Abstract
Background
Drowning-associated pneumonia (DAP) is frequent in drowned patients, and possibly increases mortality. A better understanding of the microorganisms causing DAP could improve the adequacy of empirical antimicrobial therapy. We aimed to describe the pooled prevalence of DAP, the microorganisms involved, and the impact of DAP on drowned patients.
Methods
Systematic review and meta-analysis of studies published between 01/2000 and 07/2023 reporting on DAP occurrence and microorganisms involved.
Results
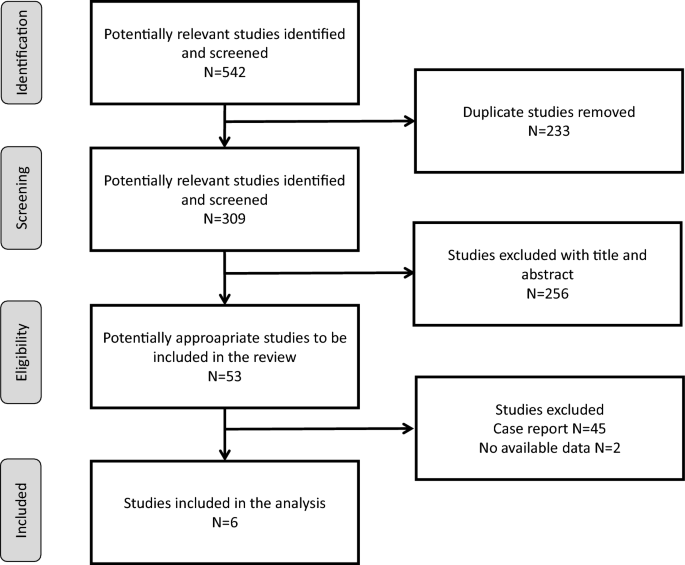
Of 309 unique articles screened, 6 were included, involving 688 patients. All were retrospective cohort studies, with a number of patients ranging from 37 to 270. Studies were conducted in Europe (France N = 3 and Netherland N = 1), United States of America (N = 1) and French West Indies (N = 1). Mortality ranged between 18 to 81%. The pooled prevalence of DAP was 39% (95%CI 29–48), similarly following freshwater (pooled prevalence 44%, 95%CI 36–52) or seawater drowning (pooled prevalence 42%, 95%CI 32–53). DAP did not significantly impact mortality (pooled odds ratio 1.43, 95%CI 0.56–3.67) but this estimation was based on two studies only. Respiratory samplings isolated 171 microorganisms, mostly Gram negative (98/171, 57%) and mainly Aeromonas sp. (20/171, 12%). Gram positive microorganisms represented 38/171 (22%) isolates, mainly Staphylococcus aureus (21/171, 12%). Water salinity levels had a limited impact on the distribution of microorganisms, except for Aeromonas sp. who were exclusively found following freshwater drowning (19/106, 18%) and never following seawater drowning (0%) (p = 0.001). No studies reported multidrug-resistant organisms but nearly 30% of the isolated microorganisms were resistant to amoxicillin-clavulanate, the drug that was the most commonly prescribed empirically for DAP.
Conclusions
DAP are commonly caused by Gram-negative bacteria, especially Aeromonas sp. which is exclusively isolated following freshwater drowning. Empirical antimicrobial therapy should consider covering them, noting than amoxicillin-clavulanate may be inadequate in about one-third of the cases. The impact of DAP on patients’ outcome is still unclear.
Similar content being viewed by others
Introduction
Drowning is defined as a respiratory impairment following immersion or submersion of the airways in a liquid, typically water [1]. The ensuing hypoxemia and cardiac arrest carry a high mortality rate even with a brief period of immersion [1]. In survivors, aspiration of water in the alveoli causes surfactant dysfunction and washout, leading to diffuse alveolar damage and pulmonary edema. In 12% to 51% of the cases [2,3,4], survivors develop drowning-associated pneumonia (DAP), following the inhalation of contaminated water, endogenous flora or gastric content [1, 5]. DAP significantly impacts patient’s evolution, with prolonged mechanical ventilation and possibly higher mortality rate [2].
