Abstract
Bone tissue engineering (BTE) emerged as one of the exceptional means for bone defects owing to it providing mechanical supports to guide bone tissue regeneration. Great advances have been made to facilitate the success of BTE in regenerating bone within defects. The use of externally applied fields has been regarded as an alternative strategy for BTE. Electromagnetic fields (EMFs), known as a simple and non-invasive therapy, can remotely provide electric and magnetic stimulation to cells and biomaterials, thus applying EMFs to assist BTE would be a promising strategy for bone regeneration. When combined with BTE, EMFs improve cell adhesion to the material surface by promoting protein adsorption. Additionally, EMFs have positive effects on mesenchymal stem cells and show capabilities of pro-angiogenesis and macrophage polarization manipulation. These advantages of EMFs indicate that it is perfectly suitable for representing the adjuvant treatment of BTE. We also summarize studies concerning combinations of EMFs and diverse biomaterial types. The strategy of combining EMFs and BTE receives encouraging outcomes and holds a promising future for effectively treating bone defects.
Similar content being viewed by others
Background
Bone is a robust organ that can regenerate completely under physiological conditions. However, large bone defects resulting from traumatic injuries [1], congenital defects [2], or tumours [3] are unable to form a callus and are commonly accompanied by high complication rates [4]. Usually, clinical interventions are required for the functional recovery of patients with large bone defects. Inert metallic bone fixation devices or autologous and allogeneic bone grafting are the gold standards for the current treatment of large bone defects [5]. However, they all possess potential risks such as pain [6], comorbidities associated with surgery [5], donor site morbidity [7], secondary surgery to remove the inert fixation [8], and the risk of disease transmission from the donor tissues [9]. To promote bone repair without causing the aforementioned risks, biomaterials functioned as bone substitutes have been substantially developed. Eventually, bone tissue engineering (BTE) emerged as a highly interdisciplinary research field [5]. The predominant role of bone tissue engineering materials (functioned as scaffolds) is dedicated to mimicking the biochemistry and structure of the natural bone extracellular matrix [10]. Thus, biomimetic scaffolds, which provide an appropriate three-dimensional environment and mechanical support for cells, can properly guide tissue regeneration [11]. Dong et al. [12] proposed that a typical strategy of BTE usually involves the following aspects: (1) construction of biomimetic scaffolds, (2) seeding of osteoprogenitor cells on scaffolds, (3) employment of exogenous pro-osteogenic factors, and (4) transplantation to bone defects sites. Mesenchymal stem cells (MSCs) are the most commonly used osteoprogenitor cells owing to their prominent capacity to proliferate and differentiate. Bone tissue engineering using MSCs has been proven to be an effective means for reconstructing rodent bone defects in many studies [13]. Besides, the successful clinical applications of MSCs-loaded BTE in enhancing bone formation within defects area have been firmly supported by a large amount of preclinical and clinical data [14]. However, there are still some obstacles and challenges in the extensive use of MSCs-loaded BTE in clinical situations such as high cost [15], comprised cell survival [3], and limited cell number for clinical cases with large defects [16]. And technological advances are required for maximizing cell, viability, vascular network formation, and osteogenic differentiation capacity [14]. Hence, alternative interventions and strategies are needed for assisting BTE, especially mesenchymal stem cells-loaded bone tissue engineering.
It is acknowledged that life on earth evolved in the company of a static magnetic field combined with a vertically oriented electrostatic field [17,18,19]. And many lifeforms, such as birds needing long-distance migration, can sense the magnetic field on earth for navigation [20]. Given the interaction between electric/magnetic fields and living organisms on earth, numerous efforts were paid to explore the biological effects induced by electromagnetic fields (EMFs). And an increasing body of studies confirmed the non-negligible impact of EMFs on various cell activities including cell proliferation, differentiation, cell cycle, apoptosis, DNA replication and expression, and cytokine expression [21]. With the progress that has been made in understanding the biological effects induced by EMFs, it has been exploited for a myriad of applications including helping bone fracture healing [22], osteoarthritis improving [23], pain-relieving [24], insulin sensitivity improving [25], intervertebral fusion [26], and wound healing [27]. Externally applied fields now emerge as promising tools for fixing complicated situations in tissue engineering applications owing to their potential in remotely manipulating the classic triad of cells, materials, and biochemical factors in engineering constructs [28]. Therefore, EMFs composed of electric fields and magnetic fields are candidate tools for the successful performance of BTE.
