Abstract
Relationships between growth increments of internal shell and age was studied in three neritic decapod cephalopods cultured in laboratory through their entire life cycles. The studied cephalopods were the nektic Sepioteuthis lessoniana d’Orbigny, 1826, Sepia pharaonis Ehrenberg, 1831 and Sepiella inermis Van Hasselt, 1835. Most of the relationship models are in cubic parabolic, except when numbers of increments were estimated from age in S. pharaonis. Differences of numbers of increments from the real age were higher in the pelagic S. lessoniana when compared to the benthic sepiids. The differences were higher in juvenile stages (< 60 days after hatching) than adult stages (> 60 days) in the three species. The increment rate is close to the “one day one increment” assumption. The differences of numbers of increments from the ages and the rate of increment apposition revealed the transition point of the life cycle from 60 days of age, corresponding to the sexual maturity or adult stages. Numbers of increments with higher accuracy are reevaluated to be reliable for age determination at least for the neritic species in the tropical zone, where environmental conditions are more stable, regarding the life styles and stages in life cycles of each species.
Similar content being viewed by others
Introduction
In most decabrachian cephalopods, the length of the internal shell (cuttlebone, gladius) is approximately equal to or at least has a close relationship, to the mantle length. The apposition of growth increments or lamella, hence “growth rings” in shells and other hard structures of cephalopods had been long studied for determination of growth (see in the following paragraph). In fact, the terms “lamella” and “increment” of sepiids used in those mentioned studies are septa, which belongs to the siphucular complex and the septum attachment sites. On the other hand, increments of the gladius in myopsid squids relate to the growth of the shell wall (conotheca). The term “increment” is considered neutral herein referring to both septal structures and structures on the conotheca.
The growth increments are likely to occur on a daily basis in sepiid cuttlefish (Bello, 2001; Bettencourt & Guerra, 2001; Boonprakob et al., 1977; Choe, 1962, 1963; Chung & Wang, 2013; Le Goff et al., 1998; Martinez et al., 2000; Natsukari et al., 1991; Re & Narciso, 1994; Richard, 1969; Satayalai & Boonprakob, 1980; Suriyawarakul, 2003; Yagi, 1960a, 1960b; Yasunaga et al., 1971), in myopsid squids (Jackson et al., 1993; Khatami et al., 2017; Perez et al., 2006; Yamrungrueng, 2004; Yamrungrueng et al., 2003) and in oegopsid squids (Arkhipkin & Bizikov, 1991; Bizikov, 1991; Gong et al., 2018; Hughes, 1998; Perez, 1995; Perez et al., 1996; Schroeder & Perez, 2013). However, the numbers of increments might vary, at different degrees, upon fluctuations of biotic and abiotic factors of the environment (Natsukari et al., 1991). Such variation discredited the use of the method of increment counts for age estimation, regardless that most of the information was obtained from a part not the whole life cycle and without the exact known age.
Culture of cephalopods in laboratory with stable water properties (temperature, salinity, and pH) is a proper method to obtain information useful for age reconstruction. Three neritic species, Sepioteuthis lessoniana d’Orbigny, 1826, Sepia pharaonis Ehrenberg, 1831 and Sepiella inermis Van Hasselt, 1835, that can be cultured from hatching through entire life cycles (Nabhitabhata & Ikeda, 2014; Nabhitabhata, 2014a, 2014b) were studied. These species have different life styles. The bigfin squid, S. lessoniana (Myopsida) has a neritic nektonic life style, while S. pharaonis and S. inermis represented sepiid cuttlefish with a nekto-benthic life style. S. lessoniana and S. pharaonis are open waters species, and the third species, S. inermis was known of its ability to inhabit brackish water or the estuarine zone (Nabhitabhata, 1997). S. lessoniana and S. pharaonis are suspected to be species complexes with several morphotypes (Cheng et al., 2014; Izuka et al., 1994, 1996; Jivalak et al., 2005; Triantafillos & Adams, 2005; Tuanapaya & Nabhitabhata, 2017), inhabiting the Indo-Pacific region. However, current molecular or morphological studies supported that the differences were of population structure, not of species (Aoki et al., 2008; Imai & Aoki, 2012; Tuanapaya & Nabhitabhata, 2017). The present study was performed on the Pacific “types” from the Gulf of Thailand, western Pacific Ocean.
