Abstract
Apis mellifera, crucial pollinators for both native and cultivated plants, also yield various products such as honey, wax, royal jelly, and propolis, extensively utilized in the food, pharmaceuticals, and cosmetics industries. Nosema ceranae, a prevalent microsporidian worldwide, stands as a significant pathogen for A. mellifera, showing resistance to conventional antibiotics. Consequently, the exploration of novel compounds for N. ceranae control becomes imperative. Dithiocarbimate derivatives emerge as promising antifungal candidates under evaluation for combating various pathogens, particularly those affecting plants. This study assessed the toxicity profile of six dithiocarbimate derivatives on A. mellifera worker survival and N. ceranae pathogen. Among these, four compounds exhibited minimal bee mortality and proceeded to further evaluation against N. ceranae. In vitro assays demonstrated their inhibitory effects on spore germination. Remarkably, the most potent compound suppressed N. ceranae spores by 62% at a concentration of 20 µmol L−1in vivo. Thus, these dithiocarbimate derivatives represent promising new antifungal agents for combatting nosemosis in honey bee populations.
Similar content being viewed by others
Introduction
Bees serve as indispensable pollinators in terrestrial ecosystems, offering significant economic benefits to industries and agriculture, with their service valued between US$ 235–577 billion annually (Potts et al. 2016). Despite being crucial agricultural pollinators globally, Apis mellifera and other bee species have faced a substantial decline in populations worldwide (Castilhos et al. 2019; Zattara and Aizen 2021).
The decline in bee populations has been attributed to a multitude of stressors, including environmental pollution, habitat loss, and fragmentation, monoculture farming, climate change, improper management practices, increased pesticide use, as well as parasites and pathogens (Grab et al. 2019; Guimarães-Cestaro et al. 2020; Grant et al. 2021). Notably, among the pathogens, Microsporidia Nosema spp. are a threat to A. mellifera, resulting in mortality and reduced honey production (Burnham 2019; Goblirsch 2018; Epilobee et al. 2016).
Two species of Microsporidia infect A. mellifera: Nosema apis, first reported in 1909 (Zander 1909), and Nosema ceranae, originally found in Apis ceranae (Fries et al. 1996). Currently, N. ceranae is the most widespread species affecting honey bee populations globally (Goulson et al. 2015; Goblirsch 2018).
N. ceranae alters carbohydrate metabolism in infected bees, ensuring nutrient availability for its benefit (Dussaubat et al. 2012). Its presence can lead to colony collapse if it exceeds a critical threshold (Higes et al. 2008; Goblirsch et al. 2013).
Nosemosis has been identified as the primary cause of mortality in 54% of A. mellifera colonies in the USA (Seitz et al. 2016), and it has caused significant colony losses in Spain (Higes et al. 2008) and Bulgaria (Parvanov et al. 2014). In Brazil, N. ceranae is the sole species infecting A. mellifera (Teixeira et al. 2013; Guimarães-Cestaro et al. 2016a; Lage et al. 2022), suggesting its successful adaptation to the tropical climate (Martín-Hernández et al. 2009).
Bees become infected with Nosema spp. by ingesting spores from contaminated food and water, often while cleaning contaminated combs (Higes et al. 2010) or during trophallaxis (Smith 2012). This pathogen induces reduced longevity in adult bees, behavioral alterations, and dysentery, ultimately resulting in individual bee mortality and potential colony collapse (Anderson and Giacon 1992; Kralj and Fuchs 2010).
The treatment of nosemosis in bees has traditionally relied on the antibiotic fumagillin (Williams et al. 2008; Higes et al. 2011). However, its efficacy has waned, particularly against N. ceranae (Huang et al. 2013; Burnham 2019). Moreover, fumagillin’s usage has been banned in the European Union, Chile, Australia, and other regions due to concerns over chromosomal changes and carcinogenicity in humans (European Commission 2009; Botías 2012; van den Heever et al. 2014; Burnham 2019). Consequently, with no alternative antibiotics available for treating nosemosis, the pursuit of new bioactive compounds capable of controlling N. ceranae in A. mellifera becomes imperative.
Dithiocarbamates have long been utilized as broad-spectrum agricultural fungicides (Gullino et al. 2010; Ajiboye et al. 2022). Among them, mancozeb stands out as a widely used fungicide, comprising a zinc and manganese complex with the ethylene bis(dithiocarbamate) ligand, employed to combat various fungal diseases across field crops, fruits, vegetables, and ornamentals (Campanale et al. 2023). However, a significant toxicological concern arises from its environmental decomposition, which releases the thyrotropic agent ethylene thiourea (van Wendel de Joode et al. 2014; Campanale et al. 2023). Furthermore, the literature highlights the potential adverse effects of dithiocarbamates on the survival and flight capacity of A. mellifera and other bee species (Porrini et al. 2003; Gomes et al. 2023).
We have turned our attention to a less-explored group of compounds: dithiocarbimates derived from sulfonamides. Zinc complexes with these ligands, as well as organic allyldithiocarbimates, have shown promise in combating various plant diseases (Alves et al. 2009; Amim et al. 2011; Oliveira et al. 2015; Tavares et al. 2016; Vidigal et al. 2020; Albuini-Oliveira et al. 2020). Specifically, zinc-N-R-sulfonyldithiocarbimates (where R represents alkyl and aryl groups) have demonstrated efficient control of coffee leaf rust disease caused by the fungus Hemileia vastatrix at remarkably low doses in vivo (Rabello et al. 2022). Since certain dithiocarbimate derivatives used in this context did not increase the mortality of pollinators like A. mellifera (Rabello et al. 2022), we deemed it worthwhile to investigate their efficacy against N. ceranae and their potential application in controlling honey bee nosemosis.
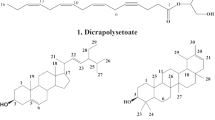
In contrast to dithiocarbamates, which are monoanions (R2N−CS2-1) and create neutral complexes with zinc(II), dithiocarbimates are dianions (RN = CS2-2). Consequently, their zinc(II) complexes (structural formula 1; Fig. 1) can be obtained as salts with various cations. This phenomenon is also observed with allyldithiocarbimates (structural formula 3; Fig. 1).
