Abstract
Background
Mesenchymal stem cells (MSCs) are one of the most widely studied adult stem cells, while MSC replicative senescence occurs with serial expansion in vitro. We determined whether miR-34a can regulate MSC senescence by directly targeting glycolytic key enzymes to influence glycolysis.
Methods
Detected the effects of miR-34a on MSC senescence and glycolytic metabolism through gene manipulation. Bioinformatics prediction and luciferase reporter assay were applied to confirm that HK1 is a direct target of miR-34a. The underlying regulatory mechanism of miR-34a targeting HK1 in MSC senescence was further explored by a cellular function recovery experiment.
Results
In the current study, we revealed that miR-34a over-expression exacerbated senescence-associated characteristics and impaired glycolytic metabolism. Then we identified hexokinase1 (HK1) as a direct target gene of miR-34a. And HK1 replenishment reversed MSC senescence and reinforced glycolysis. In addition, miR-34a-mediated MSC senescence and lower glycolytic levels were evidently rescued following the co-treatment with HK1 over-expression.
Conclusion
The miR-34a-HK1 signal axis can alleviate MSC senescence via enhancing glycolytic metabolism, which possibly provides a novel mechanism for MSC senescence and opens up new possibilities for delaying and suppressing the occurrence and development of aging and age-related diseases.
Similar content being viewed by others
Background
Aging is an inevitable, natural and complex process characterized by a progressive reduction in physiological integrity and regenerative capacity, which results in an impaired stress response and therefore increased morbidity and mortality [1, 2]. Simultaneously, aging is also a primary risky factor for multiple disorders, such as diabetes, cardiovascular and cerebrovascular diseases, and malignancies as well. Recently, it has been reported that a major contributor to organismal aging is stem cell senescence and exhaustion, which disrupts tissue homeostasis and attenuates organ regeneration [3, 4]. Notably, as individuals age, the quantity of stem cells diminishes, and their ability to proliferate and survive is also weakened [5]. Hence, how to maintain the function and homeostasis of stem cells is one of the indispensable vital factors in the prevention of aging and the treatment of age-related diseases.
Mesenchymal stem cells (MSCs) are one of the widespread studied adult stem cells, and their multipotent behavior was discovered for the first time in 1966 [6]. At present, MSCs have been considered as more feasible and safer source for cell therapy in tissue engineering and regenerative medicine in regard to minimal tumorigenic risk, the unique property of self-renewal, multi-directional differentiation and immunomodulatory ability [7, 8]. Unfortunately, the functions of MSCs are declined with serial expansion in vitro. This process is mediated by telomere-based mechanism and defined as replicative senescence, which severely impede MSC-based basic scientific research and clinical applications. Therefore, mechanistic elucidation of MSC senescence and interventions of anti-MSC senescence merit urgent exploration.
MicroRNAs (miRNAs) have been found to play critical roles in cell cycle, cell proliferation and differentiation, cellular senescence, energy metabolism, and many age-related diseases including cardiovascular and neurological diseases [9,10,11]. They can regulate post-transcriptional processes and thus mediate the expression of target genes. Among them, miR-34a is a senescence-associated miRNA, and mounting evidence indicate that miR-34a expression is enhanced in different tissues and organs with age [12,13,14]. Moreover, miR-34a deletion in mice could down-regulate the expression levels of senescence-associated biomarkers such as P16INK4a and P21 [15]. Interestingly, miR-34a may play a particularly pivotal role in metabolic diseases, such as obesity, type 2 diabetes, non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) [16]. Meanwhile, miR-34a also manipulates glucose and lipid metabolism via targeting related genes [17,18,19]. Nevertheless, whether miR-34a can ameliorate MSC senescence by targeting metabolism-related genes is not yet completely understood.
Metabolism occurs within the cells of living organisms to provide the required energy to carry out their functions. Currently, the regulatory role of metabolism in stem cell senescence has been the research focus, and increasing evidence supports that metabolic signal pathways are closely associated with aging [3, 20]. It has been revealed that MSCs are mainly dependent on glycolysis to produce ATP, and the glycolytic level or the expression levels of glycolytic key enzyme genes also display significant decline in senescent MSCs [21, 22]. Besides, the enhanced glycolysis could promote the osteogenic differentiation of MSCs [23]. As the first committed step rate-limiting enzyme in glucose metabolism, hexokinases (HKs) catalyze the phosphorylation of glucose to glucose-6-phosphate (G-6-P) and sustain a concentration gradient, facilitating glucose entry into cells and the initiation of all major pathways of glucose utilization. Among them, HK1 is a ubiquitously expressed enzyme in all living cells. The latest research reports that HK1 is intently connected with stem cell senescence [24]. However, the regulatory effect of HK1 on MSC replicative senescence and its underlying molecular mechanism remain unknown.
In our previous study, we demonstrated that the expression of miR-34a was remarkably elevated in replicative senescent MSCs and age-related natural senescent MSCs [13]. MiR-34a repletion could repress cell proliferation capability, impair osteogenic differentiation, retard cell cycle and further accelerate MSC senescence. Additionally, it also has been well-established that miR-34a exerts an important role in cellular energy metabolism including glucose metabolism [25], cholesterol efflux [26], lipid metabolism [19] and metabolic diseases [16], which has attracted widespread concerns. What’s more, our latest studies revealed metabolic disorders might be a key driving factor to boost stem cell senescence [24, 27, 28]. And the glycolysis level and the expression levels of glycolytic enzymes were both declined in MSCs derived from senile rats [24]. As mentioned above, we hypothesized that miR-34a might govern a modulatory role in MSC senescence by influencing glucose metabolism. Here, this study aimed to systematically elucidate the effects and underlying molecular mechanism of miR-34a exerting on MSC replicative senescence via gene manipulation and identify HK1 as its direct target gene.
Methods
MSC isolation and culture
The primary culture of MSCs was performed as described previously [29]. In brief, 1–2-month-old healthy, male Wistar rats were obtained from the Experimental Animal Center of Jilin University and euthanized by CO2 inhalation, followed by immersing in 75% ethanol for disinfection of the whole body. Then MSCs were isolated from the bone marrow of limbs and cultured using the whole bone marrow adherent method. MSCs were cultured in Dulbecco’s modified Eagle’s medium with nutrient mixture F-12 (DMEM-F12; HyClone, USA) consisting of 10% fetal bovine serum (FBS; Gibco, USA), 100 U/mL penicillin (Gibco, Invitrogen), and 100 μg/mL streptomycin (Gibco, Invitrogen) at 37 °C with 5% CO2 in 10-cm cell culture dishes. And the medium was replaced every 3 days. Afterward, when the cells reached about 80% confluence, cells were dissociated using 0.25% trypsin (Sigma, USA) and reseeded into multi-well plates. Finally, MSCs were consecutively expanded up to 10 passages. MSCs at P3 (early passage, EP) and P10 (late passage, LP) were used in subsequent experiments.