Stem cells are considered to be one of the basic elements in BTE [29]. Both electric fields and magnetic fields, which are components of EMFs, can stimulate the osteogenic differentiation of MSCs [30, 31]. By activating MSCs, EMFs show great potential for bone tissue engineering [21]. Additionally, mechanotransduction, the biological process of cells to sense, and respond to mechanical stimuli [32], can be affected by EMFs [33]. It is well-determined that mechanotransduction possesses a pivotal role in bone tissue homeostasis and BTE [34,35,36]. Therefore, mechanotransduction is also the connecting bridge between EMFs and BTE [33]. Conclusively, we assume that EMFs, which can be applied before and/or after implantation, are advantageous biophysical tools for BTE. This review aims to highlight the advantages associated with EMFs in assisting the performance of BTE and introduce the current studies on employing pulsed EMF (PEMF) and sinusoidal EMF (SEMF) to assist BTE in bone defects repair.
Application procedures and characteristics of EMFs
The ways in which EMFs’ stimulation was applied for BTE applications are summarized (Fig. 1). For BTE application, usually, MSCs that have been isolated and expanded would be seeded on tissue-engineered graft. Subsequently, the MSCs-laden constructs would be in vitro cultured with osteogenic medium under EMFs exposure for pre-osteogenic differentiation for a certain time. After the period of EMFs’ stimulation, MSCs-laden constructs will be implanted into the bone defects area. In addition, EMFs can also be applied after constructs implantation to sustainably promote bone regeneration in vivo.
Many kinds of EMFs currently exist, and PEMF and SEMF are two commonly used electromagnetic fields in treating bone defects. Both the two kinds of electromagnetic field show significant efficiency in helping bone regeneration. PEMF bursts are sent in an on-and-off manner, and the resulting PEMF signals refer to periodically repeated bursts which are composed of a certain amount of pulses [37]. Whilst the SEMF is non-pulsed, the sinusoidal magnetic waves are generated in a continuous manner during the exposure time [38]. Figure 2 shows the general equipment for generating electromagnetic field and the characteristics of the two electromagnetic fields.
Scheme of electromagnetic fields generation system. An electromagnetic field generation system typically composed of a waveform generator, amplifier, oscilloscope, and Helmholtz coils. Two commonly used electromagnetic fields are presented: (1) pulsed electromagnetic field signal refers to the periodically repeated bursts composed of a certain amount of pulses [37]; (2) the non-pulsed sinusoidal electromagnetic field with continuous sinusoidal waveform. B refers to the magnetic flux density, t represents time. This image was drawn by the authors. Created with BioRender.com
Biological effects of EMFs in bone tissue engineering application
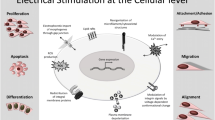
Bone regeneration is an intricate, well-orchestrated process and involves multiple cell types and their interactions. EMFs can apply non-negligible impactions on various cell types involved in bone healing and are able to induce a broad range of cell activity. Consequently, EMFs can improve the performance of bone-tissue-engineered scaffolds through the following advantages.
Cell adhesion
That stimulating cells to generate the expression of extracellular matrix for functional reconstruction of impaired tissues is a major concern of tissue engineering. The interactions between cells and engineered scaffolds, in which the biocompatibility of biomaterials is crucial, greatly affect the success of bone tissue engineering scaffolds [39]. Therefore, ensuring the biocompatibility of the engineered scaffolds is a critical prerequisite for their application. That good cell adhesion on the bone tissue engineering scaffolds’ surface is of great importance and is customarily taken as one of the important measurements for biocompatibility [40]. The positive effects of EMFs on cell adhesion have been determined by Chen et al. [41]. Their work showed that short-term exposure of EMFs (30 min/d) significantly promoted the cell adhesion and spreading of SCP-1 cell (an immortalized human mesenchymal stem cell), in addition, the short-term EMFs can even partly restore impaired SCP-1 cell adhesion caused by 5% cigarette smoke extract. Some studies further revealed that EMFs can certainly promote cell adhesion to the surface of tissue-engineered material by promoting protein adsorption [42,43,44]. Protein adsorption onto bone tissue engineering materials surface allows for cell adhesion and also possesses a vital role in determining the biocompatibility of materials [45, 46]. Wang et al. [42] reported that pulsed EMF (PEMF) actuation significantly increased protein adsorption to the titanium surface, which subsequently facilitates the initial adhesion of osteoblasts. And they assumed that PEMF stimulation induced a negatively charged surface of the titanium implant by making the dipoles aligned. Moreover, PEMF amplified the surface potential gradient of titanium implant [42]. Therefore, cations (mainly Ca2+) and proteins/peptides with positive charges would adhere to the negatively charged surface owing to the electrostatic interaction [47]. Subsequently, integrin on cell membrane, the main mediator in cell–matrix adhesion, recognizes and binds to cell adhesion-mediated motifs such as arginine-glycine-aspartic acid (RGD), which are embedded in adsorbed proteins [48, 49]. Additionally, a local higher Ca2+ concentration accelerates specific integrin receptor-mediated binding and contributes to focal adhesion formation [42, 50]. And the whole process is illustrated in Fig. 3. It is unknown whether SEMF could promote protein adsorption by such mechanism. Conclusively, using in vitro culture, it is determined that PEMF promotes cell adhesion to the material surface by promotion of protein adsorption, whereas it is still unclear whether PEMF is able to induce protein adsorption to scaffolds surface after implantation.
