Abstract
Cyanide is used in many industries despite its toxicity. Cyanide biodegradation is affordable and eco-friendly. Sampling from cyanide-contaminated areas from the Muteh gold mine and isolation of 24 bacteria were performed successfully. The selected bacteria—‘Bacillus sp. M01’—showed maximum tolerance (15 mM) to cyanide and deposited in Persian Type Culture Collection by PTCC No.: 1908. In the primary experiments, effective factors were identified through the Plackett–Burman design. In order to attain the maximum degradation by Bacillus sp. M01 PTCC 1908, culture conditions were optimized by using response surface methodology. By optimizing the effective factor values and considering the interaction between them, the culture conditions were optimized. The degradation percentage was calculated using one-way ANOVA vs t test, and was found to have increased 2.35 times compared to pre-optimization. In all of the experiments, R2 was as high as 91%. The results of this study are strongly significant for cyanide biodegradation. This method enables the bacteria to degrade 86% of 10 mM cyanide in 48 h. This process has been patented in Iranian Intellectual Property Centre under Licence No: 90533.
Similar content being viewed by others
Introduction
With the increasing population and development of various industries across the world, soil and groundwater are more prone to contamination than ever before. Cyanide is a carbon–nitrogen radical found in inorganic and organic compounds (Dash et al. 2009). It is very dangerous and toxic. Its toxicity is due to its physicochemical properties (Gurbuz et al. 2009). Because of the cyano group (–C≡N) of cyanide, there are several different forms of cyanide found in nature (Luque-Almagro et al. 2011a; Mirizadeh et al. 2014). Historically, in the First World War, cyanide was used as a chemical weapon (Gupta et al. 2010). Cyanide compounds can be used in gold mining, steel manufacturing, steel making, organic and chemical production, electroplating, polymer synthesis, and other industrial processes, in various industries such as dyes and pharmaceutical, agricultural and resins production, and cassava starch industries; hence, environmental experts also have a concern about it (Adams et al. 2001; Barclay et al. 1998; Glanpracha and Annachhatre 2016; Luque-Almagro et al. 2011b; Potivichayanon and Kitleartpornpairoat 2010; Ricaño-Rodríguez and Lepe 2015). Moreover, cellular catalase and mitochondrial cytochrome oxidase, peroxidase, ascorbic acid oxidase, and other oxidases contain cyanide (Dubey and Holmes 1995; Kao et al. 2003; Raybuck 1992). Cyanide is poisonous for all organisms. Therefore, it is crucial to remove it from industrial effluents (Nallapan Maniyam et al. 2015). In the Philippines, collection of food trades and tropical marine fish for aquarium is carried out by using sodium cyanide (Mak et al. 2005). Large amounts of cyanide are produced regularly by industries in waste water streams; it is an intense health hazard for all living things and ecosystem. Cyanides are frequently removed by biodegradation, chemical and physical methods (Kebeish et al. 2015; Pereira et al. 1996). Biodegradation of cyanide is cheaper than chemical and physical methods and faster than natural oxidation. It is more efficient and has less cost (Gupta et al. 2010; Igeño et al. 2007; Karamba et al. 2015). Chemical treatments used for cyanide degradation are toxic itself when released in ecosystem, expensive and produce some other toxic compounds. Biological treatment is thus the best alternative for cyanide degradation (Luque-Almagro et al. 2011b; Watanabe et al. 1998), because it has fewer side effects compared to other methods and is moreover less expensive (Karamba et al. 2015). Some organisms such as fungi, arthropods, plants, and bacteria can produce cyanide (Khamar et al. 2015). Cyanide produced by fungi and bacteria is used to their advantage by generating the antimicrobial cyanide compounds that inhibit rival organisms (Basile 2008). In this survey, isolation, screening, and biochemical characterization of Bacillus sp. M01 PTCC 1908 in the context of significant cyanide degradation capability were studied that can be used in many industries. Isolation and identification of cyanide degrading bacteria are a very important step, and their ability has been studied. Also, cyanide degradation has been optimized by several methods such as Plackett–Burman design and response surface methodology (RSM). Statistical analysis of the model validation was performed by one-way ANOVA vs. t test.
