: aneurysm clip or coil procedures, vasopressor use, mechanical ventilation, externalized ventricular drain (EVD), hyperdynamic (or ‘HHH’) therapy, and RBC transfusion
-
Outcome: study-specific outcomes as defined by the authors will be captured. In addition, we will abstract nadir hemoglobin, time to nadir hemoglobin, pre-transfusion hemoglobin, ICU admission, clinical complications (including vasospasm and infarction), functional recovery (including mRS, GOS, and eGOS), mortality, and other adverse events
Analysis plan
A description of all included studies, including demographic, clinical, and methodological quality (see risk of bias), will first be reported with the aid of tables and text. Our cursory review of the literature and a recent narrative review [10] suggests that several observational studies exist that will be the focus of this review. Meta-analyses of observational studies are at particular risk of bias and confounding [30]. Therefore, suitability for meta-analysis will be determined by the degree of heterogeneity (clinical and statistical) observed between the studies. Statistical heterogeneity will be described using the I2 statistic.
Primary outcome
We anticipate that the primary outcome, all-cause mortality, may be reported differently according to the study design. Authors may report the risk of mortality according to exposed/not exposed, using a threshold strategy or a cumulative exposure. Where possible, we will collect the crude numbers of dead and alive patients in each respective group (for example, exposed/non-exposed) at the latest follow-up time point per the study-specific design as well as their associated crude and adjusted effect measures including relative risk (RR), odds ratio (OR), and hazard ratio (HR). Should a meta-analysis be deemed appropriate, effect measures will be converted to obtain RRs for RCTs and ORs for observational studies with 95% confidence intervals (CIs) and pooled according to study design (for example, RCTs vs observational studies, transfused vs non-transfused, comparative threshold studies). Given that we anticipate a certain degree of heterogeneity, a random effects model will be used. Statistical heterogeneity will be reported using the I2 test with 95% confidence interval.
Secondary outcomes
Secondary outcomes will be a combination of dichotomous, ordinal, and continuous measures. Effect estimates of dichotomous outcomes will be presented as RR or ORs and 95% CIs. If appropriate, we will perform meta-analysis, and data presented as a RR will be converted to OR where possible. Neurologic outcomes (mRS, GOS, and eGOS) are expected to be presented either as ordinal data or may have already been dichotomized by the authors. Where possible, ordinal neurologic outcomes will be utilized. All continuous outcome variables will be described with means or medians and associated standard deviations or interquartile ranges as appropriate. Summaries of continuous data will be presented as mean differences with 95% confidence intervals.
Optimal transfusion hemoglobin threshold
To describe an optimal hemoglobin transfusion threshold, studies will be grouped according to whether different transfusion thresholds were assessed or if pre-transfusion hemoglobin levels were reported. When a transfusion threshold is the intervention of interest, we will group the results of the lower and higher thresholds for comparison. We will pool the results from studies using similar thresholds (for example, hemoglobin within 10 g/L). If our review includes a sufficient number of studies that assess thresholds in regard to a specific outcome, a meta-regression analysis will be performed to assess the risk of that outcome in regard to the different reported thresholds [31]. In the event of reporting of ranges of pre-transfusion hemoglobin, the exposure will be assigned as the midpoint of the range.
Risk of bias
Risk of bias will be assessed using the Downs and Black tool [32] for observational studies and the Cochrane Collaboration tool for assessing the risk of bias in RCTs [33]. Bias risk assessment will be completed in a similar fashion as the study selection process: in duplicate by two independent assessors. Cases of discordance not resolved by consensus will be reviewed by a third senior assessor. Risk of bias assessment of all included studies will be summarized and presented in table format. Meta-analysis, if possible, will be performed including all studies, with a planned sensitivity analysis (see below) to be performed using only those studies at low risk of bias. Low risk of bias will be defined as those studies with a score of ≥25 using the Downs and Black tool or those deemed low risk across all domains of the Cochrane Collaboration’s tool for assessing risk of bias. The authors recognize that no formal cutoffs exist to define low or high risk of bias with the Downs and Black tool (written correspondence with the author); however, we deem that in order for low risk of bias to exist in an observational study, there must be excellent reporting, high internal and external validity with little risk of confounding, and sufficient study power (all domains assessed with this tool) such that high scores in each of these domains are necessary to meet low-risk criteria.