Limited data is available on microorganisms causing DAP; several factors can influence the microbial composition of contaminated water, including its chemical composition, geographic location and salinity level [6]. Ignoring the microorganisms causing DAP can adversely affect patients’ outcomes, through the administration of inappropriate empirical antimicrobial treatments. Indeed, a fair proportion of the microorganisms isolated in DAP are intrinsically resistant to the antimicrobials agents commonly recommended for community-acquired inhalation pneumonia. As suboptimal empirical antimicrobial therapy may lead to an unfavorable outcome, a better understanding of the causative microorganisms is needed [3].
The study aims to summarize the current knowledge on the ecology of microorganisms involved in DAP, and to assess the repercussion of DAP on patient outcomes.
Methods
Data sources and search strategy
Pubmed and EMBASE database were searched in August 2023 for relevant peer-reviewed articles, published in English or French, between January 2000 and July 2023, with no age restriction, in accordance with the PRISMA guidelines [7]. The following items were used for searches: (drowning associated pneumonia); (near-drowning associated pneumonia); (drowning AND pneumonia); (near-drowning AND pneumonia); (drowning AND microbiology). The references of all relevant publications were reviewed, and no further articles were identified.
One reviewer (VLC) screened the titles and abstracts to determine eligibility. Inclusion criteria were the following: studies reporting microbiological data on 10 humans or more who had survived drowning and later developed a DAP while being in intensive care units (ICU). Reviews and isolated case reports were excluded.
Data collection
The following baseline data was extracted: year of publication; geographical setting; severity of drowning (i.e. occurrence of pre-admission cardiac arrest, requirement of mechanical ventilation, occurrence of acute respiratory distress syndrome [8], and admission Simplified Acute Physiology Score II (SAPS II) [9] and/or Sequential Organ Failure Assessment (SOFA) [10]); patients outcome (i.e. mortality, and duration of mechanical ventilation); water location (i.e. sea, lake, river, damp, pond, swimming pool, or miscellaneous) and salinity (i.e. seawater or freshwater).
Confirmed DAP was established by microbiology. The variables of interest for DAP included: numbers of respiratory samples; proportion of positive samples; type of microorganisms isolated; number of positive individual microorganisms isolated in respiratory samples; technic of respiratory sampling (i.e. broncho-alveolar lavage (BAL), protected specimen brush, tracheal aspirates, or sputum); antimicrobials used for empirical therapy; antimicrobial resistance in the microorganisms isolated.
Statistics analysis
Descriptive statistics were used: continuous variables were reported as median (interquartile range IQR) and categorical variables as proportion (%). Microorganisms isolated from patients with seawater DAP were compared to those isolated from patients with freshwater DAP using a Fisher’s exact test. Stata v18 meta-analysis software pack was used to calculate the pooled prevalence of DAP (overall, following freshwater drowning and following seawater drowning) and to calculate the combined mortality odds ratio following DAP. Heterogeneity was assessed using the I2 statistics. High heterogeneity was defined as a I2 > 50%. In case of high heterogeneity, random-effect analyses were done and presented using forest plots. Stata v18 (StataCorp, College Station, TX, USA) was used for graphical and statistical analyses.
Results
Review and population
Of 309 unique articles, 6 studies were included, with a period of publication ranging from 2012 to 2023 (Fig. 1) [2, 3, 11,12,13,14]. Their main characteristics are detailed in Table 1. All were retrospective cohort studies, with a number of patients ranging from 37 to 270. Studies were conducted in Europe (France N = 3 and Netherland N = 1), United States of America (N = 1) and French West Indies (N = 1). Four studies included exclusively adult patients [2, 3, 11, 14], one included a mixed population of adult and pediatric patients [12] and the last one included only pediatric patients [13].