It has been previously shown that the antifungal efficacy of dithiocarbimate derivatives can be influenced by the type of cation utilized (Oliveira et al. 2015; Rabello et al. 2022), and it also relies on the nature and length of the R group (Vidigal et al. 2020; Rabello et al. 2022). For the current investigation, the butyl chain on the dithiocarbimate group (Fig. 1) and the tetraphenylphosphonium and tetrabutylammonium cations A and B (Fig. 1) were chosen based on their documented positive effects on the antifungal activity of dithiocarbimate derivatives (Oliveira et al. 2015; Albuini-Oliveira et al. 2020; Vidigal et al. 2020; Rabello et al. 2022). Additionally, the nitro-group on the aromatic ring has been shown to enhance the antifungal potency of allyldithiocarbimates against phytopathogenic fungi (Albuini-Oliveira et al. 2020), leading to its incorporation into the selected structure 3 (Fig. 1).
Zinc-dithiocarbimates undergo a reaction with sulfur, resulting in the formation of trithiocarbimates (Castro et al. 2017; Tavares et al. 2012; Oliveira et al. 2007). Given the absence of biological investigations concerning trithiocarbimates, we also explore these sulfur-rich derivatives as potential antifungals against N. ceranae (represented by salts 2A and 2B in Fig. 1).
The objective was to assess the toxicity of six dithiocarbimate-derived compounds on A. mellifera workers, alongside their fungicidal efficacy against the microsporidian pathogen N. ceranae, both in vitro and in vivo. Among these compounds, three are novel and underwent comprehensive characterization through spectroscopic techniques.
Materials and methods
Equipments
Melting points were determined with the MQAPF-302 equipment (Microquímica, Palhoça, Brazil) and are reported without correction. Elemental analyses for C, H, and N were performed using a Leco TruSpec Micro analyzer (St. Joseph, USA). The infrared (IR) spectra were recorded on a Varian 660-IR IR (Palo Alto, USA) equipped with GladiATR (Attenuated Total Reflection) scanning from 4000 to 400 cm−1. The nuclear magnetic resonance (NMR) spectra were obtained at 300 K using a Varian 300 MHz (Palo Alto, USA) or a Brucker Advance DRX 400 MHz (Billerica, USA) from solutions in deuterated chloroform (CDCl3, Sigma-Aldrich, St. Louis, USA), with tetramethyl silane (Sigma-Aldrich, St. Louis, USA) as internal standard. The exact mass of the allylditiocarbimate was determined by high-resolution electrospray ionization mass spectrometry (HRMS-ESI) in acetonitrile solutions by direct infusion method using a Bruker Daltonics MicroTOF-QII-ESI-QQ-TOF mass spectrometer (Billerica, USA).
Reagents and synthetic precursors
The butanesulfonamide was prepared from butanesulfonyl chloride (Sigma, St. Louis, USA), in reaction with concentrated ammonia solution (Vetec, Duque de Caxias, Brazil) under reflux. The potassium N-butylsulfonyldithiocarbimate was prepared from the butanesulfonamide in reaction with carbon disulfide (Vetec, Duque de Caxias, Brazil) and two equivalents of potassium hydroxide (Vetec, Duque de Caxias, Brazil) in dimethylformamide (Vetec, Duque de Caxias, Brazil), and the spectroscopic data were by the literature (Cunha et al. 2010). A Morita-Baylis-Hillman adduct was prepared by the reaction of methyl acrylate (Sigma, St. Louis, USA) with 4-nitrobenzaldehyde (Sigma, St. Louis, USA), catalyzed by trimethylamine (Cai et al. 2002) and was converted into the methyl (Z)-2-(bromomethyl)-3-(4-nitrophenyl) acrylate, after the reaction with lithium bromide (Merck, Darmstadt, Germany) and sulfuric acid (Vetec, Duque de Caxias, Brazil), and the spectroscopic data were by the literature (Ferreira et al. 2009). The solvents (A.C.S. purity) acetone, chloroform, ethyl acetate, methanol and petroleum ether were purchased from F. Maia (Mogi das Cruzes, Brazil). The salts tetraphenylphosphonium chloride and tetrabutylammonium bromide were purchased from Alfa–Aesar (Ward Hill, USA), zinc sulfate from Merck (Darmstadt, Germany), and sulfur from Vetec (Duque de Caxias, Brazil).
Syntheses of the zinc complexes and allyldithiocarbimate salts
The salts used in the biological essays were prepared as shown in Fig. 1.
Zinc complexes
The zinc-dithiocarbimate salts 1A and 1B were prepared by adding 1 mmol of zinc sulfate to the stirring solution of the potassium N-butylsulfonyldithiocarbimate (2 mmol) in 30 mL of methanol:water (1:1 by volume). After 30 min, 2 mmol of the appropriate counterion halide were added: tetraphenylphosphonium chloride (A) or tetrabutylammonium bromide (B). After stirring for one hour, the mixtures were filtered, and the white solids were washed with distilled water (30 mL) and dried in a vacuum desiccator to constant mass.
The syntheses of the zinc-trithiocarbimate salts 2A and 2B were performed by adding elemental sulfur (1 mmol) to solutions of the zinc complexes 1A or 1B (4 mmol) in chloroform (20 mL). The mixtures were stirred for one hour at room temperature, filtered (no observable residue) and the solvent was evaporated under reduced pressure. The oily residues were triturated with 5–10 mL of petroleum ether until loose solids were obtained.
All the zinc complexes were characterized by their melting points, elemental analyses, infrared and NMR spectroscopies.
Allyldithiocarbimate salts
A solution of methyl (Z)-2-(bromomethyl)-3-(4-nitrophenyl)acrylate (1 mmol) in acetone (2 mL) was added dropwise to a stirring solution of potassium N-butylsulfonyldithiocarbimate (1.2 mmol) in acetone:water (1:1 by volume, 10 mL). The mixture was stirred for 15 min (monitored by TLC) at room temperature. Then, water (10 mL) was added and the product was extracted with ethyl acetate (3 × 20 mL). The organic phase was concentrated under reduced pressure and the residue was dissolved in water (10 mL).
For the synthesis of salt 3A, 1 mmol of tetraphenylphosphonium chloride was added to this aqueous solution containing the allyldithiocarbimate anion. After stirring for 5 min at room temperature, the yellow solid thus formed was filtered, washed with distilled water, and dried under reduced pressure for one day.
For the synthesis of salt 3B, 1 mmol of tetrabutylammonium bromide was added to the aqueous solution containing the allyldithiocarbimate anion. The mixture was stirred for 10 min, forming an oily residue stuck to the flask walls. The water phase was discarded and the oily residue was washed with water (3 × 10 mL). The oil was dried under reduced pressure for one day.