Materials and methods
Materials
All materials for this study were purchased from Merck Company. The DNA extraction kit, PCR purification kit, and Master Mix for PCR amplification were bought from Bioneer Company. The 1-Kb DNA Ladder, high pure agarose, and primers were prepared by Massruler™, Invitrogen, and Cinnagen respectively. BeckMan Spectrophotometer DU530 and GFL Shaker Incubator 3031, 3033 were also used.
Softwares
GraphPad Prism 6 (San Diego, CA, USA) was used to depict the graph. Method designing and data analysis were performed using Minitab 17.1.0 Statistical package (Minitab Inc., State College, PA, USA) and Design Expert 7.0.0 (Stat-Ease, Inc., Minneapolis, MN, USA).
Samples and culture condition
Bacterial samples were isolated from the wastewater and soil found at the Muteh gold mine (Isfahan-Iran). Bacteria were cultured in nutrient broth medium (pH 6.8) and incubated in a shaking incubator at 37 °C and 180 rpm. All strains were maintained on nutrient agar.
Screening and bacterial isolation
For screening cyanide-degrading bacteria, 0.5% (V/V) of overnight culture (OD600 = 0.86) was inoculated into the nutrient broth medium. Subsequently, different quantities of filter–sterilized KCN were added to determine the degrading ability concentration of cyanide. When the bacteria reached 0.5 McFarland standard turbidity, they were added into the nutrient broth samples containing varying concentration of KCN (1.5, 2.3, and 3.8, 7.6, 11.5, and 15.3 mM). They were then incubated at 180 rpm and 37 °C for 48 h. After this, 1 mL of harvested culture was plated onto nutrient agar. The plate containing the nutrient agar was incubated at 37 °C for 24 h.
Identification of Bacillus sp. M01
The Bacillus sp. M01 was identified by analyzing the microbiological and biochemical characteristics of the bacterial colonies.
For molecular identification, the 16S rDNA sequence was studied. The 16S rDNA gene was amplified by PCR using bacterial universal primers 27F (5′AGAGTTTGATCMTGGCTCAG3′) and 1492R (5′TACGGYTACCTTGTTACGACTT3′) (Lane 1991), with an annealing temperature of 50 °C for 30 s and an extension time of 1 min for 30 cycles.
Bacillus sp. M01 deposited in Persian Type Culture Collection (PTCC) by PTCC No.: 1908.
Biodegradation and quantifying the concentration of cyanide by Bacillus sp. M01 PTCC 1908
Bacillus sp. M01 PTCC 1908 was cultured in nutrient broth medium containing 10 mM of cyanide. After 48 h, the residual cyanide in culture supernatant was measured through picric acid colorimetric assay. Color intensity is correlated with the concentration of cyanide. Absorbance was measured at 492 nm by a UV spectrophotometer. The amount of cyanide consumed by Bacillus sp. M01 PTCC 1908 was measured by calculating the absorbance difference between the positive control and the cell-free supernatant (Fisher and Brown 1952; Kandasamy et al. 2015).
Plackett–Burman design (PBD)
The Plackett–Burman design was used to identify the most important factors in the initial phase of the experiment and to find the key factors for cyanide biodegradation (Liu and Tang 2010; Plackett and Burman 1946). The factors and values is shown in Table 1 in coded form.
Central composite design (CCD)
The most effective factors were further optimized by 20-run experimental plan using the CCD method. These factors were studied at five different levels, coded as − α, − 1, 0, + 1 and + α. The screening of factors selected through the Plackett–Burman design is shown in Table 2, in preparation for the CCD optimization step.
Moreover, the full experimental plan, with regard to their values in coded form, is provided in Table 1. The effect of variables, interaction between them and statistical analysis was performed to achieve the high degradation (Altaf et al. 2006; Dayananda et al. 2005). The equation used in this model is as follows:
where Y is the final cyanide degradation, βi is the model coefficient, and Xi depicts the variables (Modiri et al. 2015).
