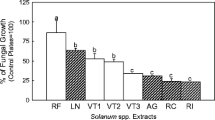
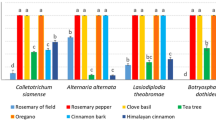
Abstract
Five plant extracts traditionally used in organic and biodynamic farming for pest control and antifungal (downy mildew) disease management were selected after a farmer survey and analyzed for their chemical composition in LC-PDA-MS-MS and using adapted analytical method from food chemistry for determination of class of component (e.g., protein, sugar, lipids…). Their antifungal activity against Penicillium expansum, Botrytis cinerea, Botrytis allii, brown rot causing agents (Monilinia laxa and Monilinia fructigena), and grape downy mildew (Plasmopara viticola) was examined in vitro. White willow (Salix alba) and absinthe (Artemisia absinthium) ethanolic extracts were found to be the most effective in particular against Plasmopara viticola, with a total inhibition of spores germination when applied at 1000 mg/L. These extracts also showed a relatively low toxicity during preliminary ecotoxicological assays on Daphnia pulex. Extract from the bark of white willow contained some flavonoids, especially flavanones (eriodyctiol and derivates) and flavanols (catechins and derivates), as major compounds, whereas absinthe extract was rich in O-methylated flavanols and hydroxycinnamic acids. Thujone content in this extract was also determined by external calibration in GC-MS analysis, and its value was 0.004% dry extract.




Similar content being viewed by others
References
Agnolet S, Wiese S, Verpoorte R, Staerk D (2012) Comprehensive analysis of commercial willow bark extracts by new technology platform: combined use of metabolomics, high-performance liquid chromatography—solid-phase extraction—nuclear magnetic resonance spectroscopy and high-resolution radical scavenging assay. J Chromatogr A 1262:130–137. https://doi.org/10.1016/j.chroma.2012.09.013
Association Of Analytic Chemistry (2005) AOAC Official Method 985.29 (A-D) In: Official Methods of Analysis of AOAC international, 18th edn. Gaithersburg
Bélanger RR, Benhamou N, Menzies JG (2003) Cytological evidence of an active role of silicon in wheat resistance to powdery mildew (Blumeria graminis f. sp. tritici). Phytopathology 93(4):402–412. https://doi.org/10.1094/PHYTO.2003.93.4.402
Blagojević P, Radulović N, Palić R, Stojanović G (2006) Chemical composition of the essential oils of Serbian wild-growing Artemisia absinthium and Artemisia vulgaris. J Agric Food Chem 54(13):4780–4789. https://doi.org/10.1021/jf060123o
Bruneton J (1999) Pharmacognosie, Phytochimie plantes médicinales, Lavoisier Tec & Doc, 3rd edn, Paris
Cai K, Gao D, Luo S, Zeng R, Yang J, Zhu X (2008) Physiological and cytological mechanisms of silicon-induced resistance in rice against blast disease. Physiol Plant 134(2):324–333. https://doi.org/10.1111/j.1399-3054.2008.01140.x
Carbonara T, Pascale R, Argentieri MP, Papadia P, Fanizzi FP, Villanova L, Avato P (2012) Phytochemical analysis of a herbal tea from Artemisia annua L. J Pharm Biomed Anal 62:79–86. https://doi.org/10.1016/j.jpba.2012.01.015
Carnat A, Heitz A, Fraisse D, Carnat AP, Lamaison JL (2000) Major dicaffeoylquinic acids from Artemisia vulgaris. Fitoterapia 71(5):587–589. https://doi.org/10.1016/S0367-326X(00)00163-5
Chen M, Zhai L, Arendrup M (2015) In vitro activity of 23 tea extractions and epigallocatechin gallate against Candida species. Med Mycol 53:194–198
Cherif M, Asselin A, Belanger RR (1994) Defense responses induced by soluble silicon in cuncumber roots infected by Pythium spp. Mol Plant Pathol 84:236–242
Clifford MN, Johnston KL, Knight S, Kuhnert N (2003) Hierarchical scheme for LC-MS identification of chlorogenic acids. J Agric Food Chem 51(10):2900–2911. https://doi.org/10.1021/jf026187q
Commision Services (2007) Working document of the Commission Services - DRAFT Comparison between EU and GHS Criteria Human Health and Environment