The allyldithiocarbimates 3A and 3B were characterized by HRMS, infrared and NMR spectroscopies. The melting point of 3A was in accordance with the literature (Vidigal et al. 2020).
Bees
Brood frames were collected from three A. mellifera colonies located in apiaries situated in Viçosa (20°45´S 42°52´W), in the state of Minas Gerais, Brazil. These frames were maintained at 32 ± 2 °C in darkness to obtain newly-emerged workers devoid of Nosema spp. Upon emergence (< 24 h old), the bees were transferred to 1 L plastic cages in groups of 320 individuals and provided with pollen and honey sourced from the colonies ad libitum for 24 h. Subsequently, the bees were moved to four cages (500 mL each) containing 80 workers per cage. They were fed ad libitum on a 50% aqueous sucrose solution and pollen grains.
Toxicity to bees
The worker bees were confined in cages, each containing 10 individuals, and were provided ad libitum with pollen grains and 50% sucrose solutions supplemented with the salts derived from dithiocarbimates at two concentrations (20 and 100 µmol L-1), along with 0.1% dimethylsulfoxide (DMSO, Merck, Darmstadt, Germany) as a co-solvent to ensure homogeneity. Additionally, toxicity tests were conducted using 0.1% DMSO in 50% sucrose solution as a control, which was compared to the control group fed on only 50% sucrose solution. Worker survival was assessed at 16 h, 24 h, and 48 h post-treatment. Each test was performed in triplicate and repeated twice.
Isolation of N. ceranae spores
To obtain N. ceranae spores, naturally infected colonies of A. mellifera were utilized. Prior confirmation of infection was conducted through the examination of workers using light microscopy, as detailed in previous studies (Guimarães-Cestaro et al. 2016b). In brief, bees were dissected, and their midguts were macerated in 1 mL of distilled water per individual. The resulting macerate was then filtered through cotton wool and centrifuged at 5000 g for 5 min, repeated three times. The pellet was resuspended in distilled water and homogenized using vortexing. Subsequently, an aliquot of the suspension was analyzed using a Neubauer chamber (Assistent, Sondheim, Germany) to count and determine the concentration of spore present in the suspension (Fries et al. 2013).
Bee infection with N. ceranae
Newly-emerged bees, aged up to 24 h, were transferred to cages containing 80 workers each and starved for three hours. Subsequently, the bees were fed for 24 h with 800 µL of a 50% sucrose solution containing isolated spores of N. ceraneae at a concentration of 12,500 spores µL−1. Considering the consumption of 10 µL per bee (Williams et al. 2013), it was estimated that each bee received approximately 125,000 spores within 24 h. Following exposure, all bees, including control, received a spore-free 50% sucrose solution until the conclusion of the experiment.
Antifungal activity in vitro
N. ceranae spores, obtained as described, were exposed in five replicates to each dithiocarbimate derivative with low toxicity for bees, as estimated previously, for 1 h at room temperature. The spore suspensions were then incubated with 10 µg mL−1 of 4′,6-diamidino-2-phenylindole (DAPI, Merk, Darmstadt, Germany) for 30 min. DAPI is a fluorescent dye that penetrates only non-viable spores. After incubation, the spores were washed twice in distilled water, centrifuged at 5000 g for 5 min, and resuspended for counting in a Neubauer chamber using a fluorescence microscope. For each evaluation, the spores were first counted under fluorescence and then in a bright field microscope. Spores stained with DAPI were considered non-viable (McGowan et al. 2016).
Antifungal activity in vivo
The dithiocarbimate derivatives, previously determined to have low toxicity for bees, were dissolved in 50% sucrose solution and provided ad libitum for feeding the 20 infected bees. Seven days after feeding, 10 bees were dissected, and their midguts were used to obtain and count the viable spores.
Statistical analysis
The data on viable spores in vitro and the quantity of spores in the midgut of A. mellifera from the control and treated groups were submitted to one-way ANOVA. The results were compared post-hoc with the Tukey test at 5% significance, using the SISVAR software version 5.6 (Ferreira 2011).
Results
Syntheses
The syntheses performed as shown in Fig. 1 were caried out in good yields: 93% for 1A and 85% for 1B, 89% for 2A, 82% for 2B, 80% for 3A and 81% for 3B. The salts 1A, 1B, and 3A have been published and their spectroscopic data were in accordance with the literature (Cunha et al. 2012; Rabello et al. 2022; Vidigal et al. 2020).
The compounds 2A, 2B and 3B are new substances and their characterization data are as follows:
Tetraphenylphosphonium bis (butylsulphonyltrithiocarbimato)zincato(II), (2A): m.p. 61.6–62.8 °C; Found (Calcd.) for C58H58N2O4P2S8Zn: C, 57.95 (56.59); H, 4.78 (4.75); N, 2.27 (2.28); IR (selected bands, ATR) ν/cm−1 1382 (νCN), 1264 (νasSO2), 1105 (νsymSO2), 925 (νCS3); 1H NMR (300 MHz, CDCl3) δ 0.81 (t, J = 6 Hz, 6 H, H4), 1.24–1.38 (m, 4 H, H3), 1.70–1.82 (m, 4 H, H2), 3.11–3.21 (m, 4 H, H1), 7.59–7.68 (m, 16 H, Hb, Hf), 7.71–7.80 (m, 16 H, Hc, He), 7.81 − 7.89 (m, 8 H, Hd); 13C NMR (75 MHz, CDCl3) δ 13.9 (C4), 22.0 (C3), 25.7 (C2), 51.6 (C1); 117.6 (d, J = 90.0 Hz, Ca), 130.8 (d, J = 7.5 Hz, Cb e Cf), 134.6 (d, J = 7.5 Hz, Cc e Ce), 135.7 (d, J = 3.0 Hz, Cd), 208.1 (C=N).
Tetrabutylammonium bis(butylsulphonyltrithiocarbimato)zincato(II), (2B): m.p. 80.2–81.5 °C; Found (Calcd.) for C42H90N4O4S8Zn: C, 47.95 (48.64); H, 9.14 (8.75); N, 5.49 (5.40); IR (selected bands, ATR) ν/cm−1 1391 (νCN), 1265 (νasSO2), 1111 (νsymSO2), 935 (νCS3); 1H NMR (300 MHz, CDCl3) δ 0.91 (t, J = 6.0 Hz, 6 H, H4 ), 0.99 (t, J = 6.0 Hz, 24 H, Hd), 1.40–1.47 (m, 20 H, H3, Hc), 1.63–1.80 (m, 20 H, H2, Hb), 3.22–3.70 (m, 24 H, H1, Ha); 13C NMR (75 MHz, CDCl3) δ 14.0 (C4, Cd), 20.0 (Cc), 22.0 (C3), 24.2 (Cb), 25.8 (C2), 52.2 (C1), 58.9 (Ca), 208.4 (C=N).