Our study focuses on the relationships of numbers of growth increments and age, hence patterns based on the “one day, one increment” assumption. The obtained relationship models were reevaluated whether the numbers of increments could be used as an alternate method for age determination of species that inhabited less fluctuated environments of tropical waters. The reason relied on advantages of this method which are with much lower expenses as well as with a simple specimen-preparation procedure compared to other methods using hard structures, i.e., beaks, statoliths and stylets. According to this, the main objective of this study is to propose a brief and/or simple model for estimation of cephalopod age for each of the three species to be used in the field and onboard. The single model for individual species is aware to be different from asymptotic biomass-growth models which vary upon ontogenetic phases (see ontogenetic growth models in Nabhitabhata, 2014a, 2014b; Nabhitabhata & Ikeda, 2014).
Materials and methods
Neritic decapod cephalopods, Sepioteuthis lessoniana and Sepia pharaonis were cultured in laboratory from hatching through their entire life cycles, obtaining specimens of exactly known age after hatching. Broodstocks and/or live eggs were collected from the wild, following Nabhitabhata and Segawa (2014). Eggs were nursed and hatchlings were reared through entire life cycles in concrete tanks with opened water systems. The cephalopods were fed ad libitum with live feeds on the first 30 days after hatching and, later, with chopped fresh fish meat. The detail of culture methods of S. lessoniana followed Nabhitabhata (1996) and Nabhitabhata and Ikeda (2014) and of Sepia pharaonis followed Nabhitabhata (2014a) and Nabhitabhata and Nilaphat (1999), respectively. The life cycles of cultured batches were approximately 120 days after hatching for S. lessoniana (Nabhitabhata, 1996) and 180 days for S. pharaonis (Nabhitabhata & Nilaphat, 1999). The water parameters were maintained, temperature 28‒30 °C, salinity 30‒32 g l−1 and pH 6.5‒8.0. Ten specimens were sampled from the culture batches every 10 days and sacrificed for their internal shells. In total 160 specimens of S. pharaonis and 170 specimens of S. lessoniana have been investigated. The study of numbers of increments of laboratory cultured Sepiella inermis based on Boonprakob et al. (1977) and Satayalai and Boonprakob (1980) from 100 specimens. Their culture method was similar to methods of Nabhitabhata (1997, 2014b).
The obtained gladii of S. lessoniana were stained by immersing in a 100 g l−1 solution of tetracycline for 2 h. The growth increments of stained gladii were marked with the Red Food-Dye (a local brand) before counting under the microscopes (Yamrungrueng, 2004; Yamrungrueng et al., 2003).
The cuttlebones of S. pharaonis were immersed in 10% KOH for 12 h. The dorsal surface of large cuttlebones was peeled off and then rubbed with an iron file and fine-sand papers to enhance the appearance of the lamellae. The preparation of the cuttlebone of S. inermis was also similar to S. pharaonis. The difference was that the dorsal surface of cuttlebones of the former species were not peeled off, because the whole cuttlebones were thinner (Boonprakob et al., 1977) than ones of the latter species. The counting of growth increment of prepared cuttlebones was performed under light microscopes (Le Goff et al., 1998; Suriyawarakul, 2003).
The relationships between number of increments and ages were based on mean values and their standard error and expressed through fitting data to the regression models, where A = Ages (days after hatching); LN = total numbers of increments (nos); ML = mantle length (mm).
Results
Number of increments of hatchling
The internal shells of neritic cephalopods already show increments at hatching which developed during the late embryonic stages. Hatchlings of the teuthiid squid S. lessoniana have on average 5.1 ± 0.9 increments and hatchlings of S. pharaonis and S. inermis have on average 7.6 ± 0.5 and 5.8 ± 0.4 increments, respectively (Table 1).
Relationships between mantle length and number of increments
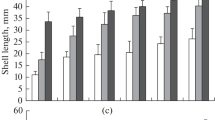
The number of increments could be estimated from their relationships with the mantle length. All obtained models were in a similar pattern, cubic parabolic, without asymptotes in the three species.
