Abstract
Background
Little interest has been paid to expiratory muscle strength, and the impact of expiratory muscle weakness on critical outcomes is not known. Very few studies assessed the relationship between maximal expiratory pressure (MEP) and critical outcomes. The aim of this study was to investigate the relationship between MEP and critical outcomes.
Methods
This work was a secondary analysis of a prospective, observational study of adult patients who required mechanical ventilation for ≥ 24 h in an 18-bed ICU. MEP was assessed before extubation after a successful, spontaneous breathing trial. The relationships between MEP and extubation failure, and short-term (30 days) mortality, were investigated. Univariate logistic regressions were computed to investigate the relationship between MEP values and critical outcomes. Two multivariate analyses, with and without maximal inspiratory pressure (MIP), both adjusted using principal component analysis, were undertaken. Unadjusted and adjusted ROC curves were computed to compare the respective ability of MEP, MIP and the combination of both measures to discriminate patients with and without extubation failure or premature death.
Results
One hundred and twenty-four patients were included. Median age was 66 years (IQR 18) and median mechanical ventilation duration was 7 days (IQR 6). Extubation failure rate was 15% (18/124 patients) and the rate for 30-day mortality was 11% (14/124 patient). Higher MEP values were significantly associated with a lower risk of extubation failure in the univariate analysis [OR 0.96 95% CI (0.93–0.98)], but not with short-term mortality. MEP was independently linked with extubation failure when MIP was not included in the multivariate model, but not when it was included, despite limited collinearity between these variables. This study was not able to differentiate the respective abilities of MEP, MIP, and their combination to discriminate patients with extubation failure or premature death (adjusted AUC for the combination of MEP and MIP: 0.825 and 0.650 for extubation failure and premature death, respectively).
Conclusions
MEP is related to extubation failure. But, the results did not support its use as a substitute for MIP, since the relationship between MEP and critical outcomes was no longer significant when MIP was included. The use of MIP and MEP measurements combined did not reach higher discriminative capacities for critical outcomes that MEP or MIP alone.
Trial Registration This study was retrospectively registered at https://clinicaltrials.gov/ct2/show/NCT02363231?cond=NCT02363231&draw=2&rank=1 (NCT02363231) in 13 February 2015
Similar content being viewed by others
Background
Mechanical ventilation (MV) is well known to cause rapid, severe, respiratory muscle dysfunction and weakness [1, 2]. Respiratory muscle weakness has been linked to poor outcomes, including ventilator-weaning failure, extubation failure and death [3,4,5,6]. Research into the impact of inspiratory muscle weakness on critical outcomes has focussed on diaphragm ultrasonography and maximal inspiratory pressure (MIP) measurements [6, 7]. In contrast, few authors have examined the impact of expiratory muscle strength on critical outcomes, despite the fact that expiratory muscles participate in respiratory system homeostasis, cough capacity, facilitate diaphragmatic contractile efficiency and reduce hyperinflation, especially in patients with impeding respiratory failure [8,9,10].
Studies into expiratory muscle strength frequently measure maximal expiratory pressure (MEP), which was recently shown to be reliable in both intubated and non-intubated patients, and strongly correlated with MIP in both conditions [11,12,13]. MEP values at the time of weaning are generally lower than predicted values; it has also been reported that MEP is reduced in patients with failed extubation, compared to those who were successfully extubated [11, 14].
Despite this, the clinical significance of a low MEP on critical outcomes is currently unknown. The primary objective of this study, therefore, was to explore the relationship between MEP values and extubation failure, and short-term mortality. Secondary objectives were (1) to explore the impact of MEP values on critical outcomes whether MIP was included or not and; (2) to compare the ability of MEP and MIP, separately and combined, to discriminate patients with extubation failure and premature death.
Methods
Study design and setting
This study was a secondary analysis of an observational study carried out between January and December 2014 in an 18-bed medical intensive care unit (ICU) [7]. The present study was authorised by the Comité de Protection des Personnes Nord-Ouest III, conducted according to the Declaration of Helsinki and registered on clinicaltrials.gov (NCT02363231). All patients participated voluntarily. The study is reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.
Participants
The same inclusion criteria were used for the secondary analysis as for the original study [7]. Briefly, participants were adults (≥ 18 years) hospitalised in ICU who had been on MV for ≥ 24 h and had undergone a successful spontaneous breathing trial (SBT). The exclusion criteria have been detailed elsewhere [7]. Briefly, they included an inability to undergo MEP measurement, a decision to withhold treatments or previously documented respiratory muscle weakness.