Tetrabutylammonium (Z)-2-(methoxycarbonyl)-3‑(4‑nitrophenyl)allyl-(N-butylsulfonyl)dithiocarbimate (3B): HRMS-ESI m/z, calcd. for C16H19N2O6S3−: 431.0411, found: 431.0355; IR (selected bands, ATR) ν/cm−1 1714 (νC= O), 1520 (νasNO2), 1382 (νCN), 1342 (νsymNO2), 1261 (νas SO2), 1149 (νSO2), 937 (νas CS2); 1H NMR (400 MHz, CDCl3) δ 0.91 (t, J = 7.4 Hz, 3H, H4),1.00 (t, J = 7.3 Hz, 12H, Hd), 1.40–1.50 (m, 10H, H3, Hc), 1.62–1.70 (m, 8H, Hb), 1.77–1.81 (m, 2H, H2), 3.25–3.29 (m, 8H, Ha), 3.50 (t, J = 8.0 Hz, 2H, H1), 3.83 (s, 3H, OCH3), 4.21 (s, 2H, H1’), 7.21 (s, 1H, H3’), 7.77 (d, J = 8.8 Hz, 2 H, H5’, H9’), 8.25 (d, J = 8.7 Hz, 2 H, H6’, H8’); 13C NMR (100 MHz, CDCl3) δ 13.7 (Cd, C4), 19.8 (Cc), 21.9 (C3), 24.0 (Cb), 25.7 (C2), 33.1 (C1’), 51.2 (C1), 52.4 (OCH3), 58.9 (Ca), 123.8 (C6’, C8’), 130.8 (C3’), 131.2 (C2’), 138.1 (C5’, C9’), 141.4 (C4’), 147.5 (C7’), 167.4 (C = O), 199.7 (C=N).
Dithiocarbimate salts and toxicity to bees and N. ceranae
Exposure of A. mellifera workers to dithiocarbimate-derived salts aimed to assess potential toxic effects. The toxicity results varied among the compounds (F = 117.81; p < 0.01; Table 1). Treatments containing salts 1B (20 µmol L−1), 2B (20 µmol L−1), 3A (20 µmol L−1), and 3B (20 µmol L−1) exhibited mortality rates similar to the control group. The predominance of safer salts featuring the tetrabutylammonium cation (B) suggests that the tetraphenylphosphonium cation (A) might contribute to the observed toxicity in other treatments (Table 1). Subsequent tests focused solely on compounds resulting in up to 5% mortality among A. mellifera worker bees.
In vitro exposure of N. ceranae spores to the four selected compounds resulted in a decrease in viability rates, with counts dropping from 12.37 ± 0.74 × 106 viable spores in the control to 11.10 ± 0.47 × 106 with 3A, 8.39 ± 0.40 × 106 with 1B, 6.96 ± 0.28 × 106 with 2B, and 7.56 ± 0.38 × 106 with 3B (F = 419.950; p < 0.001; Fig. 2).
Number (mean ± sd) of viable Nosema ceranae spores after exposure in vitro to compounds derived from dithiocarbimates and control group. Different letters above bars indicate significant difference among compounds based on Tukey test at 5% of significance level obtained from five independent biological replications
The in vitro antifungal activity of salts 1B, 2B, 3B, and 3A demonstrated reductions in N. ceranae spore viability ranging from 10 to 44%, with the most significant improvements observed in treatments containing salts with the tetrabutylammonium cation (B). Additionally, variations in spore viability were evident based on the type of dithiocarbimate derivative used, as treatments 1B, 2B, and 3B exhibited distinct reductions in N. ceranae spore viability by 32%, 44%, and 39%, respectively.
The in vivo antifungal activity was assessed by quantifying the spore quantities in the midgut of infected honey bees fed on the four compounds. Results revealed significant differences among treatments on the eighth-day post-inoculation (F = 30.688; p < 0.001), ranging from 17.66 ± 0.46 × 106 spores per midgut in the control to 9.01 ± 0.46 × 106 with 2B, 6.65 ± 0.30 × 106 with 1B, 6.56 ± 0.42 × 106 with 3B, and 16.63 ± 0.39 × 106 with 3A (Fig. 3).
Number (mean ± sd) of Nosema ceranae spores in the midgut of Apis mellifera eight days post-inoculation, after treatments in vivo with compounds derived from dithiocarbimate. Different letters above bars indicate significant difference among compounds based on Tukey test at 5% of significance level obtained from 10 independent biological replications
The in vivo test confirmed the efficacy of salts 1B, 2B, and 3B, resulting in a reduction ranging from 49 to 63% in the concentration of N. ceranae spores in the midgut of A. mellifera workers. Particularly noteworthy were the findings of treatments with salts 1B (62% inhibition) and 3B (63% inhibition), demonstrating promising antifungal activity against this Microsporidia.
Discussion
The zinc-dithiocarbimate anions (1) form stable salts with the cations A and B (Fig. 1) (Cunha et al. 2012; Rabello et al. 2022). While these substances are white solids, the zinc-trithiocarbimate salts 2A and 2B are yellow. Although analogous zinc complexes have been prepared with other trithiocarbimates (Tavares et al. 2012; Castro et al. 2017), the salts 2A and 2B are new substances. Their formulae were supported by elemental analyses, which also confirmed their purity.
In addition to the color difference, the zinc-trithiocarbimate salts 2A and 2B can be distinguished by their lower melting points compared to the parental complexes 1A and 1B. For instance, while the salt 2B melts at 80.2–81.5 °C, the corresponding zinc-dithiocarbimate salt 1B exhibits an onset melting at 122 °C (Rabello et al. 2022).