The relationships between the mantle length and number of increments (Fig. 1) were:
Relationships between age and number of increments
The number of increments could also be estimated from the relationships with the ages after hatching in similar manners to the mantle length. The obtained models were in different patterns according to species (Fig. 2).
The model was cubic parabolic in S. lessoniana:
The pattern of relationship was linear in S. pharaonis:
The relationship was also in a cubic parabola pattern in S. inermis (Fig. 2):
Relationship between number of increments and age
On the other hand, age could be estimated from relationships with number of increments (Fig. 3). The obtained models were in different patterns according to species.
The relationship between number of increments and ages was in a cubic parabolic pattern in S. lessoniana:
The model was linear in S. pharaonis:
The relationship was in a cubic parabolic pattern in S. inermis:
Differences of number of increments from age
The difference of number of increments from ages in the three species is lowest in S. inermis 5.1 ± 3.2 followed by 9.6 ± 1.2 increments in S. pharaonis, and to 25.1 ± 5.5 increments in S. lessoniana (Fig. 4, Table 1). However, the number of increments from the entire life cycles (0‒160 days of age) are significantly reduced after 60 days (71‒160 days), 11.4 ± 4.3, 11.7 ± 1.6 and 39.6 ± 5.8 increments, respectively.
The patterns of differences from the real ages were lowest in S. pharaonis and highest in S. lessoniana, and were lower in juvenile (0‒60 days) than in adult (70‒160 days) stages (Table 1).
Daily increment formation rate
For the entire life cycles, the daily increment formation rate was similar in 0.9 ± 0.1, 1.1 ± 0.1 and 1.0 ± 0.1 increment/day in S. lessoniana, S. pharaonis and S. inermis, respectively (Fig. 5, Table 1). The daily formation rate for 0‒60 days of age averaged 1.0 ± 0.1, 1.0 ± 0.1 and 0.9 ± 0.1 increment/day, respectively. The rate in adult from after 70 days of age was lower than in juveniles only in S. lessoniana, 0.8 ± 0.2 increment/day, but it is similar in the latter two species through their entire life cycles.
Discussion
The age of cuttlefish can be correctly assessed from the number of increments of their cuttlebone if they grew under known constant temperature conditions. This was proven by the studies of Martinez et al. (2000) on S. elliptica Hoyle, 1885 reared at 25 and 30 °C, and Bello (2001) on wild S. orbignyana Ferussac, 1826. It is in contrasts to the rate of increment formation in Sepia officinalis Linnaeus, 1758 that strongly depended on different water temperatures as reported by Richard (1969), Le Goff et al. (1998) and Bettencourt and Guerra (2001). The increment formation rate in S. officinalis is approximately 0.1 and 0.6 increment/day at 13 and 25 °C in the wild stocks (Le Goff et al., 1998), and 0.1‒0.2 increment/day at 13‒15 °C, 0.3 at 18‒20 °C, 0.4 at 20 °C and 0.6 at 25 °C in cultured batches (Bettencourt & Guerra, 2001; Richard, 1969). When compared to the rate 1.1 ± 0.1 increment/day in S. pharaonis at 28‒30 °C in the present study, the differences might be caused by the wider range of temperature fluctuation in cultured tanks in the former study. On the other hand, there might also be a species-specific temperature optimum as suggested by O’Dor and Wells (1987), which requires further studies.
However, the number of increments does not depend on the temperature alone, but also on nutrition and food availability. Choe (1962, 1963), Boletzky (1974), Boletzky and Wiedmann (1978) and Wiedmann and Boletzky (1982) reported that the formation rate of increment in the cuttlebone of S. esculenta Hoyle, 1885, S. lycidas Gray, 1849 (as S. subaculeata), S. officinalis and Sepiella japonica Sasaki, 1929 (as Sepiella maindori) was affected by nutritive value (food deficiency and availability) and environmental conditions (salinity, oxygen, temperature). Martinez et al. (2000) reported that food scarcity exaggerated the effect of small temperature difference on cuttlebone growth of S. elliptica. The present study (with excess feeding) agreed to Choe (1962, 1963), Boonprakob et al. (1977) and Satayalai and Boonprakob (1980) that under rearing conditions with sufficient feeding and less fluctuating temperature, the increments of S. inermis were formed at the rate of one increment per day. Moreover, Boletzky (1974), Boletzky and Wiedmann (1978), Wiedmann and Boletzky (1982) and Natsukari et al. (1991) stated that the ecological stress (starvation) on S. officinalis affected increment intervals (causing lower distance between increments, so called “lamella crowding”) and locular indices, but not the number of increments. In oceanic oegopsid squids, after rearing of Illex illecebrosus (Lesueur, 1821) for 24‒56 days and Todarodes pacificus (Steenstrup, 1880) for 15 days, Perez (1995) and Hughes (1998) supported the statement that the number of increments of gladius was accurate when the squids were actively feeding and was not affected by the temperature.