Cook R, Hennell JR, Lee S, Khoo CS, Carles MC, Higgins VJ, Govindaraghavan S, Sucher NJ (2013) The Saccharomyces cerevisiae transcriptome as a mirror of phytochemical variation in complex extracts of Equisetum arvense from America, China, Europe and India. BMC Genomics 14(1):445. https://doi.org/10.1186/1471-2164-14-445
Couteux A, Lejeune A (2015) Index phytosanitaire. ACTA, Paris
Dane Y, Mouhouche F, Canela-Garayoa R, Delpino-Rius A (2016) Phytochemical analysis of methanolic extracts from Artemisia absinthium L. 1753 (Asteraceae), Juniperus phoenicea L., and Tetraclinis articulata (Vahl) Mast, 1892 (Cupressaceae) and evaluation of their biological activtiy for stored grain protection. Arab J Sci Eng 41(6):247–2158. https://doi.org/10.1007/s13369-015-1977-2
Du Q, Jerz G, Winterhalter P (2004) Preparation of three flavonoids from the bark of Salix alba by high-speed countercurrent chromatographic separation. J Liq Chromatogr Relat Technol 27(20):3257–3264. https://doi.org/10.1081/JLC-200034917
Esatbeyoglu T, Winterhalter P (2010) Preparation of dimeric Procyanidins B1, B2, B5, and B7 from a polymeric procyanidin fraction of black chokeberry (Aronia melanocarpa). J Agric Food Chem 58(8):5147–5153. https://doi.org/10.1021/jf904354n
Francescato LN, Debenedetti SL, Schwanz TG, Bassani VL, Henriques AT (2013) Identification of phenolic compounds in Equisetum giganteum by LC–ESI-MS/MS and a new approach to total flavonoid quantification. Talanta 105:192–203. https://doi.org/10.1016/j.talanta.2012.11.072
Frey S, Carver TLW (1998) Induction of systemic resistance in pea to pea powdery mildew by exogenous application of salicylic acid. J Phytopathol 146:239–245
Garcia D, Garcia-Cela E, Ramos AJ, Sanchis V, Marín S (2011) Mould growth and mycotoxin production as affected by Equisetum arvense and Stevia rebaudiana extracts. Food Control 22(8):1378–1384. https://doi.org/10.1016/j.foodcont.2011.02.016
Garcia D, Ramos AJ, Sanchis V, Marín S (2013) Equisetum arvense hydro-alcoholic extract: phenolic composition and antifungal and antimycotoxigenic effect against Aspergillus flavus and Fusarium verticillioides in stored maize: Equisetum arvense hydro-alcoholic extract. J Sci Food Agric 93(9):2248–2253. https://doi.org/10.1002/jsfa.6033
Gonzalez-Coloma A, Bailen M, Diaz CE, Fraga BM, Martínez-Díaz R, Zuñiga GE, Contreras RA, Cabrera R, Burillo J (2012) Major components of Spanish cultivated Artemisia absinthium populations: antifeedant, antiparasitic, and antioxidant effects. Ind Crop Prod 37(1):401–407. https://doi.org/10.1016/j.indcrop.2011.12.025
Guével MH, Menzies JG, Bélanger RR (2007) Effect of root and foliar applications of soluble silicon on powdery mildew control and growth of wheat plants. Eur J Plant Pathol 119(4):429–436. https://doi.org/10.1007/s10658-007-9181-1
Han J, Ye M, Qiao X, Xu M, Wang B, Guo DA (2008) Characterization of phenolic compounds in the Chinese herbal drug Artemisia annua by liquid chromatography coupled to electrospray ionization mass spectrometry. J Pharm Biomed Anal 47(3):516–525. https://doi.org/10.1016/j.jpba.2008.02.013
Hold KM, Sirisoma NS, Ikeda T, Narahashi T, Casida JE (2000) a-Thujone (the active component of absinthe): g-aminobutyric acid type A receptor modulation and metabolic detoxification. Proc Natl Acad Sci 97(8):3826–3831. https://doi.org/10.1073/pnas.070042397
International Organization For Standardization (1973) ISO 1443. Meat and meat products. Determination of total fat content. In: International organization for standardization, Geneva
Ivanescu B, Vlase L, Corciova A, Lazar MI (2010) HPLC-DAD-MS study of polyphenols from Artemisia absinthium, A. annua, and A. vulgaris. Chem Nat Compd 46(3):468–470. https://doi.org/10.1007/s10600-010-9648-8
Julio L, Burillo J, Giménez C, Cabrera R, Díaz C, Sanz J, González-Coloma A (2015) Chemical and biocidal characterization of two cultivated Artemisia absinthium populations with different domestication levels. Ind Crop Prod 76:787–792. https://doi.org/10.1016/j.indcrop.2015.07.041