The integration curves observed in the 1H NMR spectra of salts 2A and 2B were consistent with a 2:1 ratio between the tetraphenylphosphonium or tetrabutylammonium cations and the zinc-trithiocarbimate anion. The signals corresponding to the trithiocarbimate moiety in both the 1H and 13C NMR spectra of the complexes exhibited chemical shifts similar to those observed in the parental zinc-dithiocarbimates (Rabello et al. 2022). In the 13C NMR spectra of 2A and 2B, most signals showed slight upfield shifts compared to the spectra of the parent complexes. For instance, signals attributed to the butyl group in complex 1B, observed around 13, 21, 25, and 51 ppm (Rabello et al. 2022), shifted to approximately 14, 22, 26, and 52 ppm in the spectrum of salt 2B.
The infrared data obtained for the salts 2A and 2B are also similar to those observed for the parental compounds 1A and 1B (Cunha et al. 2012; Rabello et al. 2022), though slight shifts are observed for each band. For example, while the C= N stretching band appears at 1385 cm−1 in the spectrum of 1B (Rabello et al. 2022) the value for compound 2B is 1391 cm−1.
The allyldithiocarbimate anion of 3A and 3B has been previously prepared and isolated as a tetraphenylphosphonium salt, as 3A (Vidigal et al. 2020). The spectroscopic data of the new salt of tetrabutylammonium (salt 3B) are very similar to those of 3A, concerning the signals and bands due to the allyldithiocarbimate anion. Any disparities noted between the spectra of 3A and 3B solely due to the distinct cations A and B. Nevertheless, the two substances are easily recognizable by their melting points. While the salt 3A melts at 120.7−122.6 oC, 3B is a viscous oil at room temperature.
The molecular formula of salt 3B was confirmed via HRMS, which exhibited the expected peak corresponding to the tetrabutylammonium ion in the positive mode, alongside the molecular ion peak of the allyldithiocarbimate in the negative mode. Additionally, the integration curves observed in the 1H NMR spectrum of salt 3B were consistent with a 1:1 ratio between the allyldithiocarbimate anion and the tetrabutylammonium cation.
It is worth noting the chemical shift of the C = N signal, which transitions from 223.6 ppm in the spectrum of potassium N-butylsulfonildithiocarbimate (Cunha et al. 2010) to approximately 208 ppm in the spectra of zinc complexes 1A, 1B, 2A, and 2B, and to ca. 200 ppm in the spectra of allyldithiocarbimates 3A and 3B. This shift suggests an increased shielding effect on this carbon atom (Rabello et al. 2022; Vidigal et al. 2020). This observation aligns with the IR data, which indicates a greater double bond character of the C = N bond in the allyldithiocarbimates and the complexes compared to the free dithiocarbimate ligand. The stretching of the C = N bond gives rise to a band at 1283 cm−1 in the IR spectrum of potassium N-butyldithiocarbimate (Cunha et al. 2010), with corresponding bands observed at approximately 1390 cm−1 for derivatives 1A, 1B, 2A, 2B, 3A, and 3B (Rabello et al. 2022; Vidigal et al. 2020).
Currently, the antibiotic fumigallin is the main chemical used to control nosemosis in vivo, but it has been reported to have low efficacy against N. ceranae in some apiaries (Huang et al. 2013; Burnham 2019). So, new compounds have been evaluated to control this Microsporidia, including the natural compounds resveratrol and thymol (Maistrello et al. 2008; Glavinic et al. 2022), formic acid (Underwood and Currie 2009), the phyto-pharmacological preparation Novezit (Higes et al. 2014), several plant extracts (Chaimanee et al. 2021), and the synthetic compounds fenbendazole and ornidazole (Bahreini et al. 2022), which have been showed some potential to control but almost always in high doses and with lower efficacy than fumigallin. Whereas the compound here evaluated showed a mortality to N. ceranae of ca. 40% in vitro and 60% in vivo.
The superior reduction of N. ceranae spores observed in vivo compared to the in vitro test suggests the involvement of at least two modes of action into the insect body treated with the dithiocarbimate derivatives. While the precise mode of action of these compounds on Microsporidia remains unexplored, similar compounds like dithiocarbamates have been shown to act through oxidative mechanisms on the amino acid cysteine, abundant in proteins from the polar tube of Nosema bombycis infecting silkworms (Dias et al. 2010; Lv et al. 2020). However, further studies are necessary to elucidate the specific modes of action of the compounds here evaluated.
This study sheds light on potential applications and ecological responses of salts derived from dithiocarbimates, revealing that their toxicity towards A. mellifera worker bees can vary depending on the specific derivative. Notably, the tetrabutylammonium cation (B) emerged as the preferable option over the tetraphenylphosphonium cation (A), particularly in terms of reduced toxicity to bees. Overall, the four tetrabutylammonium salts investigated in this study exhibit potential in controlling N. ceranae spores, demonstrated by the decrease of spore viability in vitro and spore quantities in the midgut of infected A. mellifera workers.
Data availability
All data generated in this study are described in the published article. Raw data may be provided upon reasonable request from the corresponding authors.