Study procedure
Following cessation of all sedatives, patients’ states of arousal were assessed several times per day. Once alert, cooperative (Ramsay score at 2) and responsive to simple orders, a SBT was undertaken using pressure support (inspiratory positive airway pressure: 7cmH2O and expiratory positive airway pressure: 0cmH2O) for 30 to 120 min [15]. If the SBT was successful, extubation was planned and patient eligibility was assessed. Expiratory muscle strength was assessed following inclusion by measurement of MEP [12, 16].
Measurement of MEP and outcome collection
MEP was measured using an electronic manometer (MicroRPM, Eolys) with a unidirectional valve connected to the endotracheal intubation tube with a catheter mount. The procedure is fully described elsewhere [7]. Three measurements were made, all with the patient disconnected from the ventilator, and the best value was recorded. MIP was also measured according to the Marini method [16]. Demographic data, reason for admission, comorbidities and other factors associated with the ICU stay were also collected.
Study outcomes
Extubation failure (defined as reintubation within 48 h) and short-term mortality (i.e. at 30 days) were recorded. Due to the observational design, reintubation indications were not protocolised in the present study, and were left to and made by the attending clinician, regardless of the study protocol. Nonetheless, reintubation indications in our ICU department encompasses respiratory failure, hypoxemia, laryngeal oedema, inability to ensure airway protection, shock, decreased level of consciousness, copious secretions that could not be cleared despite adequate treatments.
Statistical methods
Patients characteristics are reported as numbers (and/or percentages) for categorical data and as medians (interquartile ranges) for continuous data. We calculated univariable logistic regressions for the dependent variables extubation failure and mortality, including the independent variables age, sex, comorbidities, ICU-acquired weakness (ICU-Aw), SAPS II score, days of ventilator use (prior to test day), days of neuromuscular blocker use, as well as for MEP and MIP. We reported Odds Ratio with 95% confidence intervals as well as standardised odds ratios. For the standardisation, the continuous independent variables were divided by two standard deviations of the variables [17]. This allows the comparison of odds ratio across variables and to compare them also to odds ratio from binary independent variables [17]. Because the number of events did not allow for the adjustment with all relevant potential confounders, we used a method proposed by Riley et al. where a principal component analysis was performed including a set of predefined predictors, and the first component was used in the multivariable adjusted logistic regressions for MEP [18]. The same model was then computed, but with MIP included to explore the impact of the latter on the relationship between MEP and critical outcomes, after collinearity was verified. We calculated receiver operating characteristics (ROC) curves and area under the ROC curves (AUC) for the variables MEP, MIP, and the combination of MEP and MIP, for both outcomes extubation failure and mortality, unadjusted and adjusted for the principal component score. The null-hypothesis of equality of the AUCs for the different predictors was tested with a method proposed by DeLong and Clarke-Pearson (implement in the command roccomp in Stata version 16.1) [19]. This test produces a p-value from a Chi-squared test. We used Stata version 16.1. (StataCorp, Texas, USA) for all calculations and a p value < 0.05 was considered significant.
Results
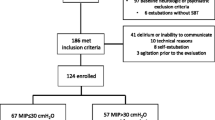
Among the 856 patients screened for the original 2014 observational study, 186 were eligible and 124 were finally included (main exclusion reasons: delirium and impossibility of measuring MEP) [7]. Patients characteristics are described in Table 1. The complete flow diagram of patient inclusions can be found elsewhere [7].
In the univariate logistic regression analyses, MEP, MIP, body mass index (BMI), neuromuscular blocker administration duration and MV duration were significantly related to extubation failure. Conversely, neither MEP nor MIP was associated with short-term mortality; and age, SAPS II and BMI were the only variables significantly related with mortality at 30 days (Table 2).
In the multivariate logistic regression models, MEP was significantly associated with extubation failure in the primary model without MIP [OR 0.96 95% CI (0.94–0.99); p = 0.01], but was not in the model that included MIP [OR 0.99 95% CI (0.96–1.02); p = 0.50] (Table 3). MEP was not significantly associated with mortality at 30 days in the multivariate analysis model, whether MIP was included in the model or not (Table 3). Collinearity diagnostics of MEP and MIP revealed very limited collinearity between both measures with a variance inflation factor (VIF) of 1.83 (square root VIF = 1.35).