Subgroup analyses
Pre-planned subgroup analyses to examine clinical heterogeneity will include transfusion in anemic patients (hemoglobin ≤100 g/L), first transfusion pre/post vasospasm and transfusion in high-grade aSAH patients (defined as Hunt and Hess grades 4 to 5, and/or Fisher grade 4, WFNS grades 4 to 5), and open surgical clipping versus endovascular coiling.
Sensitivity analyses
To test the robustness of our findings, we plan the following sensitivity analyses: 1) studies with low versus unclear/high risk of bias, 2) studies with study periods after 2005 (the year of the ISAT trial [34] publication which resulted in a management shift from surgical clipping to interventional coiling of certain aneurysms) versus before 2005, and 3) studies in which important confounders of RBC transfusion and mortality, such as aSAH severity, pre-transfusion and hemoglobin nadir, and vasospasm, are controlled for in their primary analysis.
Discussion
Subarachnoid hemorrhage is a devastating event often with lasting effects that greatly impact functionality and quality of life. The natural history of subarachnoid hemorrhage often includes complications like vasospasm that leads to further injury from ischemic damage whose very treatment may lead to hemodilution, potentially further compromising oxygen delivery. This rather uniquely sets apart this population from others in which a restrictive transfusion strategy has been shown to be at least as safe as a liberal one, and perhaps superior [21,35,36]. A restrictive transfusion strategy is not clearly beneficial for SAH patients, and doubt has been cast with small interventional studies involving other end-organ ‘at-risk’ populations [37].
This review proposes to systematically identify, gather, and summarize the observational and trial evidence that exists regarding RBC transfusion in SAH patients, using a rigorous methodology. We aim to assess the effect of RBC transfusion on all-cause mortality in addition to other clinically important outcomes which will serve as a summation of the evidence to best inform clinical decisions at the bedside.
We anticipate that the majority of available evidence will be the results of observational work rather than randomized controlled trials. As such, we have planned for a large descriptive component of this review which will include tables, figures, and charts. We recognize that the risk of bias is much higher, particularly in retrospective observational studies and as such have incorporated into the protocol two well-recognized and accepted assessment tools in addition to planned assessments of clinical and statistical heterogeneity. The results from observational studies may be inflated as was the experience in a recent systematic review of large-scale observational studies assessing the effect of transfusion on mortality in a heterogeneous patient population [38]. Although their findings were consistent across studies despite varying study designs and degrees of confounding adjustment, the magnitude of the findings were significantly larger with the observational studies. Nonetheless, clinicians are often forced to turn to observational studies to inform practice, since well-conducted randomized controlled trials are very limited in number. In fact, in SAH, the vast majority of the related recommendations in the most recent guidelines are based on observational evidence or expert opinion alone [39]. This review will formulate the best available evidence, which is dependent on the quality of the existing evidence. Careful interpretation of the findings, in light of identified limitations, is essential.
This knowledge synthesis thus will not only serve to inform the end user (that is, the clinician) but also policy and guideline producers. Finally, this review is essential to further highlight areas in need of further study. It will inform the creation of other scientific questions and help formulate other research protocols.
Abbreviations
- aSAH:
-
aneurysmal subarachnoid hemorrhage
- CI:
-
confidence interval
- DIND:
-
delayed ischemic neurologic deficit
- eGOS:
-
Extended Glasgow Outcome Scale
- EVD:
-
externalized ventricular drain
- GOS:
-
Glasgow Outcome Scale
- HHH therapy:
-
hypertension, hypervolemia, hemodilution therapy
- HR:
-
hazard ratio
- ICU:
-
intensive care unit
- mRS:
-
modified Rankin Scale
- OR:
-
odds ratio
- RBC:
-
red blood cell
- RCT:
-
randomized controlled trial
- RR:
-
relative risk
- SAH:
-
subarachnoid hemorrhage
- WFNS:
-
World Federation of Neurosurgeons
References
Findlay JM. Current management of aneurysmal subarachnoid hemorrhage guidelines from the Canadian Neurosurgical Society. Can J Neurol Sci. 1997;24:161–70.