References
Ajiboye TO, Ajiboye TT, Marzouki R, Onwudiwe DC (2022) The versatility in the applications of dithiocarbamates. Int J Mol Sci 23:1317–1353. https://doi.org/10.3390/ijms23031317
Albuini-Oliveira NM, Rubinger MMM, Guilardi S, Souza RAC, Ellena J, Alvarez N, Tavares EC, Zacchi CHC, Vidigal AEC, Lima MS, Zambolim L (2020) New allyldithiocarbimate salts: synthesis, structure and antifungal activity. J Mol Struct 1214:128149. https://doi.org/10.1016/j.molstruc.2020.128149
Alves LC, Rubinger MM, Lindemann RH, Perpétuo GJ, Janczak J, Miranda LD, Zambolim L, Oliveira MR (2009) Syntheses, crystal structure, spectroscopic characterization and antifungal activity of new N-R-sulfonyldithiocarbimate metal complexes. J Inorg Biochem 103:1045–1053. https://doi.org/10.1016/j.jinorgbio.2009.04.018
Amim RS, Oliveira MRL, Janczak J, Rubinger MMM, Vieira LMM, Alves LC, Zambolim L (2011) Syntheses, characterization, crystal structure and antifungal activity of four tetraphenylphosphonium bis(N-R-sulfonyldithiocarbimato)zincate(II) salts. Polyhedron 30:683–689. https://doi.org/10.1016/j.poly.2010.12.003
Anderson DL, Giacon H (1992) Reduced pollen collection by honey bee (Hymenoptera: Apidae) colonies infected with Nosema apis and Sacbrood virus. J Econ Ent 85:47–51
Bahreini R, Nasr M, Docherty C, de Herdt O, Feindel D, Muirhead S (2022) In vivo inhibitory assessment of potential antifungal agents on Nosema ceranae proliferation in honey bees. Pathogens 11:1375. https://doi.org/10.3390/pathogens11111375
Botías C, Martín-Hernández R, Barrios L, Garrido‐Bailón E, Nanetti A, Meana A, Higes M (2012) Nosema spp. parasitization decreases the effectiveness of acaricide strips (Apivar®) in treating varroosis of honey bee (Apis mellifera iberiensis) colonies. Environ Microbiol Rep 4:57–65. https://doi.org/10.1111/j.1758-2229.2011.00299.x
Burnham AJ (2019) Scientific advances in controlling Nosema ceranae (Microsporidia) infections in honey bees (Apis mellifera). Front Vet Sci 6:79. https://doi.org/10.3389/fvets.2019.00079
Cai JX, Zhou ZH, Zhao GF, Tang CC (2002) Dramatic rate acceleration of the Baylis-Hillman reaction in homogeneous medium in the presence of water. Org Lett 4:4723–4725. https://doi.org/10.1021/ol027197f
Campanale C, Triozzi M, Ragonese A, Losacco D, Massarelli C (2023) Dithiocarbamates: Properties, methodological approaches and challenges to their control. Toxics 11:851. https://doi.org/10.3390/toxics11100851
Castilhos D, Bergamo GC, Gramacho KP, Gonçalves LS (2019) Bee colony losses in Brazil: a 5-year online survey. Apidologie 50:263–272. https://doi.org/10.1007/s13592-019-00642-7
Castro RA, Oliveira MRL, Janczack J, Rubinger MMM (2017) Syntheses and characterization of novel heteroleptic nickel complexes with dithiocarbimates and trithiocarbimates. Inorg Chim Acta 462:195–203. https://doi.org/10.1016/j.ica.2017.03.028
Chaimanee V, Kasem A, Nuanjohn T, Boonmee T, Siangsuepchart A, Malaithong W, Sinpoo C, Disayathanoowat T, Pettis JS (2021) Natural extracts as potential control agents for Nosema ceranae infection in honeybees, Apis mellifera. J Invert Pathol 186:107688. https://doi.org/10.1016/j.jip.2021.107688
Cunha LMG, Rubinger MMM, Sabino JR, Visconte LLY, Oliveira MRL (2010) Syntheses, crystal structure and spectroscopic characterization of bis(dithiocarbimate)-nickel(II)-complexes: a new class of vulcanization accelerators. Polyhedron 29:2278–2282. https://doi.org/10.1016/j.poly.2010.04.026
Cunha LMG, Rubinger MMM, Oliveira MRL, Tavares EC, Sabino, JR, Pacheco EBAV, Visconte LLY (2012) Syntheses, crystal structure and spectroscopic characterization of bis(dithiocarbimato)-zinc(II)-complexes: a new class of vulcanization accelerators. Inorganica Chimica Acta 383:194-198. https://doi.org/10.1016/j.ica.2011.11.002
Dias PJ, Teixeira MC, Telo JP, Sa-Correia I (2010) Insights into the mechanisms of toxicity and tolerance to the agricultural fungicide mancozeb in yeast, as suggested by a chemogenomic approach. OMICS 14:211–227. https://doi.org/10.1089/omi.2009.0134
Dussaubat C, Brunet JL, Higes M, Colbourne JK, Lopez J, Choi JH, Martín-Hernández R, Botías C, Cousin M, McDonnell C, Bonnet M, Belzunces LP, Moritz RFA, Conte YL, Alaux C (2012) Gut pathology and responses to the microsporidium Nosema ceranae in the honey bee Apis mellifera. PLoS ONE 7:e37017. https://doi.org/10.1371/journal.pone.0037017
EPILOBEE Consortium, Chauzat MP, Jacques A, Laurent M, Bougeard S, Hendrikx P, Ribière-Chabert M (2016) Risk indicators affecting honeybee colony survival in Europe: one year of surveillance. Apidologie 47:348–378. https://doi.org/10.1007/s13592-016-0440-z
European Commission (2009) Commission Regulation No 470/2009 of the European Parliament and of the Council of 6 May 2009 laying down Community procedures for the establishment of residue limits of pharmacologically active substances in foodstuffs of animal origin, repealing Council Regulation (EEC) No 2377/90 and amending Directive 2001/82/EC of the European Parliament and of the Council and Regulation (EC) No 726/2004 of the European Parliament and of the Council. Official Journal of the European Union, L152/11L152/22
Ferreira DF (2011) Sisvar: a computer statistical analysis system. Ciênc Agrotecnol 35:1039–1042
Ferreira M, Fernandes L, Sá MM (2009) A highly efficient and general method for the preparation of (Z)-allylic bromides derived from Morita–Baylis–Hillman adducts. J Braz Chem Soc 20:564–568
Fries I, Feng F, Da Silva A, Slemenda SB, Pieniazek NJ (1996) Nosema ceranae n sp (Microspora, Nosematidae), morphological and molecular characterization of a Microsporidia parasite of the Asian honey bee Apis cerana (Hymenoptera, Apidae). Eur J Protistol 32:356–365. https://doi.org/10.1016/S0932-4739(96)80059-9
Fries I, Chauzat MP, Chen YP, Doublet V, Genersch E, Gisder S, Higes M, McMahon DP, Martín-Hernandez R, Natsopoulou M, Paxton RJ, Tanner G, Webster TC, Williams GR (2013) Standard methods for Nosema research. J Apicult Res 52:1–28. https://doi.org/10.3896/IBRA.1.52.1.14
Glavinic U, Blagojevic J, Ristanic M, Stevanovic J, Lakic N, Mirilovic M, Stanimirovic Z (2022) Use of thymol in Nosema ceranae control and health improvement of infected honey bees. Insects 13:574. https://doi.org/10.3390/insects13070574
Goblirsch M (2018) Nosema ceranae disease of the honey bee (Apis mellifera). Apidologie 49:131–150. https://doi.org/10.1007/s13592-017-0535-1
Goblirsch M, Huang ZY, Spivak M (2013) Physiological and behavioral changes in honey bees (Apis mellifera) induced by Nosema ceranae infection. PLoS ONE 8:e58165. https://doi.org/10.1371/journal.pone.0058165
Gomes IN, Gontijo LM, Lima MAP, Zanuncio JS, Resende HC (2023) The survival and flight capacity of commercial honeybees and endangered stingless bees are impaired by common agrochemicals. Ecotoxicology 32:937–947. https://doi.org/10.1007/s10646-023-02699-8
Goulson D, Nicholls E, Botías C, Rotheray EL (2015) Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347:1255957. https://doi.org/10.1126/science.1255957
Grab H, Branstetter MG, Amon N, Urban-Mead KR, Park MG, Gibbs J, Blitzer EJ, Poveda K, Loeb G, Danforth BN (2019) Agriculturally dominated landscapes reduce bee phylogenetic diversity and pollination services. Science 363:282–284. https://doi.org/10.1126/science.aat6016
Grant KJ, DeVetter L, Melathopoulos A (2021) Honey bee (Apis mellifera) colony strength and its effects on pollination and yield in highbush blueberries (Vaccinium corymbosum). PeerJ 9:e11634. https://doi.org/10.7717/peerj.11634
Guimarães-Cestaro L, Serrão JE, Alves MLTM, Teixeira EW (2016a) A scientific note on occurrence of pathogens in colonies of honey bee Apis mellifera in Vale do Ribeira, Brazil. Apidologie 48:384–386. https://doi.org/10.1007/s13592-016-0481-3
Guimarães-Cestaro L, Serrão JE, Message D, Matins MF, Teixeira EW (2016b) Simultaneous detection of Nosema spp., Ascosphaera apis and paenibacillus larvae in honey bee products. J Hymenopt Res 49:43–50. https://doi.org/10.3897/JHR.49.7061
Guimarães-Cestaro L, Martins MF, Martínez LC, Alves MLTMF, Guidugli-Lazzarini KR, Nocelli RCF, Malaspina O, Serrão JE, Teixeira EW (2020) Occurrence of virus, microsporidia, and pesticide residues in three species of stingless bees (Apidae: Meliponini) in the field. Sci Nat 107:1–14. https://doi.org/10.1007/s00114-020-1670-5
Gullino ML, Tinivella F, Garubaldi A, Kemmitt GM, Bacci L, Sheppard B (2010) Mancozeb: past, present, and future. Plant Dis 94:1076–1087. https://doi.org/10.1094/PDIS-94-9-1076
Higes M, Martín-Hernández R, Botías C, Bailón EG, González-Porto AV, Barrios L, Del Nozal MJ, Bernal JL, Jiménez JJ, Palencia PG, Meana A (2008) How natural infection by Nosema ceranae causes honeybee colony collapse. Environ Microbiol 10:2659–2669. https://doi.org/10.1111/j.1462-2920.2008.01687.x
Higes M, Martın-Hernández R, Meana A (2010) Nosema ceranae in Europe: an emergent type C nosemosis. Apidologie 41:375–392. https://doi.org/10.1051/apido2010019
Higes M, Nozal MJ, Alvaro A, Barrios L, Meana A, Martín-Hernandez R, Bernal J (2011) The stability and effectiveness of fumagillin in controlling Nosema ceranae (Microsporidia) infection in honey bees (Apis mellifera) under laboratory and field conditions. Apidologie 42:364–377. https://doi.org/10.1007/s13592-011-0003-2
Higes M, Gómez-Moracho T, Rodriguez-García C, Botias C, Martin-Hernández R (2014) Preliminary effect of an experimental treatment with Nozevit®, (a phyto-pharmacological preparation) for Nosema ceranae control. J Apicult Res 53:472–474. https://doi.org/10.3896/ibra.1.53.4.03
Huang WF, Solter LF, Yau PM, Imai BS (2013) Nosema ceranae escapes fumagillin control in honey bees. PLoS Pathog 9:e1003185. https://doi.org/10.1371/journal.ppat.1003185
Kralj J, Fuchs S (2010) Nosema sp. influences flight behavior of infected honey bee (Apis mellifera) foragers. Apidologie 41:21–28. https://doi.org/10.1051/apido/2009046
Lage VMGB, Santana CD, Patrocínio E, Noronha RP, Melo RL, Barbosa CJ, Lima STC (2022) Prevalence of Nosema ceranae in apiculture regions of Bahia State, Brazil. Ciênc Rural 52:e20210473. https://doi.org/10.1590/0103-8478cr20210473
Lv Q, Wang L, Fan Y, Meng X, Liu K, Zhou B, Chen J, Pan G, Zhou Z (2020) Identification and characterization a novel polar tube protein (NbPTP6) from the microsporidian Nosema bombycis. Parasit Vectors 13:1–9. https://doi.org/10.1186/s13071-020-04348-z
Maistrello L, Lodesani M, Costa C, Leonardi F, Marani G, Caldon M, Mutinelli F, Granato A (2008) Screening of natural compounds for the control of nosema disease in honeybees (Apis mellifera). Apidologie 39:436–445. https://doi.org/10.1051/apido:2008022
Martín-Hernandez R, Meana A, García-Palencia P, Marín P, Botías C, Garrido-Bailon E, Barrios L, Higes M (2009) Effect of temperature on the biotic potential of honeybee microsporidia. Appl Environ Microbiol 75:2554–2557. https://doi.org/10.1128/AEM.02908-08
McGowan J, De la Mora A, Goodwin PH, Habash M, Hamiduzzaman MM, Kelly PG, Guzman-Novoa E (2016) Viability and infectivity of fresh and cryopreserved Nosema ceranae spores. J Microbiol Methods 131:16–22. https://doi.org/10.1016/j.mimet.2016.09.021
Oliveira AA, Oliveira MR, Rubinger MMM, Perpétuo GJ, Janczack J (2007) Synthesis, structural and spectroscopic characterization of novel zinc(II) complexes with N-methylsulfonyldithiocarbimato and N-methylsulfonyltrithiocarbimato ligands. Polyhedron 26:163–168. https://doi.org/10.1016/j.poly.2006.08.002
Oliveira AA, Oliveira MR, Rubinger MMM, Piló EL, Menezes DC, Zambolim L (2015) Bis(4-fluorophenylsulfonyldithiocarbimato)zincate(II) salts: New antifungals for the control of Botrytis blight. Quim Nova 38:57–761