Statistical analysis of the model
Finally, a series of experiments was performed on optimized, predicted values that were suggested by the software as the optimized points and pre-optimized conditions in five replicates. Data were analyzed using the one-sample t test and one-way ANOVA method. The P value was greater than 0.05, implying that the three variables have a significant impact on the biodegradation of cyanide.
Molecular characterization, performed by 16S rDNA gene sequencing, confirmed the results of the microbiological and biochemical studies (Genbank Accession Number KR996794).
Results
Isolation and screening cyanide-degrading bacteria
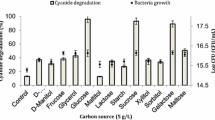
Survival of some bacterial strains in cyanide-contaminated environments implies that they may have the necessary mechanisms to tolerate and degrade cyanide compounds. In this study, cyanide-degrading bacteria were isolated from cyanide-contaminated wastewater and soil from the Muteh gold mine. Bacteria were cultured at different concentrations of KCN at 3 days in three replicates. Screening was performed among 24 isolates (data not shown), one of which was selected as the best cyanide-resistant bacterium that grows in different concentrations of cyanide within 3 days (Fig. 1).
Identification of Bacillus sp. M01 PTCC 1908
In order to identify the selected bacteria, the morphological and biochemical characteristics were studied. According to Bergey’s Manual of Systematic Bacteriology (Kersters and Vancanneyt 2005), the bacteria identified as Bacillus sp. (Table 3) belong to the GRAS bacteria family.
Factors selected by Plackett–Burman design
The factors were screened through the Plackett–Burman design. Three factors—temperature, shaking speed, and inoculation amount—were selected among the five possible variable factors (pH, temperature, cyanide concentration, shaking speed, and inoculation amount). The P values for these three factors were less than 0.05 when α was equal to 0.05. Data analysis and related figures are shown in Table 4 and Fig. 2 respectively (R2 = 96%).
Central composite design and data analysis
According to the model suggested by software, three variables will be selected to achieve the highest percentage of cyanide biodegradation. The factors were studied at five different levels. The full experimental plan, with regard to their values in absolute and cyanide-degrading percentage as a response is provided in Table 5. Models designed with the estimated parameters (e.g. P value and lack of fit) were determined using data analysis (ANOVA) for cyanide biodegradation (Table 6). ANOVA results indicate that the designed model is strongly significant (P value < 0.0001). Lack of fit is a very important parameter—it shows that the error does not have any effect on the model proposed by RSM. Lack of fit value is not significant if P value > 0.05. In this model, lack of fit has been obtained with P value = 0.35, which is an approved value. If this parameter becomes significant, the entire model loses significance.
The regression coefficient (R2) of the model implies that the obtained model is potentially correct. According to the analysis, our model is significant, since the obtained R2, AdjR2, and PredR2 are equal to 92, 87.3, and 80% respectively.
In the end, after all of the software analyses, the final equation for cultural optimization process will be obtained in ANOVA, as is shown in Table 4 and Eq. 2. The 3D response surface plots and contour plot between independent factors is shown in Fig. 3.
3D Response surface plots (I) and contour plot (II) between A: temperature and B: rotation speed (1 and 1′), also between A and C: inoculation amount (2 and 2′). The plots (I), showing the interaction of independent factors for cyanide degradation by Bacillus sp. M01. Moreover, the contour plot (II), showing the maximum rate of the cyanide degradation was shown by dark green color
One-sample t test and one-way ANOVA analysis
To confirm the optimal point predicted by the software, a series of experiments in five replicates were performed for determining the optimal point according to the software prediction and it was analyzed using one-sample t test. Before optimization, cyanide degradation by Bacillus sp. M01 PTCC 1908 was 36.7% at 10 mM initial cyanide concentration. After optimization, degradation percentage was increased to 86.4%. There was no significant difference between the predicted and experimental values, implying that the obtained data are reliable.