Ostbye T, Levy AR, Mayo NE. Hospitalization and case-fatality rates for subarachnoid hemorrhage in Canada from 1982 through 1991. The Canadian Collaborative Study Group of Stroke Hospitalizations. Stroke. 1997;28:793–8.
Reed SD, Blough DK, Meyer K, Jarvik JG. Inpatient costs, length of stay, and mortality for cerebrovascular events in community hospitals. Neurology. 2001;57:305–14.
Bederson JB, Connolly ES, Batjer HH, Dacey RG, Dion JE, Diringer MN, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40:994–1025.
Wong GKC, Poon WS, Chan MTV, Boet R, Gin T, Ng SCP, et al. Intravenous magnesium sulphate for aneurysmal subarachnoid hemorrhage (IMASH): a randomized, double-blinded, placebo-controlled, multicenter phase III trial. Stroke. 2010;41:921–6.
Van Gijn J, Rinkel GJE. Subarachnoid haemorrhage: diagnosis, causes and management. Brain. 2001;124:249–78.
Smith M. Intensive care management of patients with subarachnoid haemorrhage. Curr Opin Anaesthesiol. 2007;20:400–7.
Hop JW, Rinkel GJ, Algra A, van Gijn J. Case-fatality rates and functional outcome after subarachnoid hemorrhage: a systematic review. Stroke. 1997;28:660–4.
Sampson TR, Dhar R, Diringer MN. Factors associated with the development of anemia after subarachnoid hemorrhage. Neurocrit Care. 2010;12:4–9.
Le Roux PD. Anemia and transfusion after subarachnoid hemorrhage. Neurocrit Care. 2011;15:342–53.
Wartenberg KE, Schmidt JM, Claassen J, Temes RE, Frontera JA, Ostapkovich N, et al. Impact of medical complications on outcome after subarachnoid hemorrhage. Crit Care Med. 2006;34:617–23.
Kramer AH, Gurka MJ, Nathan B, Dumont AS, Kassell NF, Bleck TP. Complications associated with anemia and blood transfusion in patients with aneurysmal subarachnoid hemorrhage. Crit Care Med. 2008;36:2070–5.
Kramer AH, Zygun DA. Anemia and red blood cell transfusion in neurocritical care. Crit Care. 2009;13:R89.
WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and mineral nutrition information system. Geneva: World Health Organization; 2011. p. 1–6. WHO/NMH/NHD/MNM/11.1.
Keyrouz SG, Diringer MN. Clinical review: prevention and therapy of vasospasm in subarachnoid hemorrhage. Crit Care. 2007;11:220.
Dankbaar JW, Slooter AJ, Rinkel GJ, Schaaf IC Van D. Effect of different components of triple-H therapy on cerebral perfusion in patients with aneurysmal subarachnoid haemorrhage: a systematic review. Crit Care. 2010;14:R23.
Harrigan MR. Hypertension may be the most important component of hyperdynamic therapy in cerebral vasospasm. Crit Care. 2010;14:151.
Zauner A, Daugherty WP, Bullock MR, Warner DS. Brain oxygenation and energy metabolism: part I-biological function and pathophysiology. Neurosurgery. 2002;51(2):289–302.
Oddo M, Milby A, Chen I, Frangos S, MacMurtrie E, Maloney-Wilensky E, et al. Hemoglobin concentration and cerebral metabolism in patients with aneurysmal subarachnoid hemorrhage. Stroke. 2009;40:1275–81.
Smith MJ, Stiefel MF, Magge S, Frangos S, Bloom S, Gracias V, et al. Packed red blood cell transfusion increases local cerebral oxygenation. Crit Care Med. 2005;33:1104–8.
Hébert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–17.
Kramer AH, Zygun DA, Bleck TP, Dumont AS, Kassell NF, Nathan B. Relationship between hemoglobin concentrations and outcomes across subgroups of patients with aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2009;10:157–65.
Smith MJ, Le Roux PD, Elliott JP, Winn HR. Blood transfusion and increased risk for vasospasm and poor outcome after subarachnoid hemorrhage. J Neurosurg. 2004;101:1–7.
Naidech AM, Drescher J, Ault ML, Shaibani A, Batjer HH, Alberts MJ. Higher hemoglobin is associated with less cerebral infarction, poor outcome, and death after subarachnoid hemorrhage. Neurosurgery. 2006;59:775–9. discussion 779–80.