Ontogenetic growth and maturity stages also have their role on the number of increments. Chung and Wang (2013) pointed out that the temperature effect was not significant at least for hatchlings of S. pharaonis supporting our suggestion, namely that ontogenetic and life cycle stages are more significant factors than temperature. Re and Narciso (1994) reported that the number of increments was related to growth rate rather than age in juveniles S. officinalis (0‒19 days). However, the present study revealed that the number of increments from hatching to 60 days in the three species are considered to be reliable for age determination as a daily increment. The transition and subsequent fluctuation in number of increments after 60 days corresponded to sexual maturity stages and reproduction phase in the three studied species (Nabhitabhata, 1996, 1997; Nabhitabhata & Nilaphat, 1999), when energy was consequently diverted and reallocated of from growth to reproduction. There were no sex-related differences and the increments formed at a constant rate in both sexes, as also reported for S. orbignyana (Bello, 2001).
Differences of the increment formation rate were also a consequence of ontogenetic phase. In juvenile stages (0‒60 days) of S. pharaonis and S. inermis in the present study, the rate of increment formation was slower than in adult stages (after 60 days or maturity), indicating its relationship with sexual maturity. Yasunaga et al. (1971) had a similar view for S. officinalis (sic S. officinaris). This was in contrast to other species of sepiid cuttlefish. Re and Narciso (1994) and Bettencourt and Guerra (2001) reported a higher variability in the juvenile stages of S. officinalis. Yagi (1960a) reported the daily increment of cuttlebone of S. esculenta captured from Tokyo Bay was 0.7 increment/day in early life stages, and gradually decreased to 0.3 through their life cycles. It is notable that the age of the cuttlefish in Yagi (1960a) was estimated from observation of hatching in the study area and the temperature was not mentioned.
The present study agreed to Bettencourt and Guerra (2001) that subtraction of the actual number of increments with known number of increments at hatching is necessary and could enhance accuracy of age estimation. However, it should be mentioned that number of increments of hatchlings varied among species within the range of 5‒9 increments in average. The number of increments were 6‒9 in S. officinalis hatchlings (Bettencourt & Guerra, 2001; Le Goff et al., 1998; Sen, 2013), 6‒8 in S. esculenta, 7‒11 in S. lycidas, and 7‒9 in S. japonica (Choe, 1962), including the three species in the present study (Table 1). These figures reflect that the embryonic development of shells occurred at about 1 week before hatching. In other words, it is the time when the first increment was formed. In S. officinalis, the formation was observed from embryonic stages 24‒26 before hatching at stage 30 (Boletzky et al., 2016; Pabic et al., 2016; Sen, 2013).
The number of increments in the present study of the two species of cuttlefish, S. inermis and, particularly, S. pharaonis, are more reliable compared to the squid, S. lessoniana. Such differences might be consequences of the nektonic life styles of S. lessoniana with a faster growth rate requiring a higher metabolic rate, compared to the nekto-benthic life styles with a lower metabolic rate of the cuttlefish. However, this study still supported the assumption that increments were formed on a daily basis in the pelagic S. lessoniana. Perez (1995) and Perez et al. (1996) also agreed to a daily basis assumption from studies on a reared pelagic squid, I. illecebrosus.