The model suggested for this experiment follows the second-order equation. It is very difficult to distinguish the variables with the highest influence on final result. In this experiment, the effect of temperature and shaking speed as well temperature and inoculation amount on biodegradation of cyanide is shown in Fig. 2. Therefore, cyanide biodegradation efficiency is influenced by the interaction between the variables mentioned above.
One-sample t test was employed to confirm the results obtained from RSM. For this step, the P value should be higher than 0.05. After the submission of the quadratic model data, the average of five replicates was obtained from the software. For this section, the P value was evaluated about 0.197, which means that the data follow the suggested model.
For performing one-way ANOVA test, normality and equal variance must be considered; the difference between the five replicates must be insignificant and the model designed by the software should be reliable. Therefore, the P values must be more than 0.05 (in this case they are 0.292, and 0.228 respectively).
One-way ANOVA was employed after confirmation of normality and equal variances. The result of data analysis shows that P value < 0.05 (R2, Adj R2 and R2Pred) > 70% are required. Our results showed that, P value = 0.000, which implies that the experiment is very significant.
Also, R2 > 98.6, AdjR2 > 98.3% and R2Pred > 97.8, implying conformity of the suggested method and practical results. In this research, according to the results obtained from Plackett–Burman, RSM, one-way ANOVA and one-sample t test, the average percentage of cyanide biodegradation using Bacillus sp. M01 PTCC 1908 was increased by 2.35 times (Fig. 4).
Discussion
In this study, the selected bacterium, with the highest tolerance to cyanide, is shown in Fig. 1 (15 mM–976 mg L−1), although in the expression of Soni Tiwari, microorganisms cannot tolerate a high concentration of cyanide (Tiwari et al. 2017). The maximum rate of cyanide degradation was reported to be 700 mg L−1 by (Wu et al. 2014). Chen et al. showed that the K. oxytoca could tolerate a higher CN− concentration of 78 mg L−1 (Chen et al. 2008). Koksunan et al. reported that A. vinelandii could tolerate CN− concentration of 104 mg L−1 (Sarawut et al. 2013), while the Pseudomonas pseudoalcaligenes W2 could tolerate a higher cyanide concentration than 39 mg L−1; its growth was stopped at 52 mg L−1 (Tiong et al. 2015). Pseudomonas putida could grow in 4 mM concentration of cyanide and was inhibited at 8 mM (Chapatwala et al. 1998). Burkholderia cepacia sp. C-3 could tolerate up to 10 mM of cyanide concentration (Adjei and Ohta 2000). An active biological process for the maximum threshold of cyanide biodegradation was observed to be 200 mg L−1 (Kuyucak and Akcil 2013). According to the above-mentioned data, the cyanide resistance of Bacillus sp. M01 PTCC 1908 is higher than that of the others.
Optimization steps were performed, and the results of this experiments showed that at the first condition, with the points suggested by the software (130 rpm, 44 °C and 4.06% inoculation amount), cyanide biodegradation percentage is estimated to be 86%, while in the second condition proposed by the software at 30 °C, 230 rpm, and 1.98% inoculation amount, percentage of cyanide biodegradation is estimated to be about 84.5%. However, in another study, the isolated microbial species were optimized through the numerical optimization technique and the optimized condition was temperature 33.60 °C, pH 9.88 and whey-waste concentration of 14.27 g L−1, which can biodegrade an initial cyanide concentration of 500–206.53 mg L−1 in 96 h (Mekuto et al. 2015). Bacillus sp. CN-22 can tolerate the highest CN− concentration of 700 mg L−1. Under the optimized condition given by RSM, it can also achieve the highest degradation of CN− at 96.69%, by reducing CN− concentrations from 200 to 6.62 mg L−1 in 72 h (Wu et al. 2014). However, for biodegradation of cyanide with an initial CN− concentration of 1 mg L−1 in 3 min, 90% can be removed. This is used for residual cyanide from wastewater treatment with low cyanide concentrations (Hijosa-Valsero et al. 2013). When equipment in the laboratory is not sufficient for conducting the experiment under optimized conditions and the implementation of this process is limited, the second condition is preferred. With regard to Eq. 2, at any time during the performance of this experiment, the result can be obtained by the replacement of the factor values in the equation without needing to perform all the steps for the biodegradation process optimization. Therefore decision can be taken with regard to whether degradation percentage is acceptable. The results of this optimization show that Bacillus sp. M01 PTCC 1908 can degrade 651.2 mg L−1 initial cyanide concentration to 562.5 mg L−1 in 48 h (i.e. 86.4%). A study by Kao et al. reported that cyanide concentration higher than 2.6 mg L−1 can induce a longer lag phase and t test result determined that the difference between growths at cyanide concentration above 13 mg L−1 was not significant. In another research, performed by Akcil et al., the ability of degrading weak acid dissociable cyanide (CNWAD) at 400 ppm cyanide concentration was evaluated and the efficiency was found to be nearly 90% (Akcil et al. 2003).