Naidech AM, Jovanovic B, Wartenberg KE, Parra A, Ostapkovich N, Connolly ES, et al. Higher hemoglobin is associated with improved outcome after subarachnoid hemorrhage. Crit Care Med. 2007;35:2383–9.
Broessner G, Lackner P, Hoefer C, Beer R, Helbok R, Grabmer C, et al. Influence of red blood cell transfusion on mortality and long-term functional outcome in 292 patients with spontaneous subarachnoid hemorrhage. Crit Care Med. 2009;37:1886–92.
Desjardins P, Turgeon AF, Tremblay M-H, Lauzier F, Zarychanski R, Boutin A, et al. Hemoglobin levels and transfusions in neurocritically ill patients: a systematic review of comparative studies. Crit Care. 2012;16:R54.
Higgins J, Green S (Eds). Cochrane handbook for systematic reviews of interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration; 2011. www.cochrane-handbook.org.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–12.
Egger M, Smith GD, Schneider M. Systematic reviews of observational studies. In: Systematic reviews in health care. London, UK: BMJ Publishing Group; 2001. p. 211–27.
Bagnardi V, Zambon A, Quatto P, Corrao G. Flexible meta-regression functions for modeling aggregate dose–response data, with an application to alcohol and mortality. Am J Epidemiol. 2004;159:1077–86.
Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–84.
Higgins JPT, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Molyneux AJ, Kerr RSC, Yu L, Clarke M, Sneade M, Yarnold JA, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366:809–17.
Hajjar LA, Vincent J-L, Galas FRBG, Nakamura RE, Silva CMP, Santos MH, et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA. 2010;304:1559–67.
Carson JL, Terrin ML, Noveck H, Sanders DW, Chaitman BR, Rhoads GG, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365:2453–62.
Carson JL, Brooks MM, Abbott JD, Chaitman B, Kelsey SF, Triulzi DJ, et al. Liberal versus restrictive transfusion thresholds for patients with symptomatic coronary artery disease. Am Heart J. 2013;165:964–71.e1.
Hopewell S, Omar O, Hyde C, Yu L-M, Doree C, Murphy MF. A systematic review of the effect of red blood cell transfusion on mortality: evidence from large-scale observational studies published between 2006 and 2010. BMJ Open. 2013;3(5), e002154.
Connolly ES, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2012;43:1711–37.
Acknowledgements
Our research team would like to thank Ms. Risa Shorr (Librarian) for her assistance with building and conducting the electronic search strategy. We would also like to thank Ms. Marnie Gordon for the administrative assistance that she provided. S. English is the recipient of a Fellowship grant from Canadian Blood Services.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SE conceived the study, created the analytical plan, constructed the initial draft of the protocol, and contributed directly to the production, revision, and approval of the final study protocol. LM co-conceived the review and contributed to the creation of the analytical plan as well as contributed directly to the production, revision, and approval of the final study protocol. MC had substantive input on the analytical plan as well as contributed directly to the production, revision, and approval of the final study protocol. AFT, AT, AB, and GP contributed directly to the production, revision, and approval of the final study protocol. DF had substantive input on the analytical plan and contributed with the editorial revisions of the protocol leading to the final version. All authors read and approved the final manuscript.
Appendix
Search strategy
The following databases will be used to conduct our search strategy:
-
1.
MEDline + MEDline In-Process & Other Non-indexed Citations (1946 to present)
-
2.
EMBASE Classic + Embase (1947 to present)
-
3.
EBM Reviews (incl Cochrane) (2005 to present)
-
4.