Senescence-associated β-galactosidase (SA-β-gal) staining assay
Cells were stained according to a previously described method [30]. Senescence cell histochemical staining kit (Beyotime, China) was used to assess cellular SA-β-gal activity. Briefly, cells were fixed and stained overnight with x-gal solution according to the manufacturer’s instructions. Senescent cells were identified as blue-stained cells under light microscopy (OLYMPUS, Japan). Statistical analysis was performed by assessing the percentages of β-gal-positive cells in different microscopic fields.
Glucose uptake and lactate production
The glucose uptake and lactate production in cellular culture supernatants were measured with a commercial glucose assay (Jiancheng Bio, China) and lactate assay kits (Jiancheng Bio, China), respectively. In brief, 3.5 × 103 cells grew in fresh DMEM/F-12 in 96-well plates, and the cellular supernatant was collected by centrifugation after 24 h. Then, according to the manufacturer's instructions, the absorbances at 505 nm and 450 nm were recorded on a microplate reader (Thermo Fisher Scientific, USA) for glucose consumption and lactate production, respectively.
Intracellular ATP production
Intracellular ATP production was measured using ATP assay kit (Jiancheng Bio, China), as per the manufacturer's protocol. Briefly, cells were harvested by using 200–300 μl lysis buffer and vortexed for 1 min. The supernatant was mixed with detection solution in a 96-well plate with white bottom. Then plates were measured in a luminometer and ATP concentration were normalized to the corresponding total protein amounts from each sample.
Extracellular acidification rate (ECAR) analysis
The cells were seeded in 96-well plates at 4 × 104 cells per well. The following day, the medium in all the wells was replaced with a preheated liquid mixture containing reconstituted pH-Xtra reagent and fresh culture medium. The sample in each group was performed in triplicates. Then the plate was incubated in a humidified incubator at 37 °Cfor 3–4 h. The determination of extracellular acidification rates was further carried out using the pH-Xtra (Luxcel Bioscience, Cork, Ireland), as described previously[24].
Gene expression analysis by real-time quantitative PCR (RT-qPCR)
Total RNA was isolated from MSCs using RNAiso reagent (Takara, China), followed by reverse transcription of RNA using RNA PCR Kit (AMV) Ver.3.0 (Takara, China) according to the manufacturer’s instructions. The primer sequences were listed in Table 1. Quantitative RT-PCR was performed using a 7300 Real-Time PCR System (ABI, USA) with the TransStart Top Green qPCR SuperMix (TRANS, China). Data were normalized applying the 2−ΔΔCt method.
Lentiviral transduction of MSCs
The cells were seeded in 6-well plates at 1.5 × 105 cells per well and cultured overnight before transduction as previously described [13]. Then, the cells were transduced with the purchased lentiviral particles encoding rat miR-34a and its control miR-NC, or encoding rat HK1 and its vector control LV-Vector (GeneChem, China) in the presence of 1 μg/mL polybrene (GeneChem, China) for 10–12 h. Besides, in functional recovery experiments, cells were co-transfected with lentiviral particles encoding rat miR-34a or miR-NC and HK1 and its vector control LV-Vector, respectively. After 96 h of transfection, the transfection efficiency was verified by enhanced green fluorescent protein (EGFP) expression and RT-qPCR.
Target gene prediction of miRNA
Online database miRBase (http://www.mirbase.org/) was applied to predict the potential mRNAs of miR-34a. Then Miranda and RNAhybrid algorithms were performed to assess the confidence of target genes. The common genes were selected between the two algorithms and the common genes with P value < 0.01 were considered as the target genes of miR-34a. The information of miR-34a, mRNAs and P value were imported and the networks were illustrated using Cytoscape software version 3.7.1.
Luciferase reporter assay
For luciferase reporter assay, luciferase constructs were performed by inserting the full-length rat HK1 3’UTR, obtained from Imagenes in the psiCheck2 vector (Promega, Madison, USA). Then cells were cultured in 48-well plates and co-transfected with a plasmid containing luciferase marked by different promoters, miRNA expression vector in which 3’UTR containing miR-34a seed sequence of HK1, and Renilla luciferase (Promega) using Lipofectamine® 3000 (Invitrogen, Carlsbad, CA, USA). The relative luciferase activity was measured using the Dual-Luciferase Reporter Assay System (Promega) 48 h after transfection.
Statistical analysis
All experiments were performed three times independently. And at least three replicate samples were used in each experiment. Data were represented as means ± standard deviation. Two groups were compared by a two-tailed Student’s t-test. Statistical significance between groups was determined by using a two-tailed Student’s t test or a one-way ANOVA. Statistical analysis and figure drawing were performed using GraphPad Prism 8.0 software. A P value < 0.05 was considered statistically significant.
Checklist statement
The work has been reported in line with the Additional file 1: ARRIVE guidelines 2.0.
Results
Characterization of senescent MSCs during consecutive expansion in vitro
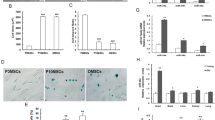
In the present study, we obtained expansion-mediated replicative senescent MSCs in vitro. Cell surface antigens were used to identify obtained MSCs by flow cytometry in our previous work. We found that serial expanded MSCs are positive for mesenchymal progenitor markers including CD44, CD90, and CD105, and negative for CD31 and CD45 [24]. Nevertheless, bone marrow-derived MSCs at early passage (EP, P3MSCs) and late passage (LP, P10MSCs) exhibited evident morphological differences. The morphology of MSCs at EP displayed fibroblast-like features, while LP MSCs presented typical senescence-like morphology including abnormal shapes, blurred cell borders, and enlarged and flattened cell bodies (Fig. 1A). Statistical analysis of morphology demonstrated that the cell area was obviously increased in LP MSCs (Fig. 1B), while the cell aspect ratio was significantly decreased alone with expansion in vitro (Fig. 1C). Next, to investigate whether LP MSCs were senescent, senescence-associated (SA)-β-gal activity and senescence-associated biomarkers were further detected. SA-β-gal staining manifested an increased number of blue-stained cells in LP MSCs compared to EP MSCs, with a higher ratio of SA-β-gal-positive cells (Fig. 1D). Simultaneously, the expression levels of senescence-associated biomarker P16INK4a and Rb1 were significantly up-regulated in LP MSCs (Fig. 1E). Furthermore, LP MSCs not only presented attenuated self-renewal and cell proliferation capability, but also were fundamentally featured by impaired multilineage differentiation compared with EP MSCs [13, 31]. In addition, miR-34a expression level was highly elevated with increasing passages (Fig. 1F). Together, these data indicated that MSCs were gradually senescent with expansion in vitro and miR-34a may exert a regulatory impact on stem cell senescence.