A total of 688 patients were available for analysis. Location of drowning included sea (N = 393), swimming pool (N = 127), river (N = 55), pond (N = 30), bathtub (N = 19), other water source (N = 9), lake (N = 5) and swamp (N = 1). Location of drowning was not documented in 49 cases. As depicted in Table 1, a high proportion of patients presented pre-admission cardiac arrest, ranging from 38 to 78%, and the overall outcome was poor with mortality rate ranging between 18 and 81%.
Drowning-associated pneumonia
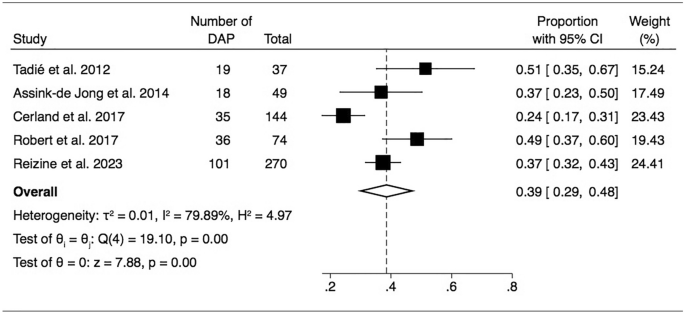
Five of the 6 studies reported the prevalence of DAP, ranging between 24 to 51%; the criteria they used to diagnose DAP are summarized in Table 2. The pooled prevalence of DAP was 39% (95% CI 29–48) (ι2 0.01) (Figure 2) and was not influenced by the water salinity level, with similar pooled prevalence following freshwater DAP (44%, 95% CI 36–52) or seawater DAP (42%, 95% CI 32–53) (Additional file 1: Figure S1).
Use of prophylactic antibiotics was not reported to be a routine procedure in any of the 6 studies. When DAP was suspected, various empirical antimicrobial therapies were used, predominately amoxicillin-clavulanate (Table 3). Only 3/6 studies evaluated the adequacy of the empirical antimicrobial therapy for the isolated microorganism, ranging from 50 to 89% [2, 3, 14] .
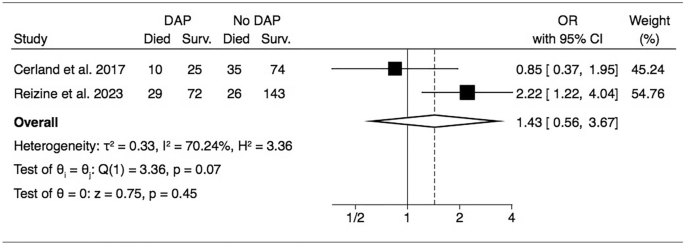
The impact of DAP on patient’s outcome was reported inconsistently across studies, with only 2 studies reporting individual-level data enabling to estimate the impact of DAP on patient outcome [2, 12]. With a total of 414 patients and 136 DAP, the meta-analysis suggests a negative impact of DAP on survival, although not statistically significant (pooled odds ratio 1.43, 95% CI 0.56–3.67) (Fig. 3).
Isolated microorganisms
A total of 171 microorganisms were isolated from 167 respiratory samples (including bronchoalveolar lavage (30%), protected specimen brush (9%), tracheal aspirates (35%), sputum (3%), and 24% not documented), as detailed in Table 4. Gram-negative were predominant (N = 98/171 (57%), primarily Aeromonas sp. (N = 20/171 (12%)), Haemophilus influenzae (N = 19/171 (11%)) and Pseudomonas aeruginosa (N = 12/171 (7%)). Gram positive followed with 38/171 (22%) isolates, mainly Staphylococcus aureus (N = 21/171 (12%)). Multiples germs were isolated in 10% of samples. Fungi were detected in a minority of samples with only 2/171 Candida sp. (1%) and 1/171 (0.5%) Aspergillus sp.
Microorganisms were compared according to the water salinity level (Table 4). The proportion of Gram positive and Gram negative was similar across both types of water. Aeromonas sp. was exclusively detected following freshwater drowning, with 19/106 (18%) of positive samples, and never following seawater drowning (0%) (p value 0.001). Enterobacter sp. were more frequently detected following seawater drowning (6/43) compared to freshwater drowning (3/106; p value 0.01). Fungi were exclusively isolated following freshwater drowning.