For removal of wastewater containing cyanide, the biodegradation method has been suggested. In this study we found that, Bacillus sp. M01 PTCC 1908, which has a high resistance (15 mM–976 mg L−1) and degrading ability (10 mM–562 mg L−1), was suggested for biodegradation process in many industries that dealing with this compound. In comparison with other studies, this study reported that if the optimal points of temperature of 44 °C, shaking speed of 130 rpm and inoculation amount of 4.04% are ensured, a higher maximum rate of biodegradation is observed than has ever been reported before. This process has been patented in Iranian Intellectual property centre under Licence No: 90533.
Abbreviations
- RSM:
-
response surface methodology
- ANOVA:
-
analyze of variance
- OD:
-
optical density
- PBD:
-
Plackett–Burman design
- CCD:
-
central composite design
- DF:
-
degree of freedom
- SS:
-
sum of squares
- MS:
-
mean square
- R2 :
-
regression coefficient
- CNWAD:
-
weak acid dissociable cyanide
References
Adams D, Komen J, Pickett T (2001) Biological cyanide degradation. Cyanide: social, industrial and economic aspects. The Metals Society, Warrendale. pp 203–213
Adjei MD, Ohta Y (2000) Factors affecting the biodegradation of cyanide by Burkholderia cepacia strain C-3. J Biosci Bioeng 89(3):274–277
Akcil A, Karahan A, Ciftci H, Sagdic O (2003) Biological treatment of cyanide by natural isolated bacteria (Pseudomonas sp.). Miner Eng 16(7):643–649
Altaf M, Naveena B, Venkateshwar M, Kumar EV, Reddy G (2006) Single step fermentation of starch to l (+) lactic acid by Lactobacillus amylophilus GV6 in SSF using inexpensive nitrogen sources to replace peptone and yeast extract–optimization by RSM. Process Biochem 41(2):465–472
Barclay M, Tett VA, Knowles CJ (1998) Metabolism and enzymology of cyanide/metallocyanide biodegradation by Fusarium solani under neutral and acidic conditions. Enzyme Microb Technol 23(5):321–330
Basile LJ (2008) Cyanide-degrading enzymes for bioremediation. Texas A&M University
Chapatwala K, Babu G, Vijaya O, Kumar K, Wolfram J (1998) Biodegradation of cyanides, cyanates and thiocyanates to ammonia and carbon dioxide by immobilized cells of Pseudomonas putida. J Ind Microbiol Biotechnol 20(1):28–33
Chen C, Kao C, Chen S (2008) Application of Klebsiella oxytoca immobilized cells on the treatment of cyanide wastewater. Chemosphere 71(1):133–139
Dash RR, Gaur A, Balomajumder C (2009) Cyanide in industrial wastewaters and its removal: a review on biotreatment. J Hazard Mater 163(1):1–11
Dayananda C, Sarada R, Bhattacharya S, Ravishankar G (2005) Effect of media and culture conditions on growth and hydrocarbon production by Botryococcus braunii. Process Biochem 40(9):3125–3131
Dubey S, Holmes D (1995) Biological cyanide destruction mediated by microorganisms. World J Microbiol Biotechnol 11(3):257–265
Fisher F, Brown J (1952) Colorimetric determination of cyanide in stack gas and waste water. Anal Chem 24(9):1440–1444
Glanpracha N, Annachhatre AP (2016) Anaerobic co-digestion of cyanide containing cassava pulp with pig manure. Bioresour Technol 214:112–121
Gupta N, Balomajumder C, Agarwal V (2010) Enzymatic mechanism and biochemistry for cyanide degradation: a review. J Hazard Mater 176(1):1–13