Senescence-associated characteristics and miR-34a expression in mesenchymal stem cell (MSCs) during serial expansion in vitro. A Morphological evaluation of MSCs at early passage (EP) and MSCs at late passage (LP) (scale bar = 50 μm). B-C Analysis of cell surface area (B) and cell aspect ratio (C). D SA-β-gal staining and the percentages of SA-β-gal-positive cells (scale bar = 100 μm). E–F The expression of P16INK4a, Rb1 (E) and miR-34a (F) at mRNA levels by RT-qPCR. All data were presented as mean ± SEM (error bars), n = 3, **P < 0.01, ***P < 0.001
Attenuated glycolytic metabolism in replicative senescent MSCs
To determine the functional alterations in cellular metabolic activity of replicative senescent MSCs, we evaluated the glucose uptake and lactate secretion in EP and LP MSCs. As shown in Fig. 2A and B, glucose uptake and lactate secretion were declined in LP MSCs, suggesting a weakened glucose metabolism in senescent MSCs. To further verify the low glycolytic activity in LP MSCs, ECAR analysis was performed, which quantified proton production as a substitute for lactate production. As expected, ECAR levels were substantially reduced in LP MSCs when compared to EP MSCs (Fig. 2C). Moreover, the cellular ATP production was also obviously lessened in LP MSCs (Fig. 2D). 2-Deoxy-D-glucose (2-DG), a glucose structural analog, competitively inhibits the glycolysis by arresting HK. With the treatment of 2-DG, the glucose uptake, lactate secretion, ECAR level and ATP production in EP and LP MSCs were all conspicuously lower (Fig. 2A–D), which indicated that glycolysis was the pivotal metabolic pattern in MSCs. What is more, glycolysis metabolic exhaustion in LP MSCs was also supported by the decreased expression of glycolytic enzymes, including hexokinase 1 (HK1), HK2, phosphofructokinase 1 (PFK1), pyruvate kinase (PK), lactate dehydrogenase A (LDHA) and LDHB (Fig. 2E). Therefore, the alterations of glucose metabolic level concomitant with glycolytic enzymes implied that MSCs undergoing replicative senescence present the reduced glycolytic metabolism in vitro.
Identification of glycolytic levels in replicative senescent MSCs. A-D Relative glucose uptake (A), lactate secretion (B), ECAR levels (C) and ATP production (D) were measured in EP and LP MSCs. E RT-qPCR results for relative mRNA expression levels of glycolysis-related enzymes in EP and LP MSCs. All data were presented as mean ± SEM (error bars), n = 3, *P < 0.05, **P < 0.01, ***P < 0.001
MiR-34a over-expression aggravates MSC senescence and represses glycolytic levels
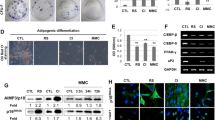
Given that miR-34a has a regulatory effect on MSC senescence, we obtained miR-34a over-expressed MSCs via transduction of lentiviral particles encoding miR-34a into EP MSCs to reveal the underlying molecular mechanism. Firstly, enhanced green fluorescent protein expression and RT-qPCR analysis were monitored to assess transduction efficiency. Notably, enhanced green fluorescent proteins were successfully expressed in miR-34a and miR-NC group (Fig. 3A). And the expression level of miR-34a was up-regulated approximately 3.82-folds in miR-34a group compared with that in miR-NC group. Next, senescence-associated phenotypic features were confirmed by morphological characteristics, SA-β-gal activity, and senescence-related biomarkers. As shown in Fig. 3B, the cell area was obviously enhanced (Fig. 3B), whereas the cell aspect ratio was significantly diminished after miR-34a repletion in EP MSCs (Fig. 3C). Moreover, our data showed that the number of blue-stained MSCs was elevated in miR-34a group, and the statistical analysis ascertained that SA-β-gal activity in miR-34a group was also seriously increased in comparison with miR-NC group (Fig. 3D). Correspondingly, it was found that miR-34a repletion up-regulated the expression at mRNA levels of senescence-associated biomarker P16INK4a and Rb1 (Fig. 3E). In the previous studies, we found that miR-34a repletion impaired the stemness properties of MSCs, including arrested cell cycle, protracted population doubling time (PDT) and diminished osteogenic differentiation[13]. Thus, we speculated that miR-34a over-expression might act as a trigger of MSC senescence. It is well known that miR-34a also participates in various metabolism pathways [32]. As a result, the glycolysis levels were further detected after enforcing miR-34a expression. As expected, the relative glucose uptake (Fig. 3F) and lactate secretion (Fig. 3G) were heavily discouraged in miR-34a group when compared with miR-NC group. Meanwhile, the ECAR level (Fig. 3H) and ATP production (Fig. 3I) were also diminished in miR-34a group. Collectively, these data suggested that miR-34a provoking MSC senescence may be linked to the impaired glycolytic metabolism.
miR-34a over-expression aggravates MSC senescence and restrains glycolysis. A Fluorescence images showed that miR-34a was successfully over-expressed in EP MSCs (scale bar = 100 µm), and miR-34a expression was determined by RT-qPCR. B-C Quantitative analysis of cell surface area (B) and cell aspect ratio (C) in miR-NC and miR-34a over-expressed EP MSCs. D SA-β-gal staining (scale bar = 50 μm) and quantification of β-gal positive cell numbers. E The mRNA expression of the senescence-associated biomarker P16INK4a and Rb1. F-I Relative glucose uptake (F), lactate secretion (G), ECAR levels (H) and ATP production (I) were measured in miR-NC and miR-34a over-expressed EP MSCs. All data were presented as mean ± SEM (error bars), n = 3, *P < 0.05, **P < 0.01
HK1 identified as a direct target gene of miR-34a
MiRNAs can bind to the specific bases of target mRNAs to be involved in the post-transcriptional regulation, which plays a critical role in a variety of biological processes [33]. To further investigate the mechanism of miR-34a regulation on MSC senescence and glucose metabolism, we predicted the potential targeting mRNAs of miR-34a using the miRBase database and identified the 3’untranslated region (3’UTR) of 35 genes containing binding sites with miR-34a (Fig. 4A). The information of predicted target genes was shown in supplementary Table 1. Among them, there were 2 genes related to the glycolytic ability, including HK1 and enolase 3 (ENO3). Besides, according to the stability and accuracy of miR-34a binding to target genes and the rate-limiting function of the target genes on the glycolytic pathway, HK1 was considered as the key gene for subsequent studies. The miRBase database revealed the binding sites of HK1 3’UTR and miR-34a-5p (Fig. 4B). Next, the dual luciferase reporter assay was conducted to confirm that HK1 was a potential target gene of miR-34a. Our results manifested that the luciferase activity in HK1-Mut with the treatment of miR-34a mimic was comparable to that in the miR-NC group, while miR-34a over-expression extraordinarily abolished the luciferase activity in HK1-Wt as compared to the control group (Fig. 4C). In addition, miR-34a replenishment in EP MSCs also down-regulated the expression level of HK1 (Fig. 4D). These observations above support the notion that miR-34a can negatively modulate HK1 expression via directly targeting the 3’UTR of HK1.