Kammerer B, Kahlich R, Biegert C, Gleiter CH, Heide L (2005) HPLC-MS/MS analysis of willow bark extracts contained in pharmaceutical preparations. Phytochem Anal 16(6):470–478. https://doi.org/10.1002/pca.873
Lachenmeier DW, Nathan-Maister D, Breaux TA, Sohnius EM, Schoeberl K, Kuballa T (2008) Chemical composition of vintage preban absinthe with special reference to thujone, fenchone, pinocamphone, methanol, copper, and antimony concentrations. J Agric Food Chem 56(9):3073–3081. https://doi.org/10.1021/jf703568f
Lee SJ, Chung HY, Maier CGA, Wood AR, Dixon RA, Mabry TJ (1998) Estrogenic flavonoids from Artemisia vulgaris L. J Agric Food Chem 46(8):3325–3329. https://doi.org/10.1021/jf9801264
Lopes-Lutz D, Alviano DS, Alviano CS, Kolodziejczyk PP (2008) Screening of chemical composition, antimicrobial and antioxidant activities of Artemisia essential oils. Phytochemistry 69(8):1732–1738. https://doi.org/10.1016/j.phytochem.2008.02.014
Melguizo-Melguizo D, Diaz-de-Cerio E, Quirantes-Piné R, Švarc-Gajić J, Segura-Carretero A (2014) The potential of Artemisia vulgaris leaves as a source of antioxidant phenolic compounds. J Funct Foods 10:192–200. https://doi.org/10.1016/j.jff.2014.05.019
Nakatani N, Kayano S, Kikuzaki H, Sumino K, Katagiri K, Mitani T (2000) Identification, quantitative determination, and antioxidative activities of chlorogenic acid isomers in prune (Prunus d omestica L.) J Agric Food Chem 48(11):5512–5516. https://doi.org/10.1021/jf000422s
OECD (2004) OECD n° 202: daphnia sp., acute immobilisation test and reproduction, test organisation for economic co-operation and development, Paris
Pobłocka-Olech L, van Nederkassel AM, Vander Heyden Y, Krauze-Baranowska M, Glód D, Baczek T (2007) Chromatographic analysis of salicylic compounds in different species of the genus Salix. J Sep Sci 30(17):2958–2966. https://doi.org/10.1002/jssc.200700137
Suárez-Quiroz ML, Alonso Campos A, Valerio Alfaro G, González-Ríos O, Villeneuve P, Figueroa-Espinoza MC (2015) Anti-Aspergillus activity of green coffee 5-O-caffeoyl quinic acid and its alkyl esters. Mic Pathogen 61-62:51–56
Rodrigues FÁ, Benhamou N, Datnoff LE, Jones JB, Bélanger RR (2003) Ultrastructural and cytochemical aspects of silicon-mediated rice blast resistance. Phytopathology 93(5):535–546. https://doi.org/10.1094/PHYTO.2003.93.5.535
Slingleton VL, Rossi JA Jr (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Tala V, Candida da Silva V, Rodrigues C, Nkengfack A, Campaner dos Santos L, Vilegas W (2013) Characterization of Proanthocyanidins from Parkia biglobosa (Jacq.) G. Don. (Fabaceae) by Flow Injection Analysis — Electrospray Ionization Ion Trap Tandem Mass Spectrometry and Liquid Chromatography/Electrospray Ionization Mass Spectrometry. Molecules 18:2803–2820. https://doi.org/10.3390/molecules18032803
Yamaji K, Ichihara Y (2012) The role of catechin and epicatechin in chemical defense against damping-off fungi ofcurrent-year Fagus crenata seedlings in natural forest. For Path 42:1–7. https://doi.org/10.1111/j.1439-0329.2010.00709.x
Acknowledgements
The spectroscopic experiments have been performed using the “Biodiversité et Biotechnologies Marines” (Bio2Mar) facilities at the University of Perpignan.
This project was support by Perpignan University Foundation and French Ministry in charge of Agriculture through the CASDAR project “4P” (Protection des Plantes Par les Plantes).
The authors gratefully thank Jeanine ALMANY for providing English language editing (as well as constructing comments) which improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Andreu, V., Levert, A., Amiot, A. et al. Chemical composition and antifungal activity of plant extracts traditionally used in organic and biodynamic farming. Environ Sci Pollut Res 25, 29971–29982 (2018). https://doi.org/10.1007/s11356-018-1320-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-1320-z