Search of trial registers for ongoing and unpublished studies
SAMPLE search strategy
Database: Embase Classic + Embase <1947 to present>, Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) <1946 to present>
Search strategy:
-
1
exp Subarachnoid Hemorrhage/ (44404)
-
2
*intracranial hemorrhages/ or *cerebral hemorrhage/ or *vasospasm, intracranial/ (45036)
-
3
*Intracranial Aneurysm/ (27317)
-
4
*Rupture, Spontaneous/ or *rupture/ or rupture$.tw. (207853)
-
5
3 and 4 (7647)
-
6
*Aneurysm, Ruptured/ (8661)
-
7
exp *brain/ or exp *meninges/ (1256700)
-
8
6 and 7 (430)
-
9
((subarachnoid or arachnoid$) adj3 (haemorrhag$ or hemorrhag$ or haematoma$ or hematoma$ or bleed$ or blood$)).tw. (40217)
-
10
((brain or cereb$ or intracranial) adj3 aneurysm$ adj3 ruptur$).tw. (6251)
-
11
((brain or cereb$ or intracranial) adj3 aneurism$ adj3 ruptur$).tw. (30)
-
12
((cerebral or intracranial or cerebrovascular) adj6 (vasospasm or spasm)).tw. (8794)
-
13
sah.tw. (15075)
-
14
1 or 2 or 5 or 8 or 9 or 10 or 11 or 12 or 13 (101951)
-
15
Erythrocyte Transfusion/ (18728)
-
16
((red blood cell$ or rbc or erythrocyte$ or red cell$) adj2 (transfus$ or therap$)).tw. (14636)
-
17
*Blood Transfusion/ (67415)
-
18
rbct.tw. (125)
-
19
(blood adj2 transfus$).tw. (89864)
-
20
(hemotransfus$ or haemotransfus$).tw. (522)
-
21
or/15-20 (148295)
-
22
14 and 21 (371)
-
23
*anemia/ or anemia.tw. or anaemia.tw. (267609)
-
24
14 and 23 (498)
-
25
*Hemoglobins/ or hemoglobin$.ti. orhaemoglobin$.ti. (100884)
-
26
14 and 25 (265)
-
27
22 or 24 or 26 (1012)
-
28
animals/ not humans/ (5123176)
-
29
27 not 28 (949)
-
30
29 use emczd (622) EMBASE
-
31
exp Subarachnoid Hemorrhage/ (44404)
-
32
intracranial hemorrhages/ or cerebral hemorrhage/ or vasospasm, intracranial/ (94172)
-
33
Intracranial Aneurysm/ (33870)
-
34
Rupture, Spontaneous/ or rupture/ or rupture$.tw. (227167)
-
35
33 and 34 (9450)
-
36
Aneurysm, Ruptured/ (15457)
-
37
exp brain/ or exp meninges/ (2087107)
-
38
36 and 37 (1927)
-
39
((subarachnoid or arachnoid$) adj3 (haemorrhag$ or hemorrhag$ or haematoma$ or hematoma$ or bleed$ or blood$)).tw. (40217)
-
40
((brain or cereb$ or intracranial) adj3 aneurysm$ adj3 ruptur$).tw. (6251)
-
41
((brain or cereb$ or intracranial) adj3 aneurism$ adj3 ruptur$).tw. (30)
-
42
((cerebral or intracranial or cerebrovascular) adj6 (vasospasm or spasm)).tw. (8794)
-
43
sah.tw. (15075)
-
44
31 or 32 or 35 or 38 or 39 or 40 or 41 or 42 or 43 (145988)
-
45
Erythrocyte Transfusion/ (18728)
-
46
((red blood cell$ or rbc or erythrocyte$ or red cell$) adj2 (transfus$ or therap$)).tw. (14636)
-
47
Blood Transfusion/ (145644)
-
48
rbct.tw. (125)
-
49
(blood adj2 transfus$).tw. (89864)
-
50
(hemotransfus$ or haemotransfus$).tw. (522)
-
51
or/45-50 (199301)
-
52
44 and 51 (1576)
-
53
anemia/ or anemia.tw. or anaemia.tw. (321603)
-
54
44 and 53 (1752)
-
55
Hemoglobins/ or hemoglobin$.ti. orhaemoglobin$.ti. (207188)
-
56
44 and 55 (1173)
-
57
52 or 54 or 56 (3847)
-
58
animals/ not humans/ (5123176)
-
59
57 not 58 (3698)
-
60
59 use prmz (625) MEDLINE
-
61
30 or 60 (1247)
-
62
remove duplicates from 61 (957)
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
English, S.W., Chassé, M., Turgeon, A.F. et al. Red blood cell transfusion and mortality effect in aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis protocol. Syst Rev 4, 41 (2015). https://doi.org/10.1186/s13643-015-0035-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13643-015-0035-1