Antimicrobial therapy resistance and adequacy
Studies did not report systematically on antibiotic resistance. Three studies reported on the proportion of microorganisms being resistant to amoxicillin-clavulanate: 31% in Robert et al. [3], 36.4% in Reizine et al. [2] and 31.6% in Tadié et al. [14]. One study reported the prevalence of cefotaxime resistance to be 12% [2]. No studies reported on the presence of multidrug-resistant microorganisms.
Studies did not consistently report on the inadequacy of antimicrobial therapy and its consequences; however, some interesting results were mentioned by two studies. Reizine et al. reported a mortality rate of 7/10 (70%) among the patients who received inadequate antimicrobial therapy, whereas the global mortality rate in that study was 20% [2]. In the publication from Tadié et al. the mortality rate was 2/6 among the patients who received inadequate antimicrobial therapy, whereas the global mortality rate in that study was 81% [14].
Discussion
In this systematic review, we assessed the impact of DAP on nearly 700 patients admitted to ICU following drowning. A variety of microorganisms were isolated, irrespective of the water salinity level, apart from Aeromonas sp. and fungi that were exclusively isolated following freshwater drowning. As the empirical antibiotic therapy used was usually not targeting the isolated microorganisms, our findings highlight the importance of early bacterial samplings in drowned patients, as inadequate treatment is likely to impact the patients’ outcome.
Drowning represents one of the leading causes of accidents worldwide and carries a high mortality rate [15]. Patients surviving the initial drowning event are often admitted to ICU and are at risk of secondary respiratory complications such as DAP. Historically, DAP prevalence was reported to be between 11% and 54% [4, 16,17,18], in line with our updated estimate of 39%. Importantly, not all patients developed DAP, possibly owing to multiple factors, including: different microorganism load, varying immersive liquid chemical composition, occurrence of laryngospasm preventing aspiration, and the nature of drowning (i.e. primary or secondary to seizure, syncope, arrhythmia, or trauma) [1, 6].
Whether DAP occurrence increases the mortality rate is still unknown. In the past, the mortality rate in patients with DAP ranged between 26% and 60%, whilst recent studies report a mortality rate of approximately 28% [2, 4, 6, 12]. We found conflicting results in our review, with a non-significant trend for an impact of DAP on mortality rate. Cerland et al. reported similar mortality rates among patients with or without DAP, while Reizine et al. suggested a detrimental impact of DAP on patient outcome [2, 12]. DAP can lead to hypotension, hypoxemia and temperature instabilities, all recognized as factors worsening patient outcome following a cardiac arrest [19, 20]. Moreover, the intricated influence of inflammation triggered by DAP and the consequence of an inflamed lung may have on brain lesions might also play a significant role in affecting the patient outcome [20, 21]. However, other factors may have more impact on patient’s outcome, such as pre-admission cardiac arrests, or patients’ comorbidities. Similarly, a study showed that the reduction of early ventilator-associated pneumonia occurrences in post-cardiac arrest patients did not improve the mortality rate or the duration of mechanical ventilation [22].
We underscore the high prevalence of Gram-negative bacteria, both in freshwater and seawater, as historically described [4, 6]. The high incidence of Enterobacter sp. and other coliform bacteria could be explained by water fecal contamination [23]. Identification of those microorganisms strongly suggests the inhalation of contaminated water. In addition, a large number of samples suggest aspiration of oro-pharyngeal secretions (Streptococcus pneumonia, Staphylococcus aureus, Haemophilus influenza). Those results underline the role of aspiration, from both water or secretion, as a source for bacterial inoculum in DAP.