miR-34a directly targets HK1 and represses its expression. A Bioinformatics-based target analysis showed the potential target gene hub of miR-34a. B Schematic representation of the predicted complimentary base pairing between miR-34a and the 3′UTR of HK1, and the mutated binding site of putative miR-34a seed sequence. C Analysis of luciferase activity in 293 T cells co-transfected with miR-34a and plasmid containing the 3′ UTR of the wild type (WT) HK1 or a mutated (Mut) HK1 sequence in luciferase reporter assay. D RT-qPCR analysis was performed to detect the expression of HK1 after miR-34a over-expression in EP MSCs. All data were presented as mean ± SEM (error bars), n = 3, *P < 0.05, **P < 0.01
HK1 sufficiency alleviates MSC senescence by enhancing glycolysis
To investigate the specific mechanism underlying how miR-34a aggravated MSC senescence by regulating glycolysis, LP MSCs were transduced with lentivirus-expressing HK1 (LV-HK1) and lentiviral vector (LV-Vector). Then the transduction efficiency was evaluated through enhanced green fluorescent protein expression and RT-qPCR. Our results displayed that enhanced green fluorescent proteins were both successfully expressed in both groups (Fig. 5A), and HK1 expression in LV-HK1 group (Fig. 5B) was up-regulated significantly compared with LV-Vector group. Next, senescence-associated phenotypes were detected to explain the regulatory role of HK1 on cellular senescence. As depicted in Fig. 5C and D, a significant decrease in cell area and an increase in cell aspect ratio were observed in LV-HK1 group when compared to those in LV-Vector group. What is more, representative images exhibited that HK1 over-expression markedly reduced the number of blue-stained senescent cells, and statistical analysis also yielded similar arguments (Fig. 5E). To further clarify that up-regulated HK1 could rescue MSC senescence, senescence-associated biomarkers were further used to validate. The data manifested that the expression levels of P16INK4a and Rb1 were both declined in the HK1over-expressed MSCs as compared to those in LV-Vector group (Fig. 5F). These findings supported that HK1 had a favorable regulatory influence on ameliorating MSC senescence. To confirm whether the vigorous effect of HK1 on cellular senescence was associated with glycolysis, the products representing glycolytic levels were checked. As expected, HK1 sufficiency up-regulated the relative lower glucose uptake (Fig. 5G) and lactate secretion (Fig. 5H) resulted from MSC serial expansion in vitro. Simultaneously, the relative ECAR levels (Fig. 5I) and ATP production (Fig. 5J) were improved in LV-HK1 group in comparison with those in LV-Vector group. The above results indicated that HK1 exerts a protective impact on MSC senescence by boosting glycolytic levels.
HK1 repletion alleviates MSC senescence by facilitating glycolytic flow. A HK1 was successfully over-expressed in LP MSCs (scale bar = 100 µm). B HK1 expression determined by RT-qPCR. C-D Statistical analysis of cell surface area (C) and cell aspect ratio (D) in LV-Vector and HK1 over-expressed LP MSCs. E. SA-β-gal staining (scale bar = 50 μm) and quantification of β-gal positive cell numbers. F The expression of the senescence-associated biomarker P16INK4a and Rb1. G-J Relative glucose uptake (G), lactate secretion (H), relative ECAR levels (I) and ATP production (J) were measured in LV-Vector and HK1 over-expressed LP MSCs. All data were presented as mean ± SEM (error bars), n = 3, *P < 0.05, **P < 0.01
MSC senescence can be manipulated by miR-34a-HK1 signal axis by ameliorating glycolysis levels
To gain a deeper understanding of the underlying molecular mechanisms how miR-34a mediated MSC senescence, we next examined senescent-associated phenotypes following miR-34a over-expression and co-transfection with miR-34a and LV-HK1 or with its control LV-Vector in young EP MSCs. We found that SA-β-gal activity were noticeably higher in miR-34a over-expressed MSCs, whereas the number of SA-β-gal staining positive cells was clearly reduced in MSCs co-transfected with miR-34a and LV-HK1, and similar conclusion was obtained from the quantitative analysis results (Fig. 6A). Meanwhile, HK1 repletion could reverse miR-34a-mediated the high expression of senescence-associated biomarkers P16INK4a and Rb1 (Fig. 6B, C). Of course, in comparison with the miR-NC, the expression of HK1was declined in miR-34a over-expressed MSCs, while it was elevated in MSCs co-transfected with miR-34a and LV-HK1 (Fig. 6D). These results demonstrated that HK1 supplementation could rescue miR-34a-induced MSC senescence, further indicating that miR-34a-HK1 signal axis might be one of the critical mechanisms modulating stem cell senescence. To further certify that miR-34a-mediated MSC senescence by targeting HK1 was related to glycolysis regulation, the relative glucose uptake, lactate secretion, ECAR level and ATP production were all investigated. Our results revealed that miR-34a over-expression led to the remarkable decline in glucose uptake (Fig. 6E), lactate secretion (Fig. 6F), ECAR level (Fig. 6G) and ATP production (Fig. 6H). Conversely, the glycolytic levels were restored in MSCs co-transfected with miR-34a and LV-HK1. Taken together, it was concluded that miR-34a can directly target HK1 to regulate MSC senescence by orchestrating glycolysis.
miR-34a-HK1 axis modulates MSC senescence via influencing glycolytic metabolism. A SA-β-gal staining (scale bar = 50 μm) and quantification in EP MSCs treated with miR-NC, miR-34a, miR-34a plus LV-Vector or miR-34a plus LV-HK1. B-D Detection of the expression levels of P16INK4a (B), Rb1 (C) and HK1 (D) by RT-qPCR. E–H Relative glucose uptake (E), lactate secretion (F), relative ECAR levels (G) and ATP production (H) were measured in EP MSCs treated with miR-NC, miR-34a, miR-34a plus LV-Vector or miR-34a plus LV-HK1. All data were presented as mean ± SEM (error bars), n = 3, *P < 0.05 vs. miR-NC; △P < 0.05 vs. miR-34a, ▲P < 0.05 vs. miR-34a plus LV-Vector
Discussion
MSCs are overwhelmingly useful tools as a unique cell-based therapy to postpone organ aging and treat age-associated diseases. However, successful MSC therapy requires large-scale cell populations and a prolonged culture in vitro, which subsequently may induce cellular replicative senescence. To investigate cellular senescence and related molecular mechanism during long-term cultivation in rat bone marrow MSCs, MSCs at early passage (EP, P3) and late passage (LP, P10) were considered as the research objects in the present study. As expected, LP MSCs exhibited the distinct morphological and molecular biological differences from EP MSCs, mainly manifesting elongated and spindle-shaped cell body, increased cell surface area and decreased aspect ratio. These results were in accordance with MSCs derived from placenta [34]. Next, the senescent status of LP MSCs was confirmed by the SA-β-gal staining assay, which showed enhanced activity of the lysosomal β-galactosidase specifically in LP MSCs. Additionally, the expression levels of senescence-associated biomarker P16INK4a and Rb1 were approximately 5.22-fold and 2.72-fold higher, respectively, in LP MSCs in comparison with those in EP MSCs. Similar findings were found in natural aging and premature senescence induced by oxidative stress [13, 35]. It has been demonstrated that aging is primarily regulated by p53 and P16INK4a/Rb pathways, which are also closely associated with bone homeostasis [36, 37]. In most mammalian tissues, the expression level of P16INK4a increases with age [38]. Simultaneously, P16INK4a-positive cells accumulating during adulthood negatively influence the lifespan and promote age-dependent changes in multiple organs, so their therapeutic removal may be an attractive approach to extending a healthy lifespan [39].
Energy metabolism has emerged as a key process involved in the stem cell function and commitment, playing an important role in both the acquisition and maintenance of stemness. Unlike most differentiated mature cells, glycolysis is the predominant source of ATP in MSCs [40, 41]. Unfortunately, the glycolytic levels in MSCs decreased as organisms aged [21, 24]. To investigate the glycolytic flux in MSCs during culture expansion in vitro, glucose uptake and lactate levels were firstly examined. The results implied that there were markedly decreased glucose consumption and lactic acid secretion in LP MSCs. What is more, the relative ECAR level and ATP production were also weakened, which were congruent with the findings of our latest research [24]. Besides, multiple genes involved in glycolysis including HK1, HK2, PFK1, PK, LDHA and LDHB were also down-regulated in LP MSCs. In other types of stem cell senescence, similar metabolic trends were obtained by RNA sequencing [24]. Furthermore, Yuan and his colleague reported that glycolytic ATP production and glycolysis-related genes such as HK2, PK and LDHA were found to be significantly reduced in LP human MSCs (hMSCs), whereas the lactate secretion, ECAR and oxygen consumption rate (OCR) values were elevated compared to those in EP hMSCs, indicating a metabolic shift from glycolysis towards oxidative phosphorylation (OXPHOS) during in vitro culture expansion of hMSCs [42]. Upon removal from the in vivo niche, MSCs start to adapt to the in vitro environment and cellular homeostasis is interrupted with serial expansion, which is associated to cellular metabolic state. Additionally, aging-mediated oxidative stress, damaged organelles and different cell culture and laboratory conditions might also be attributed to the metabolic discrepancy. 2-DG, a glucose analog, is phosphorylated by HK, thereby competitively inhibiting the production of G-6-P from glucose and ultimately restraining glycolysis. After 2-DG treatment, the glycolytic flux was blunted and characterized by reduced glucose uptake, lactate secretion, ECAR level and ATP production. Meanwhile, the metabolic alterations in EP MSCs were more conspicuous. It has been reported that embryonic stem cells (ESCs) treated with 2-DG displayed evidently lower ECAR level and decreased proliferative activity [43]. These results above indicate that the glycolytic metabolism pathway is involved in stem cell replicative senescence and may be utilized for the development of novel therapeutics to treat age-related diseases.
MiRNAs, a class of high-conserved, small and single-stranded noncoding RNAs, can bind with 3’UTR of mRNAs to repress mRNA translation or induce mRNA degradation, thus silencing gene expression at the transcription level. More recently, multiple studies documented miR-34a is a pivotal regulator of age-dependent tissues changes and an inducer of cellular senescence [44, 45]. We have previously shown that miR-34a over-expression by lentiviruses markedly abolished cell proliferation capacity, retarded cell cycle progression and impaired differentiation capacity, eventually resulting in MSC senescence [13]. Similar results were reported in human adipose derived mesenchymal stem cells (hAD-MSCs) [46, 47]. In addition, miR-34a is also involved in metabolic pathways by targeting related rate-limiting enzymes [17,18,19]. On account of these, we speculated that miR-34a may play an indispensable role in stem cell senescence by manipulating metabolic pathways. To test our speculation, we up-regulated miR-34a expression via gene manipulation in young EP MSCs. As expected, it was observed that miR-34a sufficiency in young MSCs induced senescence-like morphology alterations, including abnormal shapes, increased cell area and decreased cell aspect ratio. To further verify the occurrence of cellular senescence induced by miR-34a repletion, SA-β-gal staining was firstly performed. Our data showed that the SA-β-gal-positive rate in miR-34a over-expressed group was about 3 times higher than that in NC group. Moreover, miR-34a replenishment up-regulated the expression levels of senescence-associated biomarker P16INK4a and Rb1, indicating miR-34a had an adverse regulatory effect on MSC culture expansion in vitro and instigated MSC replicative senescence. These observations are consistent with our previous findings [13]. Similarly, Neda Mokhberian et al. reported that miR-34a inhibition in hAD-MSCs enhanced the cell proliferation, promoted the adipogenic and osteogenic differentiation, and reduced the SA-β gal activity [47]. These results illustrate the significance of miR-34a silence as an anti-senescence approach to improve the therapeutic potentials of stem cells and as a promising target for the treatment of age-related diseases or common metabolic disorders.
Dysregulation of miR-34a disrupts the homeostasis of gene regulatory network, leading to cellular senescence, metabolic syndrome and the related diseases. Previously, we found that miR-34a suppression targeted nicotinamide phosphoribosyltransferase (Nampt) to down-regulate NAD+/NADH ratio and NAD+ content, which exerted vigorous regulatory function on MSC senescence [13]. Studies have shown that miR-34a attenuated osteoblast differentiation of hMSCs through glycolysis inhibition via targeting LDHA [48]. Meanwhile, the expression levels of glycolytic enzymes, such as HK2, glucose transporter 1 (GLUT1), and LDHA were significantly declined due to miR-34a over-expression in hMSCs [48]. Consistent herewith, miR-34a sufficiency markedly suppressed glycolysis flux, as manifested by lessened glucose uptake, lactate secretion, ECAR levels and ATP production. These data are similar to those obtained in cardiomyocytes [49]. To further examine the molecular mechanisms for miR-34a-mediated glycolysis suppression, the potential targets of miR-34a were investigated. Notably, 35 potential target genes of miR-34a were predicted by the miRBase database, and HK1 was considered as the focus for subsequent studies based on the stability and accuracy of miR-34a binding to target genes and the rate-limiting function in the glycolytic pathway. To this end, the luciferase reporter assay was performed. The data exhibited that miR-34a regulated HK1 expression levels by targeting the 3’UTR of HK1, which was consistent with the published findings [33, 50]. Research has shown that miR-34a modulates lens epithelial cell (LEC) apoptosis and cataract development through the HK1/caspase 3 signaling pathway [51]. In parallel, we then investigated the effect of miR-34a on HK1 expression. The results implied that there was markedly decreased expression of HK1 in miR-34a mimic group in comparison with that in miR-NC group. The above statements illustrated that HK1 was a target gene of miR-34a, and miR-34a can directly bind to the 3’UTR of HK1 mRNA and further down-regulate its expression.
Glycolysis is a series of redox reactions in the cytosol that rapidly consumes a large amount of glucose and produces lactic acid as the main end product. As the key enzyme responsible for the first step of glycolysis, HKs are involved in the occurrence and development of cellular senescence and age-related diseases. It is widely accepted that HKs have four isomers, HK1-HK4, however, there are limited studies on HK1. On account of this, we modulated HK1 expression via gene manipulation in young and senescent MSCs to determine its protective effect on stem cell senescence. The results displayed that HK1 over-expression in LP MSCs ameliorated senescence-like morphology, including more regular shapes, clear cell bodies, decreased cell area and increased cell aspect ratio. At the same time, the lysosomal SA-β-gal activity and the expression levels of senescence marker P16INK4a and Rb1 were all shrunk in LV-HK1 group in comparison with those in LV-Vector group. However, the alterations of stemness properties after transfection with HK1 in MSCs are still unclear, which will be the focus of our future research. It has been reported that down-regulated HK1 expression could suppress the proliferation of human LECs and induces apoptosis [51]. Inconsistent herewith, De Jesus et al. demonstrated that spleen tissue from aged mice (85 weeks old) contained elevated inflammatory cytokines and increased cytosolic HK1 levels, which might be related to animal species and distinct culture conditions [52]. Furthermore, HK1 is also associated with age-related diseases. One research has shown that HK1 activity and expression were both reduced in the hippocampal tissue of 9-month-old 3 × Tg-AD mice, suggesting HK1 possessed a beneficial effect on the pathogenesis of Alzheimer’s disease (AD) [53]. Given that HK1 is the rate-limiting enzyme of glycolysis, and MSCs depend mainly on glycolysis to convert glucose to pyruvate and lactate, the glycolysis function was further measured. We discovered that the glycolytic levels including glucose uptake, lactate secretion, ECAR levels and ATP production were all elevated after HK1 replenishment in senescent MSCs. The above results indicate that over-expressed HK1 has a favorable regulatory effect on alleviating MSC senescence and remodeling glycolytic function, which will provide a new therapeutic target and theoretical basis for senility postponement and age-related tissue regenerative treatment.
Along with the MSC replicative senescence, we found intriguingly that miR-34a expression incremented whereas HK1 expression inversely decreased. And the miRNA-target prediction and the luciferase reporter assay verified that miR-34a specifically bound and interacted with its potential target HK1, which was consistent with the published findings [45, 50]. To this end, we then performed the functional recovery experiment. The results certified that miR-34a-induced senescence in young EP MSCs could be rescued by HK1 restoration, as evidenced from the significant alleviation of miR-34a-induced augmented β-gal-positive cells and the obvious down-regulated expression levels of P16INK4A and Rb1. Furthermore, HK1 supplementation could reverse the decreased glycolytic function caused by miR-34a over-expression, which was illustrated by enhanced glucose uptake, lactate secretion, ECAR levels and ATP production. Overall, these results reveal that miR-34a-HK1 signal axis ameliorates MSC senescence by boosting glycolysis function.
However, there are still several limitations to our study. At first, we only performed in vitro experiments in MSCs. Many questions remain to be answered in vivo. For example, whether the expression of HK1 is abnormal in MSCs derived from senile rats, and whether organism aging can be retarded by regulating the miR-34a-HK1 axis. Secondly, two isomers (HK1 and HK2) are expressed in the rat MSCs, we only detected the function of HK1. Whether HK2 is also involved in MSC senescence by affecting glucose metabolism requires further exploration. Thirdly, HK1 contains an N-terminal mitochondrial binding domain (MBD) that enables it to bind to the mitochondrial outer membrane (OMM). The mitochondrial binding function of HK1 allows for preferential access to mitochondrial pools of ATP and is proposed to increase tricarboxylic acid (TCA) cycle activity, further giving rise to enhanced protein translation. Whether the effect of HK1 on mitochondria can influence MSC senescence needs to be further investigated in depth. Lastly, in the whole study, we only examined the expression of HK1 not its enzyme activity. To verify the favorable impact of HK1 to attenuate stem cell senescence, HK1 activity should be evaluated. Further work needs to be done to better understand the function of HK1 and serve the stem cell applications.
Conclusions
In summary, the present study demonstrated that miR-34a exerts the direct targeted inhibition on HK1 expression, resulting in glycolytic metabolism depletion, and further contributing to MSC replicative senescence (Fig. 7). These findings provide novel insights into the mechanisms underlying stem cell senescence, opens up new possibilities for stem cell applications and therapy from the perspective of epigenetic and metabolic regulation and further highlight the significance of HK1 as a potential anti-aging agent to delay and suppress the occurrence and development of aging and age-related diseases.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- MSCs:
-
Mesenchymal stem cells
- HK1:
-
Hexokinases1
- miRNAs:
-
MicroRNAs
- NAFLD:
-
Non-alcoholic fatty liver disease
- NASH:
-
Non-alcoholic steatohepatitis
- G-6-P:
-
Glucose-6-phosphate
- ECAR:
-
Extracellular acidification rate
- EP:
-
Early passage
- LP:
-
Late passage
- Senescence-associated SA-β-gal:
-
Senescence-associated β-galactosidase
- 2-DG:
-
2-Deoxy-D-glucose
- PFK1:
-
Phosphofructokinase 1
- PK:
-
Pyruvate kinase
- LDHA:
-
Lactate dehydrogenase A
- ENO3:
-
Enolase 3
- PDT:
-
Population doubling time
- LV-HK1:
-
Lentivirus-expressing HK1
- LV-Vector:
-
Lentiviral vector
- OCR:
-
Oxygen consumption rate
- OXPHOS:
-
Oxidative phosphorylation
- ESCs:
-
Embryonic stem cells
- hAD-MSCs:
-
Human adipose derived mesenchymal stem cells
- Nampt:
-
Nicotinamide phosphoribosyltransferase
- GLUT1:
-
Glucose transporter 1
- LEC:
-
Lens epithelial cell
- AD:
-
Alzheimer’s disease
- MBD:
-
Mitochondrial binding domain
- OMM:
-
Mitochondrial outer membrane
- TCA:
-
Tricarboxylic acid
References
He X, Memczak S, Qu J, Belmonte JCI, Liu GH. Single-cell omics in ageing: a young and growing field. Nat Metab. 2020;2:293–302.
Bi S, Liu Z, Wu Z, Wang Z, Liu X, Wang S, et al. SIRT7 antagonizes human stem cell aging as a heterochromatin stabilizer. Protein Cell. 2020;11:483–504.
Ren R, Ocampo A, Liu GH, Izpisua Belmonte JC. Regulation of stem cell aging by metabolism and epigenetics. Cell Metab. 2017;26:460–74.
Dorronsoro A, Santiago FE, Grassi D, Zhang T, Lai RC, McGowan SJ, et al. Mesenchymal stem cell-derived extracellular vesicles reduce senescence and extend health span in mouse models of aging. Aging Cell. 2021;20:e13337–437.
Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–217.
Fridenshteĭn A, Piatetskiĭ S, II, Petrakova KV. [Osteogenesis in transplants of bone marrow cells]. Arkh Anat Gistol Embriol. 1969;56:3–11. Kosteobrazovanie v transplantatakh kostnomozgovykh kletok.
Lee B-C, Yu K-R. Impact of mesenchymal stem cell senescence on inflammaging. BMB Rep. 2020;53:65–73.
Li Y, Wu Q, Wang Y, Li L, Bu H, Bao J. Senescence of mesenchymal stem cells (Review). Int J Mol Med. 2017;39:775–82.
Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33.
Williams J, Smith F, Kumar S, Vijayan M, Reddy PH. Are microRNAs true sensors of ageing and cellular senescence? Ageing Res Rev. 2017;35:350–63.
Kumar S, Vijayan M, Bhatti JS, Reddy PH. MicroRNAs as peripheral biomarkers in aging and age-related diseases. Prog Mol Biol Transl Sci. 2017;146:47–94.
Badi I, Burba I, Ruggeri C, Zeni F, Bertolotti M, Scopece A, et al. MicroRNA-34a induces vascular smooth muscle cells senescence by SIRT1 downregulation and promotes the expression of age-associated pro-inflammatory secretory factors. J Gerontol A Biol Sci Med Sci. 2015;70:1304–11.
Pi C, Ma C, Wang H, Sun H, Yu X, Gao X, et al. MiR-34a suppression targets Nampt to ameliorate bone marrow mesenchymal stem cell senescence by regulating NAD(+)-Sirt1 pathway. Stem Cell Res Ther. 2021;12:271.
Ito T, Yagi S, Yamakuchi M. MicroRNA-34a regulation of endothelial senescence. Biochem Biophys Res Commun. 2010;398:735–40.
Badi I, Mancinelli L, Polizzotto A, Ferri D, Zeni F, Burba I, et al. miR-34a promotes vascular smooth muscle cell calcification by downregulating SIRT1 (Sirtuin 1) and Axl (AXL receptor tyrosine Kinase). Arterioscler Thromb Vasc Biol. 2018;38:2079–90.
Rottiers V, Näär AM. MicroRNAs in metabolism and metabolic disorders. Nat Rev Mol Cell Biol. 2012;13:239–50.
Miao LH, Lin Y, Huang X, Pan WJ, Zhou QL, Liu B, et al. In Vivo analysis of miR-34a regulated glucose metabolism related genes in megalobrama amblycephala. Int J Mol Sci. 2018;19:2417.
Cui R, Li C, Wang J, Dai J. Induction of hepatic miR-34a by perfluorooctanoic acid regulates metabolism-related genes in mice. Environ Pollut. 2019;244:270–8.
Wang L, Sun M, Cao Y, Ma L, Shen Y, Velikanova AA, et al. miR-34a regulates lipid metabolism by targeting SIRT1 in non-alcoholic fatty liver disease with iron overload. Arch Biochem Biophys. 2020;695: 108642.
Sharma R, Ramanathan A. The aging metabolome-biomarkers to hub metabolites. Proteomics. 2020;20:e1800407–507.
Li X, Wang X, Zhang C, Wang J, Wang S, Hu L. Dysfunction of metabolic activity of bone marrow mesenchymal stem cells in aged mice. Cell Prolif. 2022;55:e13191–291.
Babenko VA, Silachev DN, Danilina TI, Goryunov KV, Pevzner IB, Zorova LD, et al. Age-related changes in bone-marrow mesenchymal stem cells. Cells. 2021;10:1273.
Deng L, Yi S, Yin X, Li Y, Luan Q. MFN2 knockdown promotes osteogenic differentiation of iPSC-MSCs through aerobic glycolysis mediated by the Wnt/β-catenin signaling pathway. Stem Cell Res Ther. 2022;13:162.
Sun Y, Yu X, Gao X, Zhang C, Sun H, Xu K, et al. RNA sequencing profiles reveal dynamic signaling and glucose metabolic features during bone marrow mesenchymal stem cell senescence. Cell Biosci. 2022;12:62.
Xu CR, Fang QJ. Inhibiting glucose metabolism by miR-34a and miR-125b protects against hyperglycemia-induced cardiomyocyte cell death. Arq Bras Cardiol. 2021;116:415–22. A Inibição do Metabolismo da Glicose por miR-34a e miR-125b Protege contra a Morte Celular de Cardiomiócitos Causada por Hiperglicemia.
Xu Y, Xu Y, Zhu Y, Sun H, Juguilon C, Li F, et al. Macrophage miR-34a is a key regulator of cholesterol efflux and atherosclerosis. Mol Ther. 2020;28:202–16.
Zhang W, Li J, Duan Y, Li Y, Sun Y, Sun H, et al. Metabolic regulation: a potential strategy for rescuing stem cell senescence. Stem Cell Rev Rep. 2022;18(5):1728–42.
Gao X, Yu X, Zhang C, Wang Y, Sun Y, Sun H, et al. Telomeres and mitochondrial metabolism: implications for cellular senescence and age-related diseases. Stem Cell Rev Rep. 2022;18:1–13.
Yu X, Sun H, Gao X, Zhang C, Sun Y, Wang H, et al. A comprehensive analysis of age-related metabolomics and transcriptomics reveals metabolic alterations in rat bone marrow mesenchymal stem cells. Aging (Albany NY). 2022;14:1014–32.