The number of increments which differ among different studies might be due to different methods of counting. Jackson et al. (1993) reported the ontogenetic change in the gladius daily increment of cultured S. lessoniana (at average 24 °C), decreasing from > 1 increments/day in juveniles to 0.02 after 87 days of age. Such result although agreed to S. lessoniana of the present study, which the daily increment decreased from 1.0 to 0.8 increment/day after 60 days, but was at a much different degree. The reason for such differences might because of differences in temperature and culture conditions and/or human error in counting from gladius specimens that were not stained in the former study. Jackson et al. (1993) stated that it was not possible to ascertain the counting of the number of increments because of their faintness. The faintness of gladius increments was also reported in another myopsid squid, Uroteuthis duvaucelii (d’Orbigny, 1835) (Khatami et al., 2017). Hughes (1998) suggested that the faintness in the oegopsid, T. pacificus occurred, because the feeding pattern was disturbed by the stress in culture conditions.
From the present study, number of increments are reliable for age determination at least for juveniles (0‒60 days) of neritic species in the tropical zone, where environmental conditions relatively stable. The “one day, one increment” assumption is supported by the present study of the three studied species. In comparison between the stages life cycles, the support was stronger in juvenile stages than in (mature) adult stages. In comparison among the three species, the strongest support was from the benthic S. pharaonis through its entire life, regardless of growth stages. The present study agreed to Natsukari et al. (1991) that the number of increments is a valuable age index for tropical species for age estimation as in S. esculenta. The present study also agreed to Yagi (1960a, 1960b) that obtained models could be used for estimation of age in adult stages, as well as comparison among species of sepiid cuttlefish.
The obtained single model through the entire life cycles regardless of asymptotic growth phases should be a practical tool for preliminary estimation of age in the field as well as in laboratory aquaculture. Results of the present studies are essential in the management of wild stocks of commercially important cephalopod species, since age estimation of captured specimens is a parameter of utmost importance in most fishery biology models, i.e., growth, age-at-maturity, fishing mortality. What to be aware for further applications is that the present studies were obtained from cultured animals (in captivity) and certain degree of variations from wild animals are expected.
Conclusions
The study was performed on three species of neritic cephalopods cultured in laboratory through their entire life cycles. Relationships between growth increments of internal shell and age are closed to the “one day one increment” assumption in lesser fluctuated environmental conditions. Brief models for each species were estimated as a reevaluation for age determination for using the field and laboratory.
Availability of data and materials
All specimens are deposited at the Reference Collection of Phuket Marine Biological Center, Phuket, Thailand.
References
Aoki, M., Imai, H., Naruse, T., & Ikeda, Y. (2008). Low genetic diversity of oval squid Sepioteuthis cf. lessoniana (Cephalopoda: Loliginidae) in Japanese Waters inferred from mitochondrial DNA non-coding region. Pacific Science, 62(3), 399–407.
Arkhipkin, A. A. & Bizikov, V. A. (1991). Comparative analysis of age and growth rates estimation using statoliths and gladius in squids. In P. Jereb, S. Ragonese & S. v. Boletzky (Eds.), Squid Age Determination Using Statoliths (pp. 19‒33). Palermo: N.T.R.-I.T.P.P. special publication no. 1.
Bello, G. (2001). Dimorphic growth in male and female cuttlefish Sepia orbignyana (Cephalopoda: Sepiidae) from the Adriatic Sea. Helgoland Marine Research, 55, 124–127.
Bettencourt, V., & Guerra, A. (2001). Age studies based on daily growth increments in statoliths and growth lamellae in cuttlebone of cultured Sepia officinalis. Marine Biology, 139, 327–334.
Bizikov, V. A. (1991). A new method of squid age determination using the gladius. In P. Jereb, S. Ragonese & S. v. Boletzky (Eds.), Squid Age Determination Using Statoliths (pp. 39‒51). Palermo: N.T.R.-I.T.P.P. special publication no. 1.
Boletzky, S. V., (1974). Effets de la sous-nutrition prolongée sur le développement de la coquille de Sepia officinalis L. (Mollusca, Cephalopoda). Bulletin De La Société Zoologique De France, 99(4), 667–673.
Boletzky, S. V., Andouche, A., & Bonnaud-Ponticelli, L. (2016). A developmental table of embryogenesis in Sepia officinalis. Vie Et Milieu - Life and Environment, 66(1), 11–23.