Importantly, Aeromonas sp. was the main germ isolated following freshwater drowning. This microorganism has several chromosomal beta-lactamases, which can impact DAP management trough reduced susceptibilities to antimicrobial agents, such as amoxicillin-clavulanate (only 16% susceptible isolates in a report), the most commonly used antimicrobial agent for empirical treatment [24,25,26]. However, most of Aeromonas sp. may remain susceptible to cefepime or piperacillin-tazobactam [25]. Despite its aquatic tropism, Pseudomonas aeruginosa was isolated in less than 10% of the samples. The density of Pseudomonas spp. colony in water is highly variable and may be very low in surface waters of natural water area, while contamination may be significant in recreational waters such as swimming pools [27]. Noticeably, fungal or anaerobic identification was rare. However, in special circumstances such as natural disasters, high incidence of Aspergillus sp. has been reported [28]. Considering those germs in these specific situations seems to be a practical approach to adopt [11].
The dilemma of whether empirical antimicrobial therapies are indicated at admission of drowned patient remains unresolved, but most guidelines discourage using them systematically [4, 29, 30]. A practical approach would be to restrain the use of such antimicrobial in drowned patients, with the exception of drowning occurring in highly contaminated environments (e.g. septic tank, manure pit) or in patients presenting severe lung lesions. As only a third of patients may develop a DAP, early respiratory sampling seems reasonable when DAP is suspected, as it has been shown to be effective to reduce antimicrobial prescription in patients with aspiration pneumonia and may help to guide antimicrobial therapy or help cease it [31]. Respiratory samplings will enable to rapidly identify the causative microorganisms and its antimicrobial susceptibilities. As the main isolated germs are Gram-negative, including Aeromonas spp. or Pseudomonas spp., close follow-up of antimicrobial susceptibilities is crucial as clinicians may encounter resistant microorganisms causing DAP. Antimicrobial resistance in the environment could be frequent through acquiring and sharing antibiotic resistance genes, in addition to natural resistance [32]. When antibiotic treatment cannot be delayed, piperacillin-tazobactam or a 4th generation cephalosporin could be suggested as first-line treatment, since inadequate antimicrobial therapy seems to carry a high risk of adverse outcome, as mentioned in the reviewed studies [2, 3, 11, 14]. Importantly, antimicrobial therapy should always be tailored to local microbiological ecology and de-escalation performed as soon as possible.
It is important to note that diagnosis of DAP remains difficult as numerous criteria used for its definition can be confounded by concurring events, similarly to ventilator associated pneumonia [33]. The use of controlled temperatures after a cardiac arrest may mask any sign of hypo or hyperthermia linked to an infection, as illustrated by Reizine et al. who reported a median body temperature of 38.1 °C (IQR 35.6–38.7) at DAP diagnosis [2]. Interpreting radiological findings can be challenging in presence of lung damage and difficult to differentiate DAP from cardiogenic pulmonary edema, atelectasis and non-infective acute lung injury related to submersion [5]. In addition, inflammatory markers may be less useful following cardiac arrest as they will be deranged by the ischemia–reperfusion syndrome [34]. All these considerations highlight the importance of maintaining a low threshold for respiratory samplings in drowned patients, as it serves as a crucial criterion to initiating treatment and will be paramount to adjust the antimicrobial therapy.
Limitations
Our study has several limitations, including publication bias. All included studies were retrospectives; they differed in their methodology and their population in terms of proportion of pre-hospital cardiac arrests, severity score at admission, and whether they included drowned and/or nearly-drowned patients. The results may not apply to all geographic areas, especially tropical and/or warm temperature waters (only 1/6 studies take place in a tropical area, the one from the French West Indies). Moreover, meta-analyses on the impact of DAP on patients outcome could only be performed by 2 studies. Not all patients with DAP had a respiratory sampling done, and the microorganisms identified are not necessarily the causative agents of DAP. Finally, as mentioned above, diagnosis of pneumonia remains difficult in this population and some patients may have drowning-induced pulmonary damage misdiagnosed as DAP.
Conclusion
This study provides important information on DAP ecology, emphasizing the predominant role of Gram-negative bacteria and Aeromonas sp. who are commonly resistant to the antimicrobial frequently used empirically. As amoxicillin-clavulanate does not cover the microorganisms commonly isolated, piperacillin-tazobactam or a 4th generation cephalosporin could be more suitable for empirical treatment. When empirical therapy is required, respiratory sampling should be performed, and potential resistance should be investigated. Future studies are needed to investigate the impact of DAP on patient outcome and the role of an early antimicrobial therapy in drowned patients.