PEMF improves cell adhesion to Ti implant surface by promoting protein adsorption [42]. PEMF induces a negatively charged surface of Ti implant by making dipoles alignment. Cations mainly Ca2+ and proteins with positive charges adsorb onto negatively charged Ti surface. Cell adhesion-mediated motifs such as RGD embedded in adsorbed proteins mediate material-cell adhesion by binding to integrins located on cell membrane. PEMF pulsed electromagnetic field, Ti titanium, RGD Arg-Gly-Asp. This image was drawn by the authors. Created with BioRender.com
All cell types are capable of synthesizing and secreting matrix proteins to maintain the dynamic balance of extracellular matrix (ECM), which in turn dictates the cell fate and functions [51]. ECM is believed to mediate cell adhesion by binding to cell surface receptors (mainly integrin receptors), and ligands embedded in these ECM proteins play a significant role in that process [52]. For example, fibronectin and collagen, two principal components of ECM, are abundant in integrin receptor-binding domain and motif. Current studies showed that EMFs can directly stimulate expressions of ECM proteins such as fibronectin and collagen [53,54,55]. Additionally, a study carried out by Chen et al. [56] demonstrated the indirect effects of EMFs on ECM synthesis which were manifested by elevated expressions of collagen1, fibronectin, biglycan in SCP-1 cell cultured with conditioned medium from EMFs-exposed macrophages. These studies implied that EMFs might facilitate cell adhesion partly by promoting the expression of ECM proteins directly and indirectly. Lee et al. [57] demonstrated that EMFs significantly promoted human MSCs adhesion to the graphene substrate in vitro by stimulating expression levels of collagen type I and fibronectin. And their results of whole genome sequencing also confirmed that human MSCs stimulated by EMFs showed upregulated genes of ECM production [57]. Given that graphene is a two-dimensional crystal with unparalleled electric conductivity [58], the elevated ECM production might attribute to the electric activity between EMFs and graphene. In a recent study, it was proposed that reduced graphene oxide (RGO) under EMFs could generate magnetic moments, which subsequently evoke electric current [59]. It has long been identified that electric stimulation contributed to the increment of ECM-related protein production [60]. Therefore, the electric current induced by the combination of RGO and EMFs plays an essential role in elevated ECM production of human MSCs [59]. In conclusion, EMFs can intrinsically stimulate ECM production, which will be strengthened when EMFs combine with material with electrical responsiveness.
Stem cells activities
The unique capabilities of self-renewal and multilineage differentiation into cell lineages make stem cells the most suitable candidate for tissue engineering [61, 62]. Using the planarian regeneration model, Van Huizen et al. [63] demonstrated that weak magnetic force promoted stem cell proliferation and the subsequent differentiation by inducing changes in reactive oxygen species (ROS) accumulation and downstream heat shock protein 70 (Hsp70) expression. Similarly, it is confirmed that EMFs can induce non-negligible impacts on stem cell fate [64]. Plenty of studies carried out on MSCs from various sources suggested that EMFs’ stimulation positively affects the osteogenic differentiation and the expression of specific osteogenic markers (e.g. ALP, Runx2, osterix, and osteocalcin) [65]. Therefore, in most studies concerning the combination of EMFs and MSCs-loaded BTE, EMFs are employed to strengthen the osteogenic differentiation of stem cells (Fig. 4), whereas the clear mechanism of EMFs in osteogenic differentiation strengthening is complicated and has not been fully elucidated yet. One possible mechanism lies in ROS generation induced by EMFs. Recent studies believed that the involvement of EMFs in ROS production is a critical part of the cellular mechanisms underlying EMF-induced effects [66, 67]. Ehnert et al. [68] demonstrated that single EMF’s exposure stimulated ROS formation, and the induced ROS formation played an essential role in the improved osteogenic function of human osteoblasts by EMFs. Countless efforts were paid to explore and determine the pathways involved in stem cell osteogenic differentiation triggered by EMFs. Pathways implicated in pro-osteogenic differentiation of EMFs include Ca2+/CaM pathway, bone morphogenetic protein pathway (BMPs), tumour growth factor β (TGF-β) pathway, Wnt/β-catenin pathway, MAPK/ERK pathway, PI3K/Akt pathway [69, 70].