Finally, AUC (adjusted and unadjusted) were not significantly different for extubation failure between MEP, MIP and the combination of MEP and MIP (unadjusted AUC: 0.748 vs 0.796 and 0.798, respectively, p = 0.24; adjusted AUC: 0.775 vs 0.817 and 0.825, respectively, p = 0.14) (Fig. 1). Similarly, AUC were not different for early mortality between MEP, MIP and the combination of MEP and MIP (unadjusted AUC: 0.563 vs 0.654 and 0.648, respectively, p = 0.10; adjusted AUC: 0.561 vs 0.652 and 0.650, respectively, p = 0.23) (Fig. 2).
Discussion
The results of this study revealed that (1) higher MEP values were associated with a lower risk of extubation failure in the primary univariate logistic regression; (2) in the multivariate logistic regression models, higher MEP values were associated with reduced extubation failure in the model without MIP, but not in the model that included MIP; (3) MEP values were not associated with short-term mortality in the univariate or multivariate analysis; (4) adjusted and unadjusted AUC for MEP, MIP and the combination of MEP and MIP similarly discriminated patients with extubation failure and short-term mortality.
The present results showed that higher MEP values were associated with a lower risk of extubation failure. The primary reason for this result could be the key role of the expiratory muscles in generating a sufficiently effective cough to ensure airway clearance following extubation [20]. Indeed, previous work based on a multivariate analysis adjusted for MV duration, the presence of chronic respiratory failure and ICU-Aw indicated that higher MEP values are associated with a reduction of extubation failure [OR 0.98 95% CI (0.97–1.0); p = 0.04] [9]. Furthermore, in that study by Terzi et al., patients who required respiratory support, non-invasive ventilation (NIV) due to respiratory distress, or mechanical cough assistance due to an inability to clear secretions also had lower MEP values than those who underwent simple extubation (30 vs 53 cmH2O) [9]. Indeed, ineffective cough is a factor associated with extubation failure, even though MEP is not the only factor contributing to cough efficiency [21, 22]. Noteworthy, cough was not directly measured in the present study, and the use of mechanical cough assistance after extubation was not reported. The use of such devices was, however, based on clinicians’ decisions and was not influenced by the measures undertaken for the present study.
One hypothesis behind the present analysis was that the expiratory muscles contribute to maintaining the balance between respiratory load and respiratory system capacity by enhancing the activity of the inspiratory muscles [8]. Two multivariate regression analysis models were then computed, with and without MIP, to explore the relationship between MEP and critical outcomes, when MIP was also taken into account. Since MEP was related to extubation failure in the model without MIP, but not in the model including MIP, expiratory muscles weakness did not occur in isolation from inspiratory muscle weakness in the present study. One explanation for this could be that two of the variables related to extubation failure in the univariate analysis (namely MV duration and neuromuscular blocker administration duration) have previously been linked to both inspiratory and expiratory muscle weakness [9, 23, 24]. In a previous study by Terzi et al., patients who required mechanical cough assistance (i.e. patients with expiratory muscle weakness) had a higher MV duration compared to those who required NIV (19.8 vs 17.4 days, respectively; p = 0.02) [9]. Expiratory muscle strength could therefore decrease at a later stage than inspiratory muscle strength, explaining the lack of relationship between MEP and critical outcomes when MIP is also included in the model.
MEP is an easy-to-use bedside tool that can easily and quickly be measured by clinicians. It is, however, worthy of note that one disadvantage of MEP evaluation is that the patient must be sufficiently aroused to voluntarily generate a maximal effort. Another key point is that, contrary to MIP, MEP cannot be directly measured on modern ventilator machines without disconnecting the patient from the ventilator. According to the AUC results, we were unable in the present study to differentiate the respective abilities of MEP and MIP separated or combined to discriminate premature death, but those three AUCs were low and unlikely to provide clinical guidance for that outcome. AUCs for the discrimination of extubation failure were higher than for short-term death, but we were unable to isolate any difference between each of the measurements undertaken. One could then consider than using MIP measurement alone could lead to similar results and would be quicker than the combination of both measures, especially since the relationship between MIP and critical outcomes has been largely described for critically ill patients [6]. However, this requires further confirmation, and MEP could still provide relevant information when MIP cannot be measured, even though such situation would rarely occur in clinical practice.