Availability of data and materials
Data are available on reasonable request.
Abbreviations
- DAP:
-
Drowning-associated pneumonia
- ICU:
-
Intensive care unit
- IQR:
-
Interquartile range
- SAPS II:
-
Simplified acute physiology score II
- SOFA:
-
Sequential organ failure assessment
References
Szpilman D, Bierens JJ, Handley AJ, Orlowski JP. Drowning. N Engl J Med. 2012;366(22):2102–10.
Reizine F, Delbove A, Tattevin P, Santos AD, Bodenes L, Bouju P, et al. Clinical and microbiological features of drowning-associated pneumonia: a retrospective multicentre cohort study. Clin Microbiol Infect. 2023. https://doi.org/10.1016/j.cmi.2022.07.027.
Robert A, Danin PE, Quintard H, Degand N, Martis N, Doyen D, et al. Seawater drowning-associated pneumonia: a 10-year descriptive cohort in intensive care unit. Ann Intensive Care. 2017;7(1):45.
van Berkel M, Bierens JJ, Lie RL, de Rooy TP, Kool LJ, van de Velde EA, et al. Pulmonary oedema, pneumonia and mortality in submersion victims; a retrospective study in 125 patients. Intensive Care Med. 1996;22(2):101–7.
Gregorakos L, Markou N, Psalida V, Kanakaki M, Alexopoulou A, Sotiriou E, et al. Near-drowning: clinical course of lung injury in adults. Lung. 2009;187(2):93–7.
Ender PT, Dolan MJ. Pneumonia associated with near-drowning. Clin Infect Dis. 1997;25(4):896–907.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71.
Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–33.
Le Gall JR, Lemeshow S, Saulnier F. A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957–63.
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996. https://doi.org/10.1007/BF01709751.
Assink-de Jong E, Douma M, Beishuizen A, Hoogewerf M, Debets-Ossenkopp YJ, de Waard MC, et al. Microbiological findings and adequacy of antibiotic treatment in the critically ill patient with drowning-associated pneumonia. Intensive Care Med. 2014;40(2):290–1.
Cerland L, Megarbane B, Kallel H, Brouste Y, Mehdaoui H, Resiere D. Incidence and consequences of near-drowning-related pneumonia—a descriptive series from martinique. French West Indies: Int J Environ Res Public Health; 2017. https://doi.org/10.3390/ijerph14111402.
Moffett BS, Lee S, Woodend K, Sigdel B, Dutta A. Evaluation of antimicrobial utilization in the pediatric drowning population. J Pediatric Infect Dis Soc. 2021;10(2):179–82.
Tadie JM, Heming N, Serve E, Weiss N, Day N, Imbert A, et al. Drowning associated pneumonia: a descriptive cohort. Resuscitation. 2012;83(3):399–401.
Mortality GBD, Causes of Death C. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015. https://doi.org/10.1016/S0140-6736(14)61682-2.
Kennedy GA, Kanter RK, Weiner LB, Tompkins JM. Can early bacterial complications of aspiration with respiratory failure be predicted? Pediatr Emerg Care. 1992;8(3):123–5.
Lee KH. A retrospective study of near-drowning victims admitted to the intensive care unit. Ann Acad Med Singap. 1998;27(3):344–6.
Oakes DD, Sherck JP, Maloney JR, Charters AC 3rd. Prognosis and management of victims of near-drowning. J Trauma. 1982;22(7):544–9.
Hirsch KG, Abella BS, Amorim E, Bader MK, Barletta JF, Berg K, et al. Critical care management of patients after cardiac arrest: a scientific statement from the American heart Association and neurocritical care Society. Neurocrit Care. 2023. https://doi.org/10.1007/s12028-023-01871-6.
Sandroni C, Cronberg T, Sekhon M. Brain injury after cardiac arrest: pathophysiology, treatment, and prognosis. Intensive Care Med. 2021;47(12):1393–414.https://doi.org/10.1007/s12028-023-01871-6.
Mai N, Miller-Rhodes K, Knowlden S, Halterman MW. The post-cardiac arrest syndrome: a case for lung–brain coupling and opportunities for neuroprotection. J Cereb Blood Flow Metab. 2019;39(6):939–58.
Francois B, Cariou A, Clere-Jehl R, Dequin PF, Renon-Carron F, Daix T, et al. Prevention of early ventilator-associated pneumonia after cardiac arrest. N Engl J Med. 2019;381(19):1831–42.
Korajkic A, McMinn BR, Harwood VJ. Relationships between microbial indicators and pathogens in recreational water settings. Int J Environ Res Public Health. 2018. https://doi.org/10.3390/ijerph15122842.
Janda JM, Abbott SL. The genus Aeromonas: taxonomy, pathogenicity, and infection. Clin Microbiol Rev. 2010;23(1):35–73.https://doi.org/10.3390/ijerph15122842.
Aravena-Roman M, Inglis TJ, Henderson B, Riley TV, Chang BJ. Antimicrobial susceptibilities of Aeromonas strains isolated from clinical and environmental sources to 26 antimicrobial agents. Antimicrob Agents Chemother. 2012;56(2):1110–2.
Fernandez-Bravo A, Figueras MJ. An Update on the Genus Aeromonas: taxonomy, epidemiology, and pathogenicity. Microorganisms. 2020. https://doi.org/10.3390/microorganisms8010129.
Mena KD, Gerba CP. Risk assessment of Pseudomonas aeruginosa in water. Rev Environ Contam Toxicol. 2009;201:71–115.https://doi.org/10.3390/microorganisms8010129.
Benedict K, Park BJ. Invasive fungal infections after natural disasters. Emerg Infect Dis. 2014;20(3):349–55.
Wood C. Towards evidence based emergency medicine: best BETs from the Manchester Royal Infirmary. BET 1: prophylactic antibiotics in near-drowning. Emerg Med J. 2010. https://doi.org/10.1136/emj.2010.094920.
Thom O, Roberts K, Devine S, Leggat PA, Franklin RC. Treatment of the lung injury of drowning: a systematic review. Crit Care. 2021;25(1):253.
Lascarrou JB, Lissonde F, Le Thuaut A, Bachoumas K, Colin G, Henry Lagarrigue M, et al. Antibiotic therapy in comatose mechanically ventilated patients following aspiration: differentiating pneumonia from pneumonitis. Crit Care Med. 2017;45(8):1268–75.
Baquero F, Martinez JL, Canton R. Antibiotics and antibiotic resistance in water environments. Curr Opin Biotechnol. 2008;19(3):260–5.
Pouly O, Lecailtel S, Six S, Preau S, Wallet F, Nseir S, et al. Accuracy of ventilator-associated events for the diagnosis of ventilator-associated lower respiratory tract infections. Ann Intensive Care. 2020;10(1):6.
Mongardon N, Lemiale V, Perbet S, Dumas F, Legriel S, Guerin S, et al. Value of procalcitonin for diagnosis of early onset pneumonia in hypothermia-treated cardiac arrest patients. Intensive Care Med. 2010;36(1):92–9.
Acknowledgement
Not applicable.
Funding
Open access funding provided by University of Geneva.
Author information
Authors and Affiliations
Contributions
VLC and LFP conceived and designed the study, VLC collected the data, VLC and LFP interpreted the data, VLC and LFP wrote the first draft of the article. All authors revised the manuscript and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Table. S1. Forrest plot of included studies reporting prevalence of drowning associated pneumonia, depending on the salinity of the water DAP drowning associated pneumonia
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cousin, V.L., Pittet, L.F. Microbiological features of drowning-associated pneumonia: a systematic review and meta-analysis. Ann. Intensive Care 14, 61 (2024). https://doi.org/10.1186/s13613-024-01287-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13613-024-01287-1