Boletzky, S. V., & Wiedmann, J. (1978). Schulp-wachstum bei Sepia officinalis in abhängigkeit von ökologischen parameter. Neues Jahrbuch Fur Geologie Und Palaontologie, 157(1/2), 103–106.
Boonprakob, P., Satayalai, O., Sithigornkul, P., Siripoonya, P., & Yodyingyuad, U. (1977). Biological study of cuttlefish Sepiella inermis. Technical Report of Royal Silver Jubilee Research Fund (pp. 1–41). Chulalongkorn University.
Cheng, S. H., Anderson, F. E., Bergman, A., Mahardik, G. N., Muchlisin, Z. A., Dang, B. T., Calumpon, H. P., Mohamed, K. S., Sasikumar, G., Venkatesan, V., & Barber, P. H. (2014). Molecular evidence for co-occurring cryptic lineages within the Sepioteuthis cf. lessoniana species complex in the Indian and Indo-West Pacific Oceans. Hydrobiology, 725, 165–188.
Choe, S. (1962). The shell and the locular index of the cuttlefishes, Sepia esculenta Hoyle, Sepia subaculeata Sasaki and Sepiella maindroni De Rochebrune. Bulletin of the Japanese Society of Scientific Fisheries, 28(11), 1082–1091.
Choe, S. (1963). Daily age markings on the shell of cuttlefishes. Nature, 197, 306–307.
Chung, M. T., & Wang, C. H. (2013). Age validation of the growth lamellae in the cuttlebone from cultured Sepia pharaonis at different stages. Journal of Experimental Marine Biology and Ecology, 447, 132–137.
Gong, Y., Li, Y., Chen, X., Gao, X., & Chen, L. (2018). Gladius growth pattern and increment of jumbo squid (Dosidicus gigas) in the tropical Pacific Ocean. Aquaculture and Fisheries, 3, 156–162.
Hughes, S. (1998). Growth history patterns in squid as assessed by gladius structure (pp. 1–187). Doctor of Philosophy thesis. University of Aberdeen.
Imai, H., & Aoki, M. (2012). Genetic diversity and genetic heterogeneity of bigfin reef squid “Sepioteuthis lessoniana” species complex in northwestern Pacific Ocean. In M. Caliskan (Ed.), Analysis of genetic variation in animals (pp. 151–166). InTech.
Izuka, T., Segawa, S., & Okutani, T. (1996). Biochemical study of the population heterogeneity and distribution of the oval Squid Sepioteuthis lessoniana complex in southwestern Japan. American Malacological Bulletin, 12, 129–135.
Izuka, T., Segawa, S., Okutani, T., & Numachi, K. (1994). Evidence on the existence of three species in the oval squid, Sepioteuthis lessoniana complex in Ishigaki Island, Okinawa, South-western Japan, by isozyme analyses. Venus, 53, 217–228.
Jackson, G. D., Arkhipkin, A. I., Bizikov, V. A., & Hanlon, R. T. (1993). Laboratory and field corroboration of age and growth from statoliths and gladii of the loliginid squid Sepioteuthis lessoniana (Mollusca: Cephalopoda). In T. Okutani, R. K. O’Dor, & T. Kubodera (Eds.), Recent advances in cephalopod fisheries biology (pp. 189–199). Tokai University Press.
Jivaluk, J., Nabhitabhata, J., Nateewathana, A., & Watprasit, P. (2005). Description of Thai type of bigfin reef squid, Sepioteuthis lessoniana, hatchling with note on comparison to Japanese types. Phuket Marine Biological Center Research Bulletin, 66, 117–126.
Khatami, S., Valinassab, T., & Kaymaram, F. (2017). Morphometric variation and growth rate of Uroteuthis duvaucelii (Cephalopoda: Loliginidae) in the Persian Gulf and Oman Sea using gladius increments. Iranian Journal of Fisheries Sciences, 16(2), 851–857.
Le Goff, R., Gauvrit, E., Sel, G. P., & Daguzan, J. (1998). Age group determination by analysis of the cuttlebone of the cuttlefish Sepia officinalis L. in reproduction in the Bay of Biscay. Journal of Molluscan Studies, 64, 183–193.