The current observational study has provided new insights into the investigation of expiratory muscle strength in evaluating critical outcomes in critically ill patients by measuring MEP. However, this study did have some limitations. Firstly, the observational design may have induced bias. Furthermore, a convenience sample based on a primary observational study, designed for a 1-year period of inclusion, was used. Secondly, the multivariate logistic regression model power may have been limited by the low number of extubation failures (18/124 patients). Nevertheless, adjustment by principal component analysis was undertaken to account for this low occurrence rate [18]. Thirdly, the use of strategies aimed at avoiding reintubation (i.e. standard oxygen, NIV, mechanical in–exsufflation and chest physiotherapy) were not recorded in the present study. Nonetheless, each of these strategies was available and used whenever needed based on clinicians’ decisions for these patients, regardless of the study protocol. Finally, the MEP measurements were taken using measurement methods which are currently only known to be reliable for non-intubated patients; specific recommendations for intubated patients do not yet exist [13, 16]. Moreover, only maximal expiratory muscle strength data were reported; additional data, such as the quantification of expiratory muscle efforts during MV (e.g. by measuring breathing work or the activation of expiratory muscles using electromyography) or direct measurement of abdominal-wall muscle thickness (via ultrasound), would be necessary to more fully understand the impact of expiratory muscle strength on critical outcomes in mechanically ventilated patients.
Conclusion
Higher MEP values were associated with a lower risk of extubation failure in the univariate logistic regression analyses and also in the multivariate logistic regression analyses if MIP was excluded. MEP was no longer independently associated with extubation failure when MIP was included in the model. This study was not able to differentiate the respective abilities of MEP, MIP, and their combination to discriminate patients with extubation failure or premature death. Both tools can easily be used at patients’ bedsides, but this study did not find convincing evidence that MEP alone, or the combination of both measures, was more relevant than MIP in critically ill patients. Future studies are needed to investigate all aspects of expiratory muscle function and activation during mechanical ventilation to precisely determine the role of expiratory muscles on critical outcomes.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- MV:
-
Mechanical ventilation
- MIP:
-
Maximal inspiratory pressure
- MEP:
-
Maximal expiratory pressure
- ICU:
-
Intensive care unit
- STROBE:
-
Strengthening the reporting of observational studies in epidemiology
- SBT:
-
Spontaneous breathing trial
- ICU-Aw:
-
ICU-acquired weakness
- ROC:
-
Receiver operating characteristics
- AUC:
-
Area under the curve
- BMI:
-
Body mass index
- VIF:
-
Variance inflation factor
- NIV:
-
Non-invasive ventilation
References
Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med. 2008;358(13):1327–35.
Jaber S, Petrof BJ, Jung B, Chanques G, Berthet J-P, Rabuel C, et al. Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am J Respir Crit Care Med. 2011;183(3):364–71.
Demoule A, Jung B, Prodanovic H, Molinari N, Chanques G, Coirault C, et al. Diaphragm dysfunction on admission to the intensive care unit. Prevalence, risk factors, and prognostic impact-a prospective study. Am J Respir Crit Care Med. 2013;188(2):213–9.
Dres M, Dubé B-P, Mayaux J, Delemazure J, Reuter D, Brochard L, et al. Coexistence and impact of limb muscle and diaphragm weakness at time of liberation from mechanical ventilation in medical intensive care unit patients. Am J Respir Crit Care Med. 2017;195(1):57–66.
Jung B, Moury PH, Mahul M, de Jong A, Galia F, Prades A, et al. Diaphragmatic dysfunction in patients with ICU-acquired weakness and its impact on extubation failure. Intensive Care Med. 2016;42(5):853–61.
Medrinal C, Combret Y, Hilfiker R, Prieur G, Aroichane N, Gravier F-E, et al. ICU outcomes can be predicted by non invasive muscle evaluation: a meta-analysis. Eur Respir J. 2020;4:1902482.
Medrinal C, Prieur G, Frenoy É, Robledo Quesada A, Poncet A, Bonnevie T, et al. Respiratory weakness after mechanical ventilation is associated with one-year mortality—a prospective study. Crit Care. 2016;20(1):231.
Shi Z-H, Jonkman A, de Vries H, Jansen D, Ottenheijm C, Girbes A, et al. Expiratory muscle dysfunction in critically ill patients: towards improved understanding. Intensive Care Med. 2019;45(8):1061–71.