Gurbuz F, Ciftci H, Akcil A (2009) Biodegradation of cyanide containing effluents by Scenedesmus obliquus. J Hazard Mater 162(1):74–79
Hijosa-Valsero M, Molina R, Schikora H, Müller M, Bayona JM (2013) Removal of cyanide from water by means of plasma discharge technology. Water Res 47(4):1701–1707
Igeño MI, Orovengua E, Guijo MI, Merchán F, Quesada A, Blasco R (2007) Biodegradation of cyanide-containing wastes by Pseudomonas pseudoalcaligenes CECT5344. Commun Curr Res Educ Topics Trends Appl Microbiol 1:100–107
Kandasamy S, Dananjeyan B, Krishnamurthy K, Benckiser G (2015) Aerobic cyanide degradation by bacterial isolates from cassava factory wastewater. Braz J Microbiol 46(3):659–666
Kao C, Liu J, Lou H, Lin C, Chen S (2003) Biotransformation of cyanide to methane and ammonia by Klebsiella oxytoca. Chemosphere 50(8):1055–1061
Karamba KI, Shukor MY, Syed MA, Zulkharnain A, Adeela N, Yasid AK, Khalil KA, Ahmad SA (2015) Isolation, screening and characterisation of cyanide-degrading Serratia marcescens strain aq07. J Chem Pharm Sci 8:401–406
Kebeish R, Aboelmy M, El-Naggar A, El-Ayouty Y, Peterhansel C (2015) Simultaneous overexpression of cyanidase and formate dehydrogenase in Arabidopsis thaliana chloroplasts enhanced cyanide metabolism and cyanide tolerance. Environ Exp Bot 110:19–26
Kersters K, Vancanneyt M (2005) Bergey’s manual of systematic bacteriology
Khamar Z, Makhdoumi-Kakhki A, Gharaie MM (2015) Remediation of cyanide from the gold mine tailing pond by a novel bacterial co-culture. Int Biodeterior Biodegrad 99:123–128
Kuyucak N, Akcil A (2013) Cyanide and removal options from effluents in gold mining and metallurgical processes. Miner Eng 50:13–29
Lane D (1991) 16S/23S rRNA sequencing. Nucleic acid techniques in bacterial systematics. pp 125–175
Liu R-S, Tang Y-J (2010) Tuber melanosporum fermentation medium optimization by Plackett–Burman design coupled with Draper–Lin small composite design and desirability function. Bioresour Technol 101(9):3139–3146
Luque-Almagro VM, Blasco R, Martínez-Luque M, Moreno-Vivián C, Castillo F, Roldán MD (2011a) Bacterial cyanide degradation is under review: Pseudomonas pseudoalcaligenes CECT5344, a case of an alkaliphilic cyanotroph. Biochem Soc Trans 39(1):269–274
Luque-Almagro VM, Merchan F, Blasco R, Igeño MI, Martínez-Luque M, Moreno-Vivian C, Castillo F, Roldán MD (2011b) Cyanide degradation by Pseudomonas pseudoalcaligenes CECT5344 involves a malate: quinone oxidoreductase and an associated cyanide-insensitive electron transfer chain. Microbiology 157(3):739–746
Mak KK, Yanase H, Renneberg R (2005) Cyanide fishing and cyanide detection in coral reef fish using chemical tests and biosensors. Biosens Bioelectron 20(12):2581–2593
Mekuto L, Ntwampe SKO, Jackson VA (2015) Biodegradation of free cyanide and subsequent utilisation of biodegradation by-products by Bacillus consortia: optimisation using response surface methodology. Environ Sci Pollut Res 22(14):10434–10443
Mirizadeh S, Yaghmaei S, Nejad ZG (2014) Biodegradation of cyanide by a new isolated strain under alkaline conditions and optimization by response surface methodology (RSM). J Environ Health Sci Eng 12(1):1
Modiri S, Sharafi H, Alidoust L, Hajfarajollah H, Haghighi O, Azarivand A, Zamanzadeh Z, Zahiri HS, Vali H, Noghabi KA (2015) Lipid production and mixotrophic growth features of cyanobacterial strains isolated from various aquatic sites. Microbiology 161(3):662–673
Nallapan Maniyam M, Sjahrir F, Latif Ibrahim A, Cass AE (2015) Enzymatic cyanide degradation by cell-free extract of Rhodococcus UKMP− 5M. J Environ Sci Health Part A 50(4):357–364
Pereira P, Arrabaça J, Amaral-Collaco M (1996) Isolation, selection and characterization of a cyanide-degrading fungus from an industrial effluent. Int Biodeterior Biodegrad 37(1):45–52
Plackett RL, Burman JP (1946) The design of optimum multifactorial experiments. Biometrika 33(4):305–325
Potivichayanon S, Kitleartpornpairoat R (2010) Biodegradation of cyanide by a novel cyanide degrading bacterium. World Acad Sci Eng Technol 42:362–1365
Raybuck SA (1992) Microbes and microbial enzymes for cyanide degradation. Biodegradation 3(1):3–18
Ricaño-Rodríguez J, Lepe MR (2015) Purification and characterization of a cyanide-degrading nitrilase from Trichoderma harzianum VSL291. Turk J Biol 39(2):248–257
Sarawut K, Laopaiboon L, Han JH (2013) Growth and cyanide degradation of Azotobacter vinelandii in cyanide-containing wastewater system. J Microbiol Biotechnol 23(4):572–578
Tiong B, Bahari ZM, Lee NSIS, Jaafar J, Ibrahim Z, Shahir S (2015) Cyanide degradation by Pseudomonas pseudoalcaligenes strain W2 isolated from mining effluent. Sains Malays 44(2):233–238
Tiwari S, Tripathi A, Gaur R (2017) Bioremediation of plant refuges and xenobiotics principles and applications of environmental biotechnology for a sustainable future. Springer, Berlin, pp 85–142
Watanabe A, Yano K, Ikebukuro K, Karube I (1998) Cloning and expression of a gene encoding cyanidase from Pseudomonas stutzeri AK61. Appl Microbiol Biotechnol 50(1):93–97
Wu C-F, Xu X-M, Zhu Q, Deng M-C, Feng L, Peng J, Yuan J-P, Wang J-H (2014) An effective method for the detoxification of cyanide-rich wastewater by Bacillus sp. CN-22. Appl Microbiol Biotechnol 98(8):3801–3807
Authors’ contributions
SA and ZJS designed and performed the experiments and helped draft the manuscript. MZ and MM helped design the experiments. SA and MZ conceived the study. All authors read and approved the final manuscript.
Acknowledgements
We would like to thank National Institute of Genetic Engineering and Biotechnology (NIGEB) for providing the research facility. The authors would also like to extend their gratitude to Microbiology laboratory of NourDanesh Institute of Higher Education for help to bacterial isolation.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article.
Consent for publication
Not applicable.
Ethical approval
This article does not contain any studies with human and animals participants performed by any of the authors.
Funding
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Javaheri Safa, Z., Aminzadeh, S., Zamani, M. et al. Significant increase in cyanide degradation by Bacillus sp. M01 PTCC 1908 with response surface methodology optimization. AMB Expr 7, 200 (2017). https://doi.org/10.1186/s13568-017-0502-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13568-017-0502-2