Aside from cell differentiation, cell proliferation and migration within the engineered scaffold is crucial for bone regeneration of critical size defects [71]. Using the human bone marrow-derived MSCs, our group confirmed that EMFs significantly promoted cell migration by activating intracellular Ca2+-dependent FAK/Rho GTPase migratory signalling [72]. Additionally, higher proliferation rate of MSCs resulted from EMFs’ stimulation is well-determined and has been widely demonstrated [73,74,75]. Therefore, after EMFs’ application, MSCs loaded on bone tissue engineering scaffolds would give rise to enhanced cell proliferation and migration, both of which are beneficial for bone regeneration. Previous studies showed that paracrine factors within conditioned medium of bone marrow-derived MSCs (BMSCs) could give rise to endothelial cell tube formation and macrophage recruitment in wound healing [76, 77], which implied the crosstalk between macrophage/endothelial cell and MSC take parts in tissue healing. And such crosstalk can be affected by EMFs [78]. Our group demonstrated that conditioned medium of BMSCs stimulated by EMFs showed prominent pro-angiogenetic capacity and osteoimmunomodulation effect [79]. And we further confirmed that it was the secreted cytokines within the conditioned medium that promote angiogenesis and M2 macrophage polarization (will be introduced later) [79]. A recent study similarly indicated that EMFs augmented the pro-angiogenetic capacity of MSCs by promoting the expression of miRNAs with intrinsic pro-angiogenic effect [80]. In conclusion, EMFs can be harnessed to impose beneficial effects on MSCs thereby extending and amplifying the beneficial role of MSCs in BTE.
Angiogenesis
In light of the fact that bone is a complex, rigid, highly vascularized tissue, and skeletal vasculature plays a pivotal role in the process of bone regeneration, achieving proper blood supply is another essential element of bone tissue engineering [81]. A variety of strategies have been proposed to obtain better angiogenesis within scaffolds for effective bone regeneration [82,83,84,85]. Angiogenesis occurs when new capillaries develop from pre-existing vessels, in which process begins with growth factors (e.g. VEGF, FGF) binding to their homologous receptors on endothelial cells (ECs) followed by activation of these cells to produce relevant enzymes [86]. As early as 1988, EMF stimulation was reported to promote in vitro angiogenesis of endothelial cells [87]. It was further identified that EMFs promoted angiogenesis mainly by upregulating FGF2, and HIF-1α as well as its downstream growth factors, VEGF, in endothelial cells [88, 89]. Recently, a study carried out by Wang et al. [90] confirmed that EMFs counteract the bone loss in ovariectomy-induced osteoporosis mice by the expansion of CD31hiEmcnhi endothelial cells. CD31hiEmcnhi vessels (type H vessel) is a newly found vessel subtype in bone tissue, and it is characterized with the capability of coupling osteogenesis and angiogenesis [91, 92]. And studies suggested targeting type H vessel induction would be an effective strategy of osteoporosis prevention and BTE [83, 93]. Thus, it is possible that by EMFs’ application, the formation of type H vessel within bone tissue engineering scaffold could be augmented, which further facilitate bone regeneration. Conclusively, EMFs can induce the activation of diverse signalling pathways including the FGF and VEGF signalling pathways to enhance both osteogenesis and angiogenesis [70]. Given the pro-angiogenesis effect that EMFs possess (Fig. 4), it has been applied to facilitate angiogenesis in BTE [94, 95]. These results indicate that, by applying alone or combining with VEGF, EMFs indeed promote angiogenesis thus substantially promoting the overall performance of BTE in bone defect treating.