Parvanov P, Rusenova N (2014) Etiology and clinic epidemiological profile of apiaries with colony collapse disorder-like symptoms in Bulgaria. Bulg J Vet Med 17:199–206
Porrini C, Sabatini AG, Girotti S, Fini F, Monaco L, Celli G, Bortolotti L, Ghini S (2003) The death of honey bees and environmental pollution by pesticides: the honey bees as biological indicators. Bull Insectol 56:147–152
Potts SG, Imperatriz-Fonseca VL, Ngo HH, Aizen MA, Biesmeijer JC, Breeze TD, Dicks LV, Garibaildi LA, Hill R, Settele J, Vanbergen AJ (2016) Safe guarding pollinators and their values to human well-being. Nature 540:220–229. https://doi.org/10.1038/nature20588
Rabello AS, Rubinger MMM, Silva LF, Oliveira AH, Serrão JE, Albuini-Oliveira NM, Tavares EC, Vidigal AEC, Oliveira MRL, Zambolim L, Souza RAC, Guilardi S, Ellena J (2022) Zinc-dithiocarbimates for the control of Hemileia vastatrix: a versatile alternative. Pest Manag Sci 78:4741–5752. https://doi.org/10.1002/ps.7094
Seitz N, Traynor KS, Steinhauer N, Rennich K, Wilson ME, Ellis JD, Rose R, Tarpy DR, Sagili RR, Caron DM, Delaplane KS, Rangel J, Lee K, Baylis K, Wilkes JT, Skinner JA, Pettis JS, van Engelsdorp D (2016) A national survey of managed honey bee 2014–2015 annual colony losses in the USA. J Apicult Res 54:292–304. https://doi.org/10.1080/00218839.2016.1153294
Smith ML (2012) The honey bee parasite Nosema ceranae: transmissible via food exchange? PLoS ONE 7:e43319. https://doi.org/10.1371/journal.pone.0043319
Tavares EC, Oliveira MRL, Janczack J, Vieira CG, Alves LC, Castro RA, Vieira L, Lindemann RH, Perpétuo GJ, Visconte LLY, Rubinger MMM (2012) Syntheses, structural and spectroscopic characterization of novel zinc(II)-bis(trithiocarbimato) complexes and bis(N-methylsulfonyldithiocarbimate)-sulfide. Polyhedron 31:494–501. https://doi.org/10.1016/j.poly.2011.10.004
Tavares EC, Rubinger MMM, Venturini E, Oliveira MRL, Piló-Veloso D, Ellena J, Guilardi S, Souza RAC, Zambolim L (2016) Tetraphenylphosphonium allyldithiocarbimates derived from Morita-Baylis-Hillman adducts: synthesis, characterization, crystal structure and antifungal activity. J Mol Struct 1106:130–140. https://doi.org/10.1016/j.molstruc.2015.10.097
Teixeira EW, Santos LG, Sattler A, Message D, Alves ML, Martins MF, Grassi-Sella ML, Francoy TM (2013) Nosema ceranae has been present in Brazil for more than three decades infecting africanized honey bees. J Invertebr Pathol 114:250–254. https://doi.org/10.1016/j.jip.2013.09.002
Underwood RM, Currie RW (2009) Indoor winter fumigation with formic acid for control of Acarapis Woodi (Acari: Tarsonemidae) and nosema disease, Nosema Sp. J Econ Ent 102:1729–1736. https://doi.org/10.1603/029.102.0501
van den Heever JP, Thompson TS, Curtis JM, Ibrahim A, Pernal SF (2014) Fumagillin: an overview of recent scientific advances and their significance for apiculture. J Agric Food Chem 62:2728–2737. https://doi.org/10.1021/jf4055374
van Wendel de Joode B, Mora AM, Córdoba L, Cano JC, Quesada R, Faniband M, Wesseling C, Ruepert C, Öberg M, Eskenazi B, Mergler D, Lindh CH (2014) Aerial application of mancozeb and urinary ethylene thiourea (ETU) concentrations among pregnant women in Costa Rica: the infants’ Environmental Health Study (ISA). Environ Health Perspect 122:1321–1328. https://doi.org/10.1289/ehp.1307679
Vidigal AEC, Rubinger MMM, Da Silva LF, Zambolim L, Pereira ABD, Guilardi S, Souza RAC, Ellena J (2020) New allyldithiocarbimates: synthesis, structure and antifungal activity against Phakopsora pachyrhizi and hemileia vastatrix. J Braz Chem Soc 31:703–715. https://doi.org/10.21577/0103-5053.20190234
Williams GR, Sampson MA, Shutler D, Rogers REL (2008) Does fumagillin control the recently detected invasive parasite Nosema ceranae in western honey bees (Apis mellifera)? J Invertebr Pathol 99:342–344. https://doi.org/10.1016/j.jip.2008.04.005
Williams GR, Alaux C, Costa C, Csaki T, Doublet V, Eisenhardt D, Fries I, Kuhn R, McMahon DP, Medrzycki P, Murray TE, Natsopoulou ME, Neumann P, Oliver R, Paxton RJ, Pernal SF, Shutler D, Tanner G, van der Steen JJM, Brodschneider R (2013) Standard methods for maintaining adult Apis mellifera in cages under in vitro laboratory conditions. J Apicult Res 52:52104. https://doi.org/10.3896/IBRA.1.52.1.04
Zander E (1909) Tierische Parasiten als Krankenheitserreger Bei Der Biene. Münchener Bienen 31:96–204
Zattara EE, Aizen MA (2021) Worldwide occurrence records suggest a global decline in bee species richness. One Earth 4:114–123. https://doi.org/10.1016/j.oneear.2020.12.005
Acknowledgements
We thank the Núcleo de Análise de Biomoléculas of the Universidade Federal de Viçosa for providing the facilities for HRMS experiments.
Funding
This study was supported by Brazilian research agencies, CAPES (code 001) FAPEMIG (APQ-02382-17, APQ-02367-18), and CNPq (303243/2022-8).
Author information
Authors and Affiliations
Contributions
AHO, MMMR and JES: conceptualization, investigation, validation, writing—original draft, writing - review and editing. AHO, ASR, AECV and ECT: methodology, investigation, Writing—review and editing. MRLO: conceptualization, methodology, funding acquisition, Writing—review and editing. JES, MMMR: project administration, supervision, data curation, funding acquisition. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This article does not involve any studies with human participants or animals
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Oliveira, A.H., Rubinger, M.M.M., da Silva Rabello, A. et al. Action of dithiocarbimates salts on the honey bee and its pathogen Nosema ceranae. AMB Expr 14, 82 (2024). https://doi.org/10.1186/s13568-024-01734-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13568-024-01734-z