Martinez, P., Bettencourt, V., Guerra, A., & Moltschaniwskyj, N. A. (2000). How temperature influences muscle and cuttlebone growth in juvenile cuttlefish (Sepia elliptica) (Mollusca: Cephalopoda) under conditions of food stress. Canadian Journal of Zoology, 78, 1855–1861.
Nabhitabhata, J. (1996). Life cycle of cultured big fin squid, Sepioteuthis lessoniana Lesson. Phuket Marine Biological Center Special Publication, 16, 83–95.
Nabhitabhata, J. (1997). Life cycle of three cultured generations of spineless cuttlefish, Sepiella inermis (Ferrussac & d’Orbigny, 1848). Phuket Marine Biological Center Special Publication, 17(1), 289–298.
Nabhitabhata, J. (2014a). Main cultured cephalopods: Sepia pharaonis. In J. Iglesias, L. Fuentes, & R. Villanueva (Eds.), Cephalopod culture (pp. 205–224). Springer.
Nabhitabhata, J. (2014b). Main cultured cephalopods: Sepiella inermis. In J. Iglesias, L. Fuentes, & R. Villanueva (Eds.), Cephalopod culture (pp. 225–240). Springer.
Nabhitabhata, J., & Ikeda, Y. (2014). Main cultured cephalopods: Sepioteuthis lessoniana. In J. Iglesias, L. Fuentes, & R. Villanueva (Eds.), Cephalopod culture (pp. 315–347). Springer.
Nabhitabhata, J., & Nilaphat, P. (1999). Life cycle of cultured pharaoh cuttlefish, Sepia pharaonis Ehrenberg, 1831. Phuket Marine Biological Center Special Publication, 19(1), 25–40.
Nabhitabhata, J., & Segawa, S. (2014). Aquaculture to restocking. In J. Iglesias, L. Fuentes, & R. Villanueva (Eds.), Cephalopod culture (pp. 113–130). Springer.
Natsukari, Y., Hirata, S., & Washizaki, M. (1991). Growth and seasonal change of cuttlebone characters of Sepia esculenta. In E. Boucaud-Camou (Ed.), La seiche the cuttlefish (pp. 49–67). Universite de Caen.
O’Dor, R. K., & Wells, M. J. (1987). Energy and nutrient flow. In P. R. Boyle (Ed.), Cephalopod life cycles comparative reviews (Vol. II, pp. 109–133). Academic Press.
Pabic, Le., Rousseau, M., Bonnaud-Ponticelli, L., & Boletzky, S. V. (2016). Overview of the shell development of the common cuttlefish Sepia officinalis during early-life stages. Vie Et Milieu - Life and Environment, 66(1), 35–42.
Perez, J. A. A. (1995). The early life history of the short-finned squid, Illex illecebrosus (Cephalopoda: Ommastrepidae), as reconstructed from the gladius structure (pp. 1–150). Doctor of Philosophy thesis. Dalhousie University.
Perez, J. A. A., Aguiar, D. C., & Santos, J. A. T. (2006). Gladius and statolith as tools for age and growth studies of the squid Loligo plei (Teuthida: Loliginidae) off southern Brazil. Brazilian Archives of Biology and Technology, 49(5), 747–755.
Perez, J. A. A., O’Dor, R. K., Beck, P., & Dawe, E. G. (1996). Evaluation of gladius dorsal surface structure for age and growth studies of the short-finned squid, Illex illecebrosus (Teuthoidea: Ommastrephidae). Canadian Journal of Fisheries and Aquatic Science, 53, 2837–2846.
Re, P., & Narciso, L. (1994). Growth and cuttlebone microstructure of juvenile cuttlefish, Sepia officinalis L., under controlled conditions. Journal of Experimental Marine Biology and Ecology, 177, 73–78.
Richard, A. (1969). The part played by temperature in the rhythm of formation of markings on the shell of cuttlefish (Sepia officinalis L.) (Mollusca, Cephalopoda). Experientia, 25(10), 1051–1052.
Satayalai, O., & Boonprakob, P. (1980). Relationships between cuttlebone lamellae and age of spineless cuttlefish Sepiella inermis. Science Journal, 34(3), 276–281.