M2 macrophage polarization
As a member of several cell lines that reside in bone marrow, monocytes/macrophages play a crucial role in bone homeostasis, repair, and anabolism, thus possessing the promising potential to aid bone regeneration [96]. At the early stage of inflammation, macrophages would swiftly migrate to the injured site in response to chemical cues after implant insertion [97]. Consequently, the response of macrophage to the engineered graft surface greatly determines the fate of the implant [98]. As an increasing body of evidence indicated the essential role of macrophages in bone regeneration, a broad range of strategies concerning macrophage manipulating were explored to facilitate better bone regeneration in BTE [99,100,101,102]. Moreover, the designing of engineered scaffolds favouring M2 macrophage polarization is one of the most effective ways. Stimulating by various signalling, macrophages characterized with diversity and plasticity may undergo M1 activation or M2 activation [103]. M2 macrophages are considered to be a pro-healing phenotype due to their ability to release high levels of the passivating cytokine such as IL-10 and TGF-β while generating low levels of inflammatory cytokines [104]. It has been reported that M2 macrophage polarization can be induced by magnetic force or electrical cues [105, 106]. Therefore, it is reasonable to consider that EMFs may modulate macrophage activity and induce macrophage polarization. Chen et al. [56] identified that EMFs with specific parameters significantly increased the protein levels of arginase I (a marker of M2 macrophage) in human peripheral blood mononuclear cells (hPBMC). Meanwhile, using the human Cytokine Array C5, they further found that the specific EMFs favoured a pro-healing secretome in hPBMC [56]. In another study carried out by Vinhas et al. [107], macrophages cultured on magnetic responsive materials can respond to PEMF and show an M2 phenotype. Their results showed that a marker associated with M2 macrophage (CD206) can only be detected in human macrophages stimulated by PEMF. And their another study also indicated that PEMF significantly promotes the expression of genes associated with M2 macrophage (Arg-1, MRC-1, Singlec-1) in THP-1 cells, a human monocytic cell line [108]. In addition, the study carried by Fu et al. [109] identified that microwave irradiation, a kind of electromagnetic field, combined with magnetic nanoparticles would conduct M2 macrophage polarization on RAW 264.7 cell line. In this study, macrophage membrane-enveloped Fe3O4/Au nanoparticles showed activation of macrophage to produce less inflammatory cytokines due to its property of electromagnetic field under microwave irradiation. In general, EMFs possess the potential to dictate M2 macrophage polarization (Fig. 4), which is one of the advantages of EMFs in improving the performance of BTE.
EMFs’ applications in assisting bone tissue engineering
A wide range of material kinds and their combinations have been considered as potential options for bone tissue engineering applications. Koons et al. [5] summarized the material types for bone tissue engineering including natural polymers, synthetic polymers, bioceramics, and carbon-based nanomaterials. The combinations of EMFs and these material types as well as their combinations (composites) have been demonstrated by numerous studies.
Common material types
EMFs have been concomitantly applied with diverse common material types including natural polymers (chitosan, keratin), synthetic polymers (poly(ε-caprolactone), poly(lactic-co-glycolic acid), poly(l-lactic acid)), calcium phosphates(hydroxyapatite, β-tricalcium phosphate), metal(titanium), and graphene (see Table 1).
Synthetic polymers
Synthetic polymers can mimic the normal extracellular matrix, and possess a pivotal position in tissue engineering [110]. The significant role of poly(ε-caprolactone) (PCL) in tissue engineering has already been well determined because of its facility of processing into long-term degradable engineered graft and its authorized security approved by the US Food and Drug Administration (FDA) [111]. Biological factors are essential players in BTE due to their role of providing correct biological signalling to guide cell differentiation and tissue growth; however, their application also brings some concerns about potential side effects [112]. In most studies concerned with a combination of BMSCs-loaded BTE and EMFs, the osteogenic medium is required for osteogenic induction, whereas, in a study carried out by Ardeshirylajimi et al. [113], EMFs’ stimulation without osteogenic medium management promoted expressions of osteogenic-related gene markers in human induced pluripotent stem cells (iPSCs) seeded on PCL nanofibres. And surprisingly, for iPSCs cultured on PCL nanofibres, EMFs’ stimulation showed a similar effect as an osteogenic medium on ALP activity, calcium content as well as expression of osteogenic gene markers [113]. The similar pro-osteogenic effect of a combination of EMFs and PCL nanofibres was also confirmed within adipose-derived mesenchymal stem cells (ADSCs) [112]. Among adult stem cells (stem cells resided in practically all organs and tissues of the adult organism, e.g. BMSCs), ADSCs are believed to be the most advantageous for tissue engineering. And it is ascribed to its ease of harvesting and high abundance in corresponding derived source tissue in comparison to BMSCs [62]. Consequently, the combination of EMFs and ADSCs-load PCL nanofibres seems a promising strategy in BTE as EMFs could be a potential alternative option for osteogenic growth factors [112].
Poly(lactic-co-glycolic acid) (PLGA) is a copolymer of lactic acid and glycolic acid. Its degradation rate can be adjusted by changing the ratio of lactic acid to glycolic acid [114,115,116]. With tunable degradation rates, good mechanical properties, and processability, PLGA is regarded as a popular and biodegradable biomaterial in BTE [117]. It has been reported that EMFs exposure with specific parameters promoted proliferation and ALP expression of rodent osteoblasts/BMSCs seeded on PLGA scaffold, which indicated the potential of EMFs to aid PLGA applied-BTE [118, 119].