Terzi N, Lofaso F, Masson R, Beuret P, Normand H, Dumanowski E, et al. Physiological predictors of respiratory and cough assistance needs after extubation. Ann Intensive Care. 2018;8(1):18.
Lai C-C, Chen C-M, Chiang S-R, Liu W-L, Weng S-F, Sung M-I, et al. Establishing predictors for successfully planned endotracheal extubation. Medicine (Baltimore). 2016;95(41):e4852.
De Jonghe B, Bastuji-Garin S, Durand M-C, Malissin I, Rodrigues P, Cerf C, et al. Respiratory weakness is associated with limb weakness and delayed weaning in critical illness. Crit Care Med. 2007;35(9):2007–15.
Savi A, Teixeira C, Silva JM, Borges LG, Pereira PA, Pinto KB, et al. Weaning predictors do not predict extubation failure in simple-to-wean patients. J Crit Care. 2012;27(2):221.e1-8.
Medrinal C, Prieur G, Combret Y, Quesada AR, Bonnevie T, Gravier FE, et al. Reliability of respiratory pressure measurements in ventilated and non-ventilated patients in ICU: an observational study. Ann Intensive Care. 2018;8(1):14.
Chao C-M, Lai C-C, Cheng A-C, Chiang S-R, Liu W-L, Ho C-H, et al. Establishing failure predictors for the planned extubation of overweight and obese patients. PLoS ONE. 2017;12(8):e0183360.
Boles J-M, Bion J, Connors A, Herridge M, Marsh B, Melot C, et al. Weaning from mechanical ventilation. Eur Respir J. 2007;29(5):1033–56.
Caruso P, Friedrich C, Denari SD, Ruiz SA, Deheinzelin D. The unidirectional valve is the best method to determine maximal inspiratory pressure during weaning. Chest. 1999;115(4):1096–101.
Gelman A. Scaling regression inputs by dividing by two standard deviations. Stat Med. 2008;27(15):2865–73.
Riley RD, Snell KI, Ensor J, Burke DL, Harrell FE Jr, Moons KG, et al. Minimum sample size for developing a multivariable prediction model: PART II - binary and time-to-event outcomes. Stat Med. 2019;38(7):1276–96.
DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–45.
McCool FD. Global physiology and pathophysiology of cough: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 Suppl):48S-53S.
Su W-L, Chen Y-H, Chen C-W, Yang S-H, Su C-L, Perng W-C, et al. Involuntary cough strength and extubation outcomes for patients in an ICU. Chest. 2010;137(4):777–82.
Vivier E, Muller M, Putegnat J-B, Steyer J, Barrau S, Boissier F, et al. Inability of diaphragm ultrasound to predict extubation failure. Chest. 2019;155(6):1131–9.
Petrof BJ, Hussain SN. Ventilator-induced diaphragmatic dysfunction: what have we learned? Curr Opin Crit Care. 2016;22(1):67–72.
Dres M, Goligher EC, Heunks LMA, Brochard LJ. Critical illness-associated diaphragm weakness. Intensive Care Med. 2017;43(10):1441–52.
Acknowledgements
The authors would like to thank the ADIR Association for their support and Johanna Robertson and Jennifer Dandrea Palethorpe for language assistance and constructive criticism.
Funding
No funding was obtained for these secondary analyses.
Author information
Authors and Affiliations
Contributions
YC, GP, OC, TB and CM designed this ancillary study. CM, GP, PS, TB and FG were responsible for patient screening, enrolment, maximal expiratory pressure assessment, and follow-up. RH designed the statistical plan for this manuscript and computed the statistical analysis. YC, RH, GP, BL, OC and CM analysed the data and wrote the manuscript. All authors contributed to interpretation of the data and provided comments on the report at various stages of development. All authors approved this manuscript in its final form.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval was granted by the French Comité de Protection des Personnes Nord-Ouest 3 (A13-D42-VOL.18). All patients or their relatives provided informed consent to participate.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Combret, Y., Prieur, G., Hilfiker, R. et al. The relationship between maximal expiratory pressure values and critical outcomes in mechanically ventilated patients: a post hoc analysis of an observational study. Ann. Intensive Care 11, 8 (2021). https://doi.org/10.1186/s13613-020-00791-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13613-020-00791-4