Schroeder, R., & Perez, J. A. A. (2013). Individual growth of the squid Illex argentinus off Brazil as reconstructed from the gladius microstructure. Journal of the Marine Biological Association of the United Kingdom, 93(6), 1653–1662.
Sen, H. (2013). Sübye [Sepia officinalis (Linneaus, 1758)]’nin iç kabuk gelişimi. Ege Journal of Fisheries and Aquatic Sciences, 30(3), 105–108.
Suriyawarakul, J. (2003). Relationship of age of pharaoh cuttlefish, Sepia pharaonis Ehrenberg, 1831 with cuttlebone lamellae and beak morphometric (pp. 1–97). Master of Science (Marine Science) thesis. Kasetsart University.
Triantafillos, L., & Adams, M. (2005). Genetic evidence that the northern calamary, Sepioteuthis lessoniana, is a species complex in Australian waters. ICES Journal Marine Science, 62, 1665–1670.
Tuanapaya, S., & Nabhitabhata, J. (2017). Morphometry and morphological phylogeny of Sepia pharaonis Ehrenberg, 1831 complex in Thai waters. Marine Biodiversity, 47, 763–777.
Wiedmann, J., & Boletzky, S. V. (1982). Wachstum und differenzierung des schulps von Sepia officinalis unter künstlichen aufzuchtbedingungen—grenzen der anwendung im palökologischen modell. Neues Jahrbuch Fur Geologie Und Palaontologie, 164(1/2), 118–133.
Yagi, T. (1960a). On the growth of the shell in Sepia esculenta Hoyle caught in Tokyo Bay. Bulletin of the Japanese Society of Scientific Fisheries, 26(7), 646–652.
Yagi, T. (1960b). Studies on the identification method of races in Sepia esculenta Hoyle by using the locular index of shells. Bulletin of the Japanese Society of Scientific Fisheries, 26(7), 640–645.
Yamrungrueng, A. (2004). Age determination and growth of bigfin squid (Sepioteuthis lessoniana Lesson, 1830) from gladius lamellae. Master of Science (Fisheries Science) thesis (pp. 1–83). Kasetsart University.
Yamrungrueng, A., Nabhitabhata, J., Chotiyaputta, C., Tappanand, T., Boonwanit, T., Promboon, P., & Jaroongpattananon, C. (2003). Age determination from gladius lamellae of reared bigfin reef squid, Sepioteuthis lessoniana Lesson. In J. Nabhitabhata & P. Nilaphat (Eds.), Programme and Abstracts International Workshop and Symposium CIAC 2003 Biology, Recruitment and Culture of Cephalopods, 17–21 February 2003 (p. 127). Department of Fisheries.
Yasunaga, Y., Kajihara, T., & Nishiwaki, M. (1971). Biological studies of squids and cuttle-fish stocks—III. On the growth patterns of the shell of Sepia officinaris, a cuttle-fish trawled in the western coastal sea of Africa. Bulletin of Tokai Regional Fisheries Research Laboratory, 66, 155–159.
Acknowledgements
This article is dedicated to Sigurd von Boletzky, a long-time mentor, colleagues and friend of the first author, who encouraged the studies and publishing the outcome of cephalopod research in Thailand.
Funding
JN, KT and ST were supported by Prince of Songkla University research fund. JS was supported by Rajamangala University of Technology research fund and AY by Department of Fisheries research fund.
Author information
Authors and Affiliations
Contributions
JN had the idea of the concept of the paper, estimated the statistical models. JS cultured the squids, collected specimens and data and performed the descriptive statistics. AY did similar work on the cuttlefish. KT and ST prepared the preservation of internal shells and figures. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
All authors have no competing interests.
Additional information
Editorial handling: René Hoffmann.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nabhitabhata, J., Suriyawarakul, J., Yamrungrueng, A. et al. Relationships of growth increments of internal shells and age through entire life cycles in three cultured neritic cephalopods (Mollusca: Cephalopoda) with re-evaluation as application for age determination. Swiss J Palaeontol 141, 5 (2022). https://doi.org/10.1186/s13358-022-00249-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13358-022-00249-z