Natural polymers
Natural polymers possess some prominent features of higher biocompatibility, excellent biodegradability, and no toxicity, and they have been widely applied for BTE and receive encouraging results [120].
Chitosan, the partially deacetylated form of chitin, is capable of promoting tissue growth and proliferation [121]. Due to its novel properties such as biocompatibility, biodegradability, and wound-healing activity, chitosan holds a promising future in tissue engineering applications [122]. Furthermore, chitosan is suitable for fabricating biomimetic scaffolds because it has a similar structure to glycosaminoglycans, an essential structural component of bone matrix [123]. A study demonstrated that EMFs enhanced the proliferation and mineralization of osteoblasts seeded on chitosan scaffold, which indicated the applicability of a combination of EMFs and chitosan scaffold in large bone defects treatment [124].
Keratin, which is the main component of feathers, hair, hoofs, and wool, has some intrinsic characteristics to assist cell adhesion, proliferation, and tissue regeneration. With these inherent biological characteristics and excellent biocompatibility, keratin-based biomaterials are widely applied for many biomedical applications such as bone morphogenetic protein carriers, ocular surface reconstruction, wound healing, and nerve regeneration [125, 126]. Bloise et al. identified that wool keratin scaffold can be applied as osteoconductive biomaterials under the joint action of PEMF and osteogenic factors. The combination of PEMF and osteogenic factors rendered osteoblast-like cells with higher calcified bone matrix deposited on wool keratin scaffolds [127].
Calcium phosphates
Calcium phosphates can mimic carbonated hydroxyapatite-the inorganic composites of bones, which makes it the most common bioceramics in BTE. And a broad range of biomaterials featuring calcium phosphates such as hydroxyapatite and β-tricalcium phosphate has been demonstrated [5]. It is generally believed that the osteoinductive characteristic of calcium phosphates comes from its releasing of Ca2+ and PO43−, both of which facilitate the bone bone-like apatite to form on the surface of calcium phosphates, and these two inorganics are critical factors in bone matrix mineralization [128]. Due to the prominent biomimicking property, calcium phosphates are commonly used as culture substrates to mimic a bone-like environment. And with the calcium phosphates substrates, the effects of EMFs on MSCs, osteoblast, and osteoclasts in a bone-like environment were elucidated [129,130,131]. Generally, EMFs promote the osteogenic differentiation while inhibiting bone resorption in a bone-like environment.
Hydroxyapatite (HAp) is a well-known and biocompatible bioceramic that suits for fabricating orthopaedic and dental implants [132]. However, HAp implants would be very slowly replaced by host bone after implantation; thus, the induction of bone growth into hydroxyapatite is difficult [133], whereas the success of osteointegration, namely the connection between the peri-implant bone and the implant surface, substantially influences the long-term usage of implants [134]. As EMFs are capable of non-invasively promoting osteogenesis, some studies demonstrated that better osteointegration of HAp implants and host bone could be achieved by applying EMFs’ stimulation [135, 136]. These studies identified the feasibility of employing EMFs to avoid implant clinical failure by improving the implant osteointegration. Compared to conventional hydroxyapatite implants, nanoscale HAp is likely a better choice for biomedical applications [133]. And the combination of HAp nanoparticles and EMFs seems to be an effective way to counteract bone loss. Prakash et al. [137] suspended rat tails for 45 days to mimic bone lose cause by microgravity and treated these hindlimb suspension (HLS) rats with different treatments (PEMF, HAp nanoparticles, PEMF + HAp nanoparticles) for another 45 days. They found that PEMF failed in restoring bone mineral density (BMD) in HLS rat femur (P < 0.5, compared with control) while HAp nanoparticles partially restored BMD in osteoporotic femur (P < 0.02, compared with control), whereas HLS rat treated by PEMF + HAp nanoparticles completely restored BMD and have a comparable BMD level (P < 0.01, compared with control) to rat without HLS management. The inadequate mechanical strength of HAp restricts its abroad application, and to overcome this drawback, HAp-reinforced nanomaterials obtained by doping with metals (magnetic particles) or other biomaterials have been demonstrated and received increasing attention [138]. A recent study by Fernandes Patrício et al. [139] indicated that magnetic microspheres obtained by doping HAp with iron were capable of carrying and releasing BMP-2 at a low dose for a certain time, and the release efficiency of BMP-2 could be elevated by EMFs’ stimulation. And they pointed out that such a combination of EMFs and magnetic HAp microspheres would be a new therapeutic strategy to meet clinical needs in bone cement and scaffolds for local bone replacements [139]. β-tricalcium phosphate(β-TCP) is an osteoinductive ceramic that can combine with EMFs to treat rat skull defects [140]. It is reported that iron-doped β-TCP significantly upregulates ALP expression and calcium deposition when an external PEMF was applied [141], which implies that a combination of EMFs and iron-doped bioceramics may be a new method for bone regeneration.
Titanium
Ti and its alloys are ideal biomaterials for bone-tissue-engineered implants [142, 143]. Implant loosening resulting from inadequate bone integration or the development of fibrous tissue is still one of the leading reasons for titanium implant failures in its clinical application [144]. As mentioned above, EMFs employment is efficient for promoting the osteointegration of hydroxyapatite implants and host bones. Thus, EMFs are similarly expected to prevent Ti implant loosening resulting from diverse conditions by improving osteointegration [145, 146]. For instance, osteolysis-induced looseness around the implants prosthesis is a common complication of orthopaedics [147]. Veronesi et al. [148] demonstrated that PEMF was a safe, and conservative treatment for counteracting periprosthetic osteolysis in rats. In their study, the histological and histomorphometric results demonstrated that bone-to-implant contact (BIC), a significant indicator of osteointegration, was significantly elevated (P < 0.0005, compared to osteolysis rats without treatments) after employing PEMF for 6 h/day for 60 days. And both fibrous capsule thickness (P < 0.005) and osteoclasts number (P < 0.0005) were dramatically decreased by PEMF [148]. The reduction in fibrous tissue formation around implants may ascribe to the induction of EMFs in the attenuation of mast cell infiltration around implants [149]. It is acknowledged that metabolic abnormalities detrimentally affect implant osteointegration and patients with diabetes mellitus are more associated with implant failure [150, 151]. Considering the increasing prevalence of diabetes mellitus, effective methods for improving osseointegration in diabetic patients are in urgent need. A study carried out by Cai et al. [152] indicated that EMFs management seemed a valuable method for combatting Ti implant loosening within type 1 diabetic rabbits. Their results confirmed that EMFs significantly improved the impaired bone architecture and mechanical properties incited by diabetic mellitus, which would subsequently help for the success of implant therapy in diabetic rabbits. Moreover, EMFs employment is also effective in improving glucocorticoid-impaired Ti implant osseointegration [153]. Conclusively, externally applied EMFs can effectively counteract Ti implant loosening resulting from diverse pathological conditions.
A vast variety of implant surface modification techniques such as topography modification has been developed to accelerate Ti implant osseointegration [144]. Wang et al. explored the effects of EMFs on the functions of osteoblasts on three types of titanium surfaces (flat, micro, and nano). They identified the positive impaction of EMFs on osteoblasts seeded on all three topographies including increasing cell adhesion, cell proliferation, extracellular matrix mineralization nodules, and expression of osteogenesis-related genes (BMP-2, OCN, Col-1, ALP, Runx2 and OSX) [42]. Bloise et al. similarly demonstrated that EMFs with clear pro-osteogenic effects can combine with Ti implants of nano-topography surface and might be an effective adjuvant treatment to improve the osseointegration of Ti implants [154].
Composites
Composite materials have been considered a better choice for bone tissue engineering due to their ability to outperform their individual constituents [5]. For example, by adding poly(l-lactic acid) (PLLA), a member of synthetic polymers, into HAp, the resulting PLLA/HAp composite scaffold can not only overcome the disadvantages of HAp in unsatisfactory degradability and brittleness but also possesses higher cell viability in comparison to pure PLLA polymer scaffolds [155]. Hence, the studies on combining EMFs with composites including magnetic and non-magnetic composites are extensive (see Table 2).
Non-magnetic composites
Non-magnetic composites here refer to conventional composites without magnetic properties. As mentioned above, PLLA/HA is superior to its individual constituents and thus represents a promising composite for BTE. Our group has demonstrated the feasibility of a combination of EMFs and PLLA/HAp scaffold in dealing with rat critical-sized calvarial defects [95]. Besides PLLA, PCL is another commonly used synthetic polymer to overcome the brittleness of HA owing to its high mechanical properties and approved safety. Our work showed that the combination of EMFs and PCL/HAp scaffold also obtained pleasing outcomes in treating rat bone defects [79, 94]. In the study carried out by Li et al., rat BMSCs-laden-PCL/HAp scaffolds were pre-treated with osteogenic medium and SEMF for 7 days (4 h/d) before in vivo implantation. And 8 weeks later, calvarial defect implanted with such scaffolds had more new bone area (P < 0.01) than rats received BMSCs-laden-PCL/HAp scaffolds pre-treated with osteogenic medium only [79]. Another example of composites composed of synthetic polymers and natural polymers is PCL/carboxymethyl chitosan (CMC). It has been reported that PCL/CMC scaffold fabricated by the electrospinning technique is suitable for BTE [156]. By grafting CMC onto PCL nanofibres, Shapourzadeh et al. revealed that grafted CMC endowed the composite scaffold with the ability to self-differentiate stem cells, and the osteoinductivity could be further augmented by EMFs employment or β-carotene management [157]. Collagen, the most abundant protein in the extracellular matrix, is the major structural element of all connective tissues [158]. Hence, collagen is believed to be an ideal biomaterial for tissue engineering related to skin, bone, and cartilage [159]. However, pure collagenous materials lack adequate mechanical strength and stiffness, and to counteract these flaws of collagen, inorganic materials are usually added to collagen for fabricating composite scaffolds [160]. And it is usually to disperse HAp into collagen to fabricate HA/collagen scaffold, a bioinspired composite suited for BTE application [161]. Our group previously demonstrated that EMFs employment was promising for improving the effectiveness of HA/collagen scaffold in rabbit femur defects repairment [162]. And the results indicated that BMSCs-laden-HA/collagen scaffold stimulated by SEMF successfully promote bone regeneration within the defect and bone integration between the graft and host bone in rabbit femur [162]. Other composites such as polyvinyl alcohol/polyethersulfone is also feasible for constructing tissue-engineered scaffolds and for combining with EMFs to stimulate osteogenesis [163].
Piezoelectricity refers to the ability to generate electric activity in response to mechanical stress and vice versa [164]. Piezoelectric materials have attracted much attention for providing electrical stimulation, a vital player in cell biological activity, to cells to promote tissue regeneration [165]. The natural bone has prominent piezoelectricity and it can physiologically generate electrical stimulation to enhance bone growth in response to mechanical stress, thus piezoelectric materials are suitable for fabricating bone tissue-engineered scaffolds [166]. Externally applied electrical field is capable of inducing the deformation of piezoelectric materials, which in turn provides mechanical stimulation for osteoblasts to aid bone healing [164]. Hence, the combination of EMFs and piezoelectric materials has been investigated. Among the piezoelectric materials, polyvinylidene fluoride (PVDF) is an attractive synthetic polymer in BTE owing to its excellent piezoelectricity and good biocompatibility [167]. Mirzaei et al. [168] fabricated a scaffold composed of PVDF and polyaniline (PANI)-a polymer with excellent electrical conductivity. Their results showed that both the PVDF scaffold and PVDF/PANI scaffold had improved cell attachment and osteogenic differentiation under EMFs exposure, while the combination of EMFs and PVDF/PANI scaffold had a more significant effect [168]. Similar results were obtained by Dong et al. [47], and they speculated that the mechanical stimulation resulting from the interplay between EMFs and PVDF-coated PCL-TCP scaffolds contributed to the well cellular response. The prominent effect of a combination of PVDF-based material and EMFs is inspiring and holds promises in the field of BTE.
Magnetic nanocomposites (MNC)
Magnetic nanocomposites (MNC) are smart materials typically composed of magnetic nanoparticles (MNPs) and the other component which is either organic or inorganic [169]. It is believed that the incorporation of MNPs endows magnetic composite scaffolds with magnetic properties and better mechanical properties, both of which improve cell spreading, adhesion, differentiation, and ability to respond to an external magnetic field [170, 171]. Combined with externally applied magnetic fields, MNC can interact with cells and modulate cell biological processes, thus MNC have been exploited for a large range of biomedical applications such as BTE [172]. Both static magnetic fields and EMFs can provide magnetic cues for MNC thus possessing great promise for assisting BTE [173]. Research works on applying static magnetic fields to combine with MNC attract a great amount of attention and obtain satisfactory results in this field [174,175,176]. There are also quite a few scientific reports on concomitantly applying EMFs with MNC for BTE applications. Huang et al. [177] identified that BMSCs seeded on porous scaffolds that are composed of Fe2O3 nanoparticles, n-HAp and PLLA have higher ALP activity after PEMF stimulation. Correspondingly, Zeng et al. [178] further demonstrated that in all PEMF-stimulated groups, cells cultured on MNPs-dispersed HA scaffolds had higher ALP values than that on HA scaffolds. These two reports implied the interactions between externally applied EMFs and MNPