Abstract
One of Egypt’s most notable and historically significant vegetable crops is the Liliaceae plant, Allium cepa L. In this study, the effectiveness of methanolic extracts of Artemisia absinthium leaves, Calotropis procera latex, Moringa oleifera seeds, and Syzygium aromaticum clove was investigated in vitro and, in a greenhouse, setting against Fusarium oxysporum, the pathogen that causes onion basal rot in Assiut Governorate, Egypt. The S. aromaticum extract exhibited the inhibition peak (63.3%), whereas the A. absinthium extract had the lowest inhibition impact against F. oxysporum growth (41.1%). The gas chromatography-mass spectroscopy (GC–MS) analysis revealed that 82 important compounds, with abundances ranging from low to high, were present in the tested S. aromaticum’s methanolic extract. The primary components were acetaldehyde, hydroxy- and 2-propanone, 1,1,3,3-tetrachloro-(42.71%), 1,2-ethanediol, and methyl alcohol (34.01%). In comparison to the infected control, the disease severity was significantly reduced by 20% with the use of a plant extracts mixture and Dovex 50% and increased by 62.22% with the use of an extract from A. absinthium. When compared to the infected control, onion plant fresh weight and dry weight were considerably higher under the clove extract therapy. The plant extracts used in this study’s testing contain a number of active ingredients, including amino acids, vitamins, minerals, antioxidants, and enzymes, which is probably why they have such positive impacts. The application of a combination of plant extracts was suggested as a feasible strategy for improving the growth and productivity of onion plants by the study’s findings. More research is needed to comprehend the mechanisms by which plant extracts promote plant development and to optimize the concentration and timing of administration.
Similar content being viewed by others
Introduction
One of the world’s most important bulb crops, the onion (Allium cepa), is regarded as one of the most important commercial vegetable crops in the world including Egypt (Hussein et al. 2014). It is one of the oldest vegetables that has been used as spice and in medicine for thousands of years (Keusgen 2002). It is a rich source of carbohydrates and minerals including protein, vitamin C, phosphorus and calcium (Chakraborty et al. 2022), as well as has several medicinal uses (Gupta et al. 2015). In the field and in storage, several pathogens attack onions, lowering their quality and yield (Khalifa et al. 2016). The basal rot of onions caused by Fusarium oxysporum holds economic significance due to its impact, resulting in the decay of the basal plate of the bulb, further infection of the bulb scales, and causing the most significant losses during storage. Several Allium species, including chive, garlic, and shallot, as well as onion, are affected by F. oxysporum, which causes Fusarium basal rot.
Onion bulbs and shallots are susceptible to Fusarium basal rot of onion at all stages of growth (Cramer 2000), which is a disease with significant economic impact. According to Bayraktar and Dolar (2011), the fungi F. oxysporum, F. solani, F. acuminatum, F. culmorum, F. equiseti, F. proliferatum, F. subglutinans, F. redolens, and F. tricinctum can all cause basal rot disease of onions. For managing fungal diseases in crops, fungicides, extended rotations, and resistant cultivars have been used. Because several of them have been associated with increased rates of a number of cancers and are regarded to pose significant health hazards, using synthetic fungicides is not environmentally friendly (Beckerman et al. 2023; Rongai et al. 2015). Antimicrobial effects of medicinal plants have long been established. Plant extracts may be the ideal treatment because they are straightforward to test in vitro (Dissanayake 2014). Alkaloids, flavonoids, phenolic compounds, and tannins are a few of the chemical substances that can be found in medicinal plants. Alkaloids, a class of organic compounds containing nitrogen, are recognized in phytochemical research for their potential antibacterial properties, distinct from phenolic compounds (Dubale et al. 2023).
Syzygium aromaticum, or cloves (Family: Myrtaceae) are unopened, fragrant, dried flower buds that are native to India, Indonesia, Zanzibar, Mauritius, and Ceylon. It has been documented that the herbal plants demonstrated antifungal and anti-mycotoxigenic actions in addition to having potential antioxidant properties (Dikhoba et al. 2019). Additionally, S. aromaticum methanol extract displayed remarkable antibacterial effectiveness against Bacillus subtilis, Pseudomonas aeruginosa, and Staphylococcus aureus (Vizhi et al. 2016). In tropical areas of Asia, Africa, and South America, the Moringa plant (Moringa oleifera) is frequently farmed (Sato et al. 2013). The leaves, roots, and bark of the Moringa tree have been demonstrated coagulant and antibacterial properties (Taiwo et al. 2020), as well as being useful for treating water (Sato et al. 2013). Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus mitis, Streptococcus pneumoniae, Enterococcus faecalis, Escherichia coli, and Legionella pneumophila can all be successfully treated with M. oleifera seed extract and recombinant protein.