Wang H, Sun Y, Pi C, Yu X, Gao X, Zhang C, et al. Nicotinamide mononucleotide supplementation improves mitochondrial dysfunction and rescues cellular Senescence by NAD(+)/Sirt3 pathway in mesenchymal stem cells. Int J Mol Sci. 2022;23:14739.
Pi C, Yang Y, Sun Y, Wang H, Sun H, Ma M, et al. Nicotinamide phosphoribosyltransferase postpones rat bone marrow mesenchymal stem cell senescence by mediating NAD(+)-Sirt1 signaling. Aging (Albany NY). 2019;11:3505–22.
Khan AA, Gupta V, Mahapatra NR. Key regulatory miRNAs in lipid homeostasis: Implications for cardiometabolic diseases and development of novel therapeutics. Drug Discov Today. 2022;27:2170–80.
Li S, Zhu K, Liu L, Gu J, Niu H, Guo J. lncARSR sponges miR-34a-5p to promote colorectal cancer invasion and metastasis via hexokinase-1-mediated glycolysis. Cancer Sci. 2020;111:3938–52.
Seok J, Jung HS, Park S, Lee JO, Kim CJ, Kim GJ. Alteration of fatty acid oxidation by increased CPT1A on replicative senescence of placenta-derived mesenchymal stem cells. Stem Cell Res Ther. 2020;11:1.
Ma C, Sun Y, Pi C, Wang H, Sun H, Yu X, et al. Sirt3 attenuates oxidative stress damage and rescues cellular senescence in rat bone marrow mesenchymal stem cells by targeting superoxide dismutase 2. Front Cell Dev Biol. 2020;8: 599376.
Tjempakasari A, Suroto H, Santoso D. Mesenchymal stem cell senescence and osteogenesis. Medicina (Kaunas). 2021;58:61.
Zindy F, Quelle DE, Roussel MF, Sherr CJ. Expression of the p16INK4a tumor suppressor versus other INK4 family members during mouse development and aging. Oncogene. 1997;15:203–11.
Rayess H, Wang MB, Srivatsan ES. Cellular senescence and tumor suppressor gene p16. Int J Cancer. 2012;130:1715–25.
Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530:184–9.
Aravinthan A. Cellular senescence: a hitchhiker’s guide. Hum Cell. 2015;28:51–64.
Nuschke A, Rodrigues M, Wells AW, Sylakowski K, Wells A. Mesenchymal stem cells/multipotent stromal cells (MSCs) are glycolytic and thus glucose is a limiting factor of in vitro models of MSC starvation. Stem Cell Res Ther. 2016;7:179.
Yuan X, Liu Y, Bijonowski BM, Tsai A-C, Fu Q, Logan TM, et al. NAD(+)/NADH redox alterations reconfigure metabolism and rejuvenate senescent human mesenchymal stem cells in vitro. Commun Biol. 2020;3:774–874.
Zhou W, Choi M, Margineantu D, Margaretha L, Hesson J, Cavanaugh C, et al. HIF1α induced switch from bivalent to exclusively glycolytic metabolism during ESC-to-EpiSC/hESC transition. Embo j. 2012;31:2103–16.
Raucci A, Macrì F, Castiglione S, Badi I, Vinci MC, Zuccolo E. MicroRNA-34a: the bad guy in age-related vascular diseases. Cellular Mol Life Sci : CMLS. 2021;78:7355–78.
Yang J, Chen D, He Y, Meléndez A, Feng Z, Hong Q, et al. MiR-34 modulates Caenorhabditis elegans lifespan via repressing the autophagy gene atg9. Age (Dordr). 2013;35:11–22.
Park H, Park H, Pak HJ, Yang DY, Kim YH, Choi WJ, et al. miR-34a inhibits differentiation of human adipose tissue-derived stem cells by regulating cell cycle and senescence induction. Differentiation. 2015;90:91–100.
Mokhberian N, Bolandi Z, Eftekhary M, Hashemi SM, Jajarmi V, Sharifi K, et al. Inhibition of miR-34a reduces cellular senescence in human adipose tissue-derived mesenchymal stem cells through the activation of SIRT1. Life Sci. 2020;257: 118055.
Hong M, Zhang XB, Xiang F, Fei X, Ouyang XL, Peng XC. MiR-34a suppresses osteoblast differentiation through glycolysis inhibition by targeting lactate dehydrogenase-A (LDHA). In Vitro Cell Dev Biol Anim. 2020;56:480–7.
Zhang Y, Liu G, Gao X. Attenuation of miR-34a protects cardiomyocytes against hypoxic stress through maintenance of glycolysis. Biosci Rep. 2017;37:BSR20170925.
Zhou Y, Ding BZ, Lin YP, Wang HB. MiR-34a, as a suppressor, enhance the susceptibility of gastric cancer cell to luteolin by directly targeting HK1. Gene. 2018;644:56–65.
Feng L, Wei Y, Sun Y, Zhou L, Bi S, Chen W, et al. MIR34A modulates lens epithelial cell apoptosis and cataract development via the HK1/caspase 3 signaling pathway. Aging (Albany NY). 2023;15:6331–45.
De Jesus A, Keyhani-Nejad F, Pusec CM, Goodman L, Geier JA, Stoolman JS, et al. Hexokinase 1 cellular localization regulates the metabolic fate of glucose. Mol Cell. 2022;82:1261-77.e9.
Ji XH, Liu TT, Wei AH, Lei HP, Chen Y, Wu LN, et al. Suppression of hnRNP A1 binding to HK1 RNA leads to glycolytic dysfunction in Alzheimer’s disease models. Front Aging Neurosci. 2023;15:1218267.
Funding
This study was supported by Jilin Provincial Science and Technology Projects (20220402074GH).
Author information
Authors and Affiliations
Contributions
Ya-nan Sun: Methodology and software, writing—original draft preparation. Chang Zhang and Qian-hui Ma: validation. Xing-yu Gao: investigation. Xiao Yu: resources. Hai-Ying Zhang: data curation. Yan Li: visualization. Ying-ai Shi: supervision. Xu He: writing—review and editing, conceptualization and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This research does not contain clinical experiments. All the animal experiments are performed at College of Basic Medical Sciences, Jilin University and approved under the project of “The regulation of glucose metabolic reprogramming mediated by miR-34a in the senescence of mesenchymal stem cells via targeting HK1” by the Animal Ethic Committee of Jilin University. The approval date for these animal experiments is 2020–03-08, and the Approval Number is 202027.
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sun, Y., Zhang, C., Ma, Q. et al. MiR-34a-HK1 signal axis retards bone marrow mesenchymal stem cell senescence via ameliorating glycolytic metabolism. Stem Cell Res Ther 15, 238 (2024). https://doi.org/10.1186/s13287-024-03857-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-024-03857-3