Calotropis procera (Family Asclepiadaceae) is also known as milk weeds due to the latex it produces, and it is distributed in several regions of the world (Kumar 2015). With the help of the powerful medication C. procera, leucoderma, leprosy, ulcers, tumors, piles, and disorders of the spleen, liver, and abdomen can all be effectively treated. The plant has undergone phytochemical analysis for cardenolides, anthocyanins, hydrocarbons, and triterpenoids, and it has been shown to exhibit a number of pharmacological properties including cardiac tonic, hepatoprotective, antibacterial, as well as anticancer (Ahmed et al. 2004; Karela 2023). According to reports, the plant’s latex has purgative effects (Karela 2023; Rajesh et al. 2005), procoagulant activity and wound healing activity (Nalwaya et al. 2009; Obagu and Ajiboso 2023), antibacterial activity (Ibrahim et al. 2023; Kumar et al. 2010), and anti-inflammatory and anti-oxidant activity.
Artemisia absinthium L., (wormwood), is a significant perennial shrubby medicinal plant that is indigenous to Asia, the Middle East, Europe, and North Africa, and belongs to the family Asteraceae (Beigh and Ganai 2017; Sharopov et al. 2012). It was a common Greek social prescription throughout the ancient era and served as an antiseptic, antimalarial, anthelmintic, antioxidant, hepatoprotective, antipyretic, anti-breast cancer, and neuroprotective. Research has shown that several Artemisia species, including A. kulbadica, A. sieberi, A. turanica, A. santolina, and A. diffusa, have deadly effects on human Caucasian hepatocyte carcinoma (HepG-2) and human Caucasian larynx cancer (Hep-2) cell lines (Emami et al. 2009). Consequently, for determination of the antifungal activity of Syzygium aromaticum, Artemisia absinthium, Moringa oleifera, and Calotropis procera methanol extracts against the pathogenic F. oxysporum, the responsible pathogen for onion basal rot, this study was designed.
Materials and methods
Isolation and identification of the pathogen
Using the direct plating method, the pathogenic fungus that causes the disease was isolated on potato dextrose agar (PDA) (Smith and Onions 1994). The diseased onion roots were placed on PDA-containing Petri plates, and the plates were then incubated at 25 °C until fungal growth was observed. The single spore isolation technique was used to create pure cultures from the generated colonies of the pathogenic fungal isolates. The pure culture of the pathogen was preserved as frozen mycelia stored at − 86 °C and lyophilized cultures, and it is available in the culture collection of the Assiut University Mycological Centre as AUMC 15798, as well as on cotton balls (Al-Bedak et al. 2019).
The process for molecular identification of the pathogen involved extracting fungal DNA using the procedures outlined by Moubasher et al. (2019), followed by PCR experiments conducted with SolGent EF-Taq at SolGent Company (SolGent Co., Ltd.; Daejeon, South Korea). The ITS region was amplified using the universal primers ITS1 and ITS4 (White et al. 1990). DNASTAR (version 5.05) was used to generate contiguous sequences of the Fusarium isolate from this study. The most similar sequences in GenBank database in addition to the Fusarium sequence in this study and the sequence of Nectria dematiosa CBS 126570 (as outgroup) were all aligned by Katoh and Standley (2013) applying the default option, and optimized by Criscuolo and Gribaldo (2010). MEGA X (version 10.2.6) was used to conduct maximum-likelihood (ML) and maximum-parsimony (MP) phylogenetic analyses (Kumar et al. 2018), and the robustness of the most parsimonious trees was evaluated by 1000 replications (Felsenstein 1985). Utilizing Modeltest 3.7’s Akaike Information Criterion (AIC), the optimum nucleotide substitution model for ML analysis was identified (Posada and Crandall 1998).
Pathogenicity test
In the greenhouse at the Department of Plant Pathology, Faculty of Agriculture, Assiut University, Egypt, during the growing season 2022–2023, the pathogenicity test was conducted on 60-day-old onion seedlings (Giza Sabeeni cultivar). Four sets of plastic pots (each measuring 25 cm in diameter) were employed, one set serving as a negative control. Each container was filled with 3 kg of pre-sterilized sand: clay soil (1:2) before the five onion seedlings were planted. Fusarium oxysporum AUMC 15798 was cultured to create the inoculum, which was subsequently incubated for 21 days at 25 °C in Erlenmeyer conical flasks containing a 1:1 mixture of barley and sand. Seven days before planting the seedlings,, 3% (w/w) of the pathogen’s inoculum was mixed with the soil. After 120 days of the trial, when the percentage of disease severity in each treatment was noted, the basal rot disease grading scale was as follows:
-
1.
Symptoms-free.
-
2.
Up to 10% rotted roots.
-
3.
10–30% rotted roots with up to 10% rotted basal plates.
-
4.
Completely rotted roots and 10–30% rotted basal plates.
-
5.
Completely rotted roots and more than 30% rotted basal plate.
Preparation of methanolic extracts
All plant components were cleaned and dried at 37 °C before being extracted with methanol. These dried plant components were then ground into a fine powder. Over 100 g of air-dried powder was used in each batch, which was macerated in 1000 mL of methanol following the method outlined by Handa (2008). The batches were left overnight at 37 °C in a shaking incubator. The methanol was evaporated at reduced pressure to collect the residuals for use in chemical analysis.
Detection of different secondary metabolites in the plant extracts
A fraction of the aqueous filtrate from each plant extract was mixed with 5 mL of diluted ammonia solution, and then concentrated H2SO4 were added. This procedure was used to identify flavonoids. Each extract had a yellow color that showed flavonoids were present. For detection of tannins, 5 mL of distilled water, 2.5 g of the plant extract were dissolved, filtered, and the filtrate was then mixed with ferric chloride reagent, tannins were assumed to be present if a blue–black, green, or blue-green precipitate formed. Saponins were detected by boiling 2 g of the powdered sample in 20 mL of distilled water and then filtered. 10 mL of the filtrate was mixed with 5 mL of distilled water and shaken vigorously. The frothing was mixed with 3 drops of olive oil and shaken vigorously, then observed for the formation of emulsion (Mathew et al. 2012; Osman et al. 2022).
Determination of total phenolic compounds (TPCs)
After refluxing with 30 mL of methanol containing 1.0% HCl for 10 min and centrifugation at 5000 rpm for 10 min, the total phenolic compounds were separately extracted from the plant extracts (0.5 g). According to a conventional procedure, the amount of total phenolic compounds present in the methanolic extracts is quantified as gallic acid equivalents (Maurya and Singh 2010).
In vitro effect of methanolic extracts on the pathogen’s radial growth and determination of the minimal inhibitory concentration (MIC)
The residues from the four studied plants’ methanolic extracts were separately dissolved in DMSO at 100, 150, and 200 ppm. 1.0 mL of each concentration was separately mixed with 20 mL PDA in sterilized Petri dish to obtain 5, 7.5, and 10 ppm/plate. The plates were inoculated with a 6 mm diameter discs obtained from 4-day-old culture of F. oxysporum. The plates were subsequently incubated at 25 °C for 96 h. The percentage of radial colony growth suppression against control was then calculated according the following Equation, and the MIC was determined (Shivapratap et al. 1996).
where D1, Colony diameter in control plate and D2, Colony diameter in treated plate.
Gas chromatography–mass spectrometry (GC–MS) analysis
The methanolic extract of clove (whole plant) was prepared according to Wilson et al. (1995). In order to identify the active ingredients, the most potent methanol extract of S. aromaticum was analyzed using GC–MS. The analysis was conducted at the Chemistry Department, Faculty of Science, Assiut University. Using a fused-silica capillary column, the previously prepared supernatant was separated (DB-5MS: 30 mm 0.25 mm 0.25 m, Agilent J & W Scientific, Folsom, CA, USA). A steady flow rate of 1 mL per minute was used for high-purity (> 99.999%) helium as a carrier gas. 1 µL of the sample was injected at a final temperature of 300 °C after the oven’s initial temperature of 50 °C for 4 min. A temperature of 250 °C was chosen for the injection. For a total runtime of 75 min, EI-ionization was set at 70 eV. By comparing mass spectra of chemical compounds to the MAINLIB and replib databases, chemical compositions were deduced. The mass spectrum of unknown components was compared to that of recognized components based on name, structure, molecular weight, intensities, and fragmented ions. All internal standards and any pseudo-positive peaks were eliminated from the ‘data array’. The total peak area of each sample was used to calibrate the normalization process.
Greenhouse experiment
T1 = methanolic extract of C. procera latex; T2 = methanolic extract of A. absinthium leaves; T3 = methanolic extract of M. oleifera seeds; T4 = methanolic extract of S. aromaticum clove; T5 = T1 + T2 + T3 + T4; T6 = methanolic extract of Dovex 50%; T7 = methanolic extracts of Dovex 50% + T5; T8 = negative control; T9 = positive control. In addition, Dovex 50% (Azoxystrobin 20% + Tebuconazole 30%) was used at the rate of 25 cm3 100 L−1) as a reference for comparison.
Determination of onion morphological parameters
120 days after planting the seedlings, the growth parameters were evaluated. The length of the shoots and roots was measured from the base of the bulb to the top of the plant using a centimeter ruler. The diameter of the bulb and neck were measured with digital calipers in millimeters. The fresh and dry mass was determined by weighing using a precise electronic balance with a sensitivity of 0.001 g. The freshly harvested shoots and roots were placed in paper bags and subjected to a drying process in an oven set at 75 ℃ for a minimum of 48 h to obtain the dry weight.
Statistical analysis
One-way analysis of variance (ANOVA), was performed using the software (Statistix 8.1) for statistical analysis and assess for significance (Kim 2014). To look at the variations between the means that were statistically significant, Duncan’s multiple range tests with 95% confidence were performed.
Results
Isolation and identification of the pathogen
Infected onion bulbs were procured from recently harvested fields in Sohag Governorate, Egypt. The isolation of fungi from the infected onion plants yielded five Fusarium isolates. The five isolates had morphological characteristics of F. oxysporum, including fast growth rate, and violet colony color on PSA, short phialides, irregular and elliptical conidia, as well as chlamydospore production (Fig. 1).
The internal transcribed spacer-based sequencing (ITS) was employed to identify the most virulent isolate, which resulted in disease severity of 88.9%. The final ITS data set had 16 sequences that yielded 534 characters, of which 473 could be successfully aligned, 108 were deemed variable characters, and 9 were classified as instructive characters. With a very good support value of 99% ML/96% MP, the Fusarium isolate used in this investigation was assigned to the F. oxysporum clade and placed on the same branch as F. oxysporum AUMC 9262 (Fig. 2). It was determined to be F. oxysporum, and the ITS sequence was entered as OR489022 in GenBank.
Evolutionary tree was created using the maximum parsimony analysis of the ITS sequence of F. oxysporum AUMC 15798 in comparison to the most related sequences of Fusarium species in GenBank (using 1000 replications of a heuristic search). Near each node, the bootstrap support values for ML/MP 50% are displayed. The Nectria dematiosa CBS 126570 outgroup is the tree’s primary node (shown in red).
Pathogenicity test
In the experiment to test for pathogenicity, all isolates were used. The severity of the disease in onions caused by all five isolates ranged from 66.6 to 88.88% (Table 1).
Methanol extraction and determination of secondary metabolites
In the extraction process, it was possible to produce 32, 15, 14.95, and 45 g from S. aromaticum, M. oleifera, A. absinthium, and C. procera, respectively, from 100 g of each studied plant’s components. All of the plant sections under study had extracts that contained phenolic compounds, alkaloids, tannins, saponins, and flavonoids, with the exception of saponins, which were missing from the extract of A. absinthium. The current results showed that clove’s total phenolic content (240.25 mg g−1) is higher than what found in the other plant extracts. While the largest concentrations of alkaloids, tannins, saponins, and flavonoids were found in the seed extract of M. oleifera (100.5, 110.5, 180.75, and 70.25 mg g−1, respectively).
Antifungal activity of the plant extracts against F. Oxysporum and MIC determination
The in vitro results showed that, although to varying degrees in comparison to the control, plant extracts may suppress the radial growth of the Fusarium pathogen. The S. aromaticum, M. oleifera, C. procera, and A. absinthium’s methanolic extract showed 63.3, 50, 46.6, and 41.1% of inhibition, respectively (Table 2).
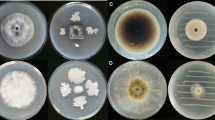
Methanol extract of S. aromaticum with 3 concentrations, 100, 150, and 200 ppm and commixture from secondary metabolites of methanolic extracts of M. oleifera, A. absinthium and C. procera with three concentrations (100, 150, and 200) were assessed on radial growth of F. oxysporum the causal pathogen of onion basal rot. The S. aromaticum methanolic extract outperformed the combination of A. absinthium, C. procera, and M. oleifera methanolic extracts in terms of effectiveness. F. oxysporum was most effectively inhibited (82.2%) from growing when administered at a dose of 200 ppm. The combination of methanolic extracts from A. absinthium, C. procera, and M. oleifera, however, showed the lowest growth inhibition (67.7%) of F. oxysporum at a concentration of 200 ppm (Table 3; Fig. 3).
Biocontrol of onion basal rot disease by plant extracts in greenhouse conditions
The ability of the plant extracts used in this research to prevent F. oxysporum, which causes onion basal rot, was tested in a greenhouse environment. Since they make the disease less severe, methanolic extracts have been demonstrated to have an effect on disease management. The disease severity was significantly reduced (20%) when using a combination of the four extracts and Dovex 50% (T7) as opposed to a combination of the four extracts (22.22 ± 3.85) or Dovex 50% alone (22.22 ± 3.85), when compared to the infected control (T9). While using the C. procera’s extract caused the greatest disease severity (62.22 ± 3.85) (Fig. 4).
Effects of various treatments using plant methanol extracts and/or Dovex 50% control agents against F. oxysporum AUMC 15798-caused onion basal rot disease in greenhouse circumstances. Where: T1 = methanolic extract of C. procera latex; T2 = methanolic extract of A. absinthium leaves; T3 = methanolic extract of M. oleifera seeds; T4 = methanolic extract of S. aromaticum clove; T5 = T 1 + T 2 + T 3 + T 4; T6 = methanolic extract of Dovex 50%; T7 = methanolic extracts of Dovex 50% + T 5; T8 = negative control; T9 = positive control.
GC–MS analysis of S. aromaticum’s methanol extract
The characterization of S. aromaticum’s methanol extract was carried out by GC–MS analysis which revealed the presence of 82 compounds which ranged from low occurrence to high abundance (Fig. 5; Table S1). Acetaldehyde, hydroxy- and 2-propanone, 1,1,3,3-tetrachloro-(42.71%), 1,2-ethanediol, and methyl alcohol (34.01%) were the main constituents detected. Also, the clove extract contained 3-allyl-6-methoxyphenyl acetate (4.1%), phenol, 2-methoxy-4-(2-propenyl)-, acetate (4.1%), and eugenol (2.25%). Some medicinally-important bioactive constituents such as propanoic acid and cyclodecasiloxane were also detected (1.0%). Some hydrocarbons were present in concentrations ranging from 0.5 to 1.5%. Peak areas for octadecane, 6-methyl undecane, tetradecane, 2,6,10-trimethyl-, dodecane, 5,8-diethyl, heptadecane, 9-hexyl-, tetradecanoic acid, 2-hydroxy-, and hexadecanoic acid, 1-(hydroxymethyl)-1,2-ethanediyl ester were all 1.41%. The presence of certain steroids (ergosta-5,22-dien-3-ol, acetate, and fatty acids (2-bromotetradecanoic acid, oleic acid) was also detected in this investigation by GC–MS (Table S1; Figure S1).
Effect of plant extracts on the onion’s growth parameters
Onion plants’ development characteristics and morphology were estimated to be affected by the plant extracts used in this investigation. The infected control had the considerably lowest values for the parameters shoot length, root length, and bulb height, whereas the mixture of plant extracts produced the significantly highest values. The application of S. aromaticum methanol extract treatment produced statistically significantly longer roots (19.96 cm) than the other treatments (Table 4). The smallest root length of plants was seen under infected control. Plants treated with the mixture of plant extracts had onion neck diameters that were much larger (1.36 cm) than those of the other treatments, whereas plants in infected control had the smallest onion neck diameters (1.04 cm). The number of leaves, fresh weight, and dry weight (g.) were all statistically substantially higher in case of S. aromaticum methanol extract treatment than they were under the infected control (Table 4).
Discussion
It is critically important to develop innovative, safe, and efficient strategies to control plant diseases, such as onion basal rot disease, that can be integrated into management programs for root-rot diseases. In this study, methanol extracts of A. absinthium leaves, C. procera latex, M. oleifera seeds, and S. aromaticum clove were produced, and their antifungal effects against F. oxysporum, the pathogen responsible for onion basal rot disease, were assessed. The methanol extract of S. aromaticum (clove) was identified as the powerful antifungal extract. The causative pathogen’s radial growth was inhibited to the greatest extent (63.3%) by the methanolic extract of S. aromaticum, followed by M. oleifera (50%). Numerous researchers have examined the phytoconstituents of S. aromaticum, and they have found that clove extracts include a wide variety of secondary metabolites. Vizhi et al. (2016) demonstrated that clove extracts in methanol and ethyl acetate contained flavonoids, phenolics, tannins, terpenoids, and steroids but did not contain alkaloids, coumarins, or quinones. Alkaloids, which are naturally occurring nitrogen-containing chemical compounds, have been found in M. oleifera together with flavonoids, tannins, and saponins (Adekanmi et al. 2020).
Due to its ability to yield comprehensive results and its typically sufficient identifying power compared to other techniques, GC–MS is strongly recommended for analytical determinations (Proestos and Komaitis 2013; Socrates and Mohan 2019), GC–MS analysis in the present investigation found the existence of several chemical substances which have diverse biological activities. The buildup of these substances in S. aromaticum is mostly connected to the metabolism of the plant. One naturally occurring, volatile organic molecule that is released from the leaves of many plant species is the highly abundant methanol (Nemecek-Marshall et al. 1995). According to research (Cossins 1964), organic acids, sugars, and amino acids like serine and methionine are the principal substrates for the incorporation of 14C-methanol. In several cases, the antimicrobial potentials of certain GC–MS identified substances were documented. For instance, S. aromaticum demonstrated observable antifungal efficacy with greater anti-candida activity than nystatin (Mansourian et al. 2014). Despite using methanol as an extraction solvent, which also contained other major chemicals, eugenol was still detected in this investigation.
In addition, 3-allyl-6-methoxyphenyl acetate was detected in the S. aromaticum extract, which has regarded as a potent antioxidant (Al-azem et al. 2019), and showed anti-melanogenic properties (Alam et al. 2023). Furthermore, eugenol was also detected in this analysis, a compound known for its antifungal properties (Manohar et al. 2001). According to a recent study (Mostafa et al. 2023), eugenol is identified as the primary active component in acetone and ethanol extracts of clove, demonstrating anti-candidal properties more pronounced than those of clotrimazole. The presence of phytochemicals the S. aromaticum clove, including alkaloids, tannins, flavonoids, anthocyanins, saponins, terpenes, steroids, and coumarins has been demonstrated (Jimoh et al. 2017). According to Cox et al. (2001), the cellular membrane appears to be the site of the primary antifungal action. The biologically-active substance cyclononasiloxane, which has hepato-protective properties (Adnan et al. 2021), was also detected from the S. aromaticum methanol extract.
The less abundant components in the clove’s methanol extract in this study were hydrocarbons. However, several of them have been found to have the ability to treat illnesses in both humans and animals. The investigation of Najibullah et al. (2022), which revealed the presence of hydrocarbons in related plants, lends weight to these conclusions. Additionally, the presence of a few steroids and fatty acids in this investigation was noted, with varying biological effects on lowering cholesterol and preventing heart disease. In vivo study (Venkatachalam et al. 2013), has demonstrated that propanoic acid, which detected in this study, exerted a hypoglycemic action when taken orally as it is a protein tyrosine phosphatase 1B inhibitor (PTP 1B), and maintained as anti-diabetic agent.
In this study, a combination of plant extracts and Dovex 50% decreased onion basal rot severity to 20% when compared to the infected control, while also significantly enhancing the growth and productivity of onion plants. Due to their phenolic and flavonoid components, S. aromaticum has been demonstrated to strongly scavenge DPPH activity in the methanol extract (Temesgen et al. 2022). Additionally, phenolic and flavonoid chemicals enhanced nutrient uptake, increased stress tolerance, and supported plant defense against pathogens and pests, among other positive effects on plant growth (Kumar et al. 2023; Pratyusha 2022).
Many plants’ vegetative growth and production have been found to be considerably enhanced by foliar application of plant extracts. In this way, using the methanol extract from M. oleifera improved the growth characteristics and production as well as the garlic bulb’s quality (Hegazi et al. 2016). Additionally, Silybum marianum plants under salt stress can grow and yield more when using Moringa leaf extract, which also boosts the production of secondary metabolites (Yap et al. 2021). Licorice root extract application greatly enhanced the physiological and biochemical characteristics, oxidative marker accumulations, antioxidant activities, nutraceutical quality, growth- and yield-related characteristics of onion cultivars (Younes et al. 2021). Garlic production and growth were stimulated by licorice root extract, and as a result the marketable bulb percentage rose while the non-marketable bulb percentage fell (Hamam et al. 2021). Wheat and barley growth has been demonstrated to be affected by C. procera aqueous extract in a biphasic manner, with low concentrations boosting germination and high amounts limiting growth (Radwan et al. 2019).
Amino acids, vitamins, minerals, antioxidants, enzymes, and other active components may all have a role in the positive effects of the plant extracts employed in this study. According to the study’s findings, applying a combination of plant extracts to onion plants may be a fruitful way to increase their development and output. However, additional study is required to determine the best concentration and timing for applying plant extracts as well as to explore the mechanisms by which they stimulate plant development.
In this study, it was determined if methanolic extracts of A. absinthium leaves, C. procera latex, M. oleifera seeds, and S. aromaticum clove were effective against F. oxysporum, the pathogen that causes onion basal rot, both in vitro and in a greenhouse setting. The extract from S. aromaticum exhibited the maximum inhibitory action, whereas that of A. absinthium achieved the lowest growth inhibition percentage. The studied methanolic extract of S. aromaticum contained 82 important compounds, with abundances ranging from low to high, as determined by the GC–MS analysis. When treated with a combination of plant extracts and Dovex 50%, the percentage of disease severity was significantly reduced; but, when treated with an extract of A. absinthium, it increased. When compared to the infected control, onion plant fresh weight and dry weight were considerably higher under the clove extract therapy. The application of the plant extracts combination was suggested as a feasible strategy for improving the growth and productivity of onion plants by the study’s findings. More research is needed to comprehend the mechanisms by which plant extracts promote plant development and to optimize the concentration and timing of administration. Therefore, we can include these plant extracts in integrated disease care to successfully avoid onion basal rot.
Availability of data and materials
All data related to this manuscript is incorporated only in the manuscript.
References
Adekanmi AA, Adekanmi SA, Adekanmi OS (2020) Qualitative and quantitative phytochemical constituents of moringa leaf. Int J Eng Inform Syst 4(5):10–17
Adnan M, Siddiqui AJ, Arshad J, Hamadou WS, Awadelkareem AM, Sachidanandan M, Patel M (2021) Evidence-based medicinal potential and possible role of selaginella in the prevention of modern chronic diseases: Ethnopharmacological and ethnobotanical perspective. Records Nat Prod 15(5):355
Ahmed KM, Rana A, Dixit V (2004) Effect of Calotropis procera latex on isoproterenol induced myocardial infarction in albino rats. Phytomedicine 11(4):327–330
Al-azem DA, Al-Derawi KH, Malik Al-Saadi SA (2019) The Protective effects of Syzygium aromaticum essential oil extract against Methotrexate Induced hepatic and renal toxicity in rats. J Pure Appl Microbiol 13(1)
Al-Bedak O, Sayed R, Hassan S (2019) A new low-cost method for long-term preservation of filamentous fungi. Biocatal Agric Biotechnol 22:101417
Alam MB, Park NH, Song B-R, Lee S-H (2023) Antioxidant potential-rich Betel leaves (Piper betle L.) exert depigmenting action by triggering autophagy and downregulating MITF/tyrosinase in vitro and in vivo. Antioxidants 12(2):374
Bayraktar H, Dolar FS (2011) Molecular identification and genetic diversity of Fusarium species associated with onion fields in Turkey. J Phytopathol 159(1):28–34
Beckerman J, Palmer C, Tedford E, Ypema H (2023) Fifty years of fungicide development, deployment, and future use. Phytopathology 113(4):694–706
Beigh YA, Ganai AM (2017) Potential of wormwood (Artemisia absinthium Linn.) Herb for use as additive in livestock feeding: a review. The Pharma Innov 6(8):176
Cossins EA (1964) The utilization of carbon-1 compounds by plants: I. The metabolism of methanol-c14 and its role in amino acid biosynthesis. Can J Biochem 42(12):1793–1802
Cramer CS (2000) Breeding and genetics of Fusarium basal rot resistance in onion. Euphytica 115:159–166
Criscuolo A, Gribaldo S (2010) BMGE (block mapping and gathering with entropy): a new software for selection of phylogenetic informative regions from multiple sequence alignments. BMC Evol Biol 10(1):210
Chakraborty AJ, Uddin TM, Zidan M, Redwan BM, Mitra S, Das R, Nainu F, Dhama K, Roy A, Hossain MJ (2022) Allium cepa: a treasure of bioactive phytochemicals with prospective health benefits. Evid Based Complement Altern Med
Cox SD, Mann CM, Markham JL (2001) Interactions between components of the essential oil of Melaleuca alternifolia. J Appl Microbiol 91(3):492–497
Dissanayake M (2014) Inhibitory effect of selected medicinal plant extracts on phytopathogenic fungus Fusarium oxysporum (Nectriaceae) Schlecht Emend. Snyder and Hansen. Annu Res Rev Biol 4:133–142
Dikhoba PM, Mongalo NI, Elgorashi EE, Makhafola TJ (2019) Antifungal and anti-mycotoxigenic activity of selected South African medicinal plants species. Heliyon 5(10)
Dubale S, Kebebe D, Zeynudin A, Abdissa N, Suleman S (2023) Phytochemical screening and antimicrobial activity evaluation of selected medicinal plants in Ethiopia. J Exp Pharmacol 51–62
Emami SA, Vahdati-Mashhadian N, Vosough R, Oghazian MB (2009) The anticancer activity of five species of Artemisia on Hep2 and HepG2 cell lines. Pharmacologyonline 3:327–339
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39(4):783–791
Gupta R, Khokhar MK, Lal RAM (2015) Management of the black mould disease of onion. J Plant Pathol Microbiol 3(5):1000133
Handa SS (2008) An overview of extraction techniques for medicinal and aromatic plants. Extr Technol Med Aromatic Plants 1(1):21–40
Hamam MA, Moustafa YMM, Ali MAM, Sh AS (2021) Effect of foliar spray with licorice root extract on production and quality of Egyptian and Chinese garlic. J Plant Prod 12(8):929–940
Hegazi AZ, Hasan SKH, El-Said NAM (2016) Response of garlic plants to foliar application of moringa leaves extract, glutamine and cysteine. J Plant Prod 7(1):1–6
Hussein MAM, Hassan MHA, Abo-Elyousr KAM (2014) Biological control of Botrytis allii by Trichoderma viride on onion Allium cepa. World Appl Sci J 32(3):522–526
Ibrahim H, Hamza A, Salawudeen A, Ummu R, Hanifa M (2023) Susceptibility of microbial infectious agents to the latex and leaf extract of Calotropis procera. Bima J Sci Technol 7(02):12–24
Jimoh SO, Arowolo LA, Alabi KA (2017) Phytochemical screening and antimicrobial evaluation of syzygium aromaticum extract and essential oil. Int J Curr Microbiol Appl Sci 6:4557–4567
Keusgen M (2002) Health and alliums. Allium Crop Sci Recent Adv 357–378
Kim H-Y (2014) Analysis of variance (ANOVA) comparing means of more than two groups. Restor Dent Endod 39(1):74–77
Kumar D (2015) Morpho taxonomical studies on some members of family Asclepiadaceae
Karela P (2023) Phytochemical constituent and therapeutic uses of Calotropis gigantea
Kumar G, Karthik L, Rao KB (2010) Antimicrobial activity of latex of Calotropis gigantea against pathogenic microorganisms-an in vitro study. Pharmacologyonline 3(3):155–163
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30(4):772–780
Khalifa M, Mahmoud N, Abou-Zeid N (2016) Management of onion bulb rots during storage using pre-and post-harvest control treatments. Egypt J Phytopathol 44(2):1–16
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35(6):1547–1549
Kumar K, Debnath P, Singh S, Kumar N (2023) An overview of plant phenolics and their involvement in abiotic stress tolerance. Stresses 3(3):570–585
Maurya S, Singh D (2010) Quantitative analysis of total phenolic content in Adhatoda vasica Nees extracts. Int J PharmTech Res 2(4):2403–2406
Manohar V, Ingram C, Gray J, Talpur NA, Echard BW, Bagchi D, Preuss HG (2001) Antifungal activities of origanum oil against Candida albicans. Mol Cell Biochem 228:111–117
Mansourian A, Boojarpour N, Ashnagar S, Beitollahi JM, Shamshiri A (2014) The comparative study of antifungal activity of Syzygium aromaticum, Punica granatum and nystatin on Candida albicans; an in vitro study. J Mycol Med 24(4):e163–e168
Mathew BB, Jatawa SK, Tiwari A (2012) Phytochemical analysis of Citrus limonum pulp and peel. Int J Pharm Pharm Sci 4(2):369–371
Moubasher A, Ismail M, Al-Bedak O, Mohamed R (2019) Ramophialophora Chlamydospora, a new species from an alkaline lake of Wadi-El-Natron, Egypt. Asian J Mycol 2(1):110–117
Najibullah SNM, Ahamad J, Sultana S, Zafar A (2022) Chemical characterization and evaluation of anticancer activity of Pistacia terebinthus Linn. fruits essential oil. J Essent Oil Bear Plants 25(1):180–187
Mostafa AA-F, Yassin MT, Al–Askar AA, Al-Otibi FO (2023) Phytochemical analysis, antiproliferative and antifungal activities of different Syzygium aromaticum solvent extracts. J King Saud University-Science 35(1):102362
Nalwaya N, Pokharna G, Deb L, Jain NK (2009) Wound healing activity of latex of Calotropis gigantea. Int J Pharm Pharm Sci 1(1):176–181
Nemecek-Marshall M, MacDonald RC, Franzen JJ, Wojciechowski CL, Fall R (1995) Methanol emission from leaves (enzymatic detection of gas-phase methanol and relation of methanol fluxes to stomatal conductance and leaf development). Plant Physiol 108(4):1359–1368
Obagu E, Ajiboso S (2023) Evaluation of Wound Healing activity of crude latex obtained from Calotropis Procera Stem. Int J Adv Eng Manag 5(1):46–53
Osman A, Mansour A, Ahmed A (2022) Antimicrobial activity of methanolic extract from clove and the test of preservative power of ras cheese. J Food Dairy Sci 13(12):181–185
Pratyusha S (2022) Phenolic compounds in the plant development and defense: an overview. Plant Stress Physiol Perspect Agric 125–140
Proestos C, Komaitis M (2013) Analysis of naturally occurring phenolic compounds in aromatic plants by RP-HPLC coupled to diode array detector (DAD) and GC–MS after silylation. Foods 2(1):90–99
Posada D, Crandall KA (1998) Modeltest: testing the model of DNA substitution. Bioinformatics (Oxford England) 14(9):817–818
Rajesh R, Gowda CR, Nataraju A, Dhananjaya B, Kemparaju K, Vishwanath B (2005) Procoagulant activity of Calotropis gigantea latex associated with fibrin (ogen) olytic activity. Toxicon 46(1):84–92
Rongai D, Pulcini P, Pesce B, Milano F (2015) Antifungal activity of some botanical extracts on Fusarium oxysporum. Open Life Sci 10(1)
Radwan AM, Alghamdi HA, Kenawy SKM (2019) Effect of Calotropis procera L. plant extract on seeds germination and the growth of microorganisms. Ann Agric Sci 64(2):183–187
Smith D, Onions AH (1994) The preservation and maintenance of living fungi. CAB International
Socrates SH, Mohan SC (2019) Phytochemical analysis of flower extracts of different Cassia species by using gas chromatography-mass spectrometry. Int J Biol Chem 13:1–11
Shivapratap HR, Philip T, Sharma DD (1996) The species concept in Fusarium. Indian J Seri 35(2):107–110
Sharopov FS, Sulaimonova VA, Setzer WN (2012) Composition of the essential oil of Artemisia absinthium from Tajikistan. Records Nat Prod 6(2)
Sato T, Qadir M, Yamamoto S, Endo T, Zahoor A (2013) Global, regional, and country level need for data on wastewater generation, treatment, and use. Agric Water Manag 130:1–13
Taiwo AS, Adenike K, Aderonke O (2020) Efficacy of a natural coagulant protein from Moringa oleifera (Lam) seeds in treatment of Opa reservoir water, Ile-Ife, Nigeria. Heliyon 6(1)
Temesgen S, Sasikumar JM, Egigu MC (2022) Effect of extraction solvents on total polyphenolic content and antioxidant capacity of Syzygium Aromaticum L. Flower Bud from Ethiopia. BioMed Research Int
Venkatachalam M, Singaravelu G, Govindaraju K, Ahn JS (2013) PTP 1B inhibitory action of a phytochemical propanoic acid, 2-(3-acetoxy-4, 4, 14-trimethylandrost-8-en-17-yl). Curr Sci 827–832
Vizhi D, Irulandi K, Mehalingam P, Kumar N (2016) In vitro antimicrobial activity and phytochemical analysis of fruits of Syzygium aromaticum (L.) Merr. & LM Perry-an important medicinal plant. J Phytopharm 5(4):137–140
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc Guide Methods Appl 18(1):315–322
Wilson RM, Runciman WB, Gibberd RW, Harrison BT, Newby L, Hamilton JD (1995) The quality in Australian health care study. Med J Aust 163(9):458–471
Yap Y-K, El-Sherif F, Habib ES, Khattab S (2021) Moringa oleifera leaf extract enhanced growth, yield, and silybin content while mitigating salt-induced adverse effects on the growth of Silybum marianum. Agronomy 11(12):2500
Younes NA, Rahman MM, Wardany AA, Dawood MFA, Mostofa MG, Keya SS, Abdel Latef AAH, Tran L-SP (2021) Antioxidants and bioactive compounds in licorice root extract potentially contribute to improving growth, bulb quality and yield of onion (Allium cepa). Molecules 26(9):2633
Acknowledgements
Thanks to all the technicians at the Faculty of Agriculture and Science, Al-Azhar University branch in Assiut, Egypt. The authors appreciate everyone who contributed to the completion of this research.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors participated equally in data analysis, authoring, and revising the article. The final version of the manuscript has been reviewed and approved by all authors. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that there are no potential conflicts of interest regarding.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hegazy, M.G.A., Ahmed, AR.M., Yousef, A.F. et al. Effectiveness of some plant extracts in biocontrol of induced onion basal rot disease in greenhouse conditions. AMB Expr 14, 72 (2024). https://doi.org/10.1186/s13568-024-01721-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13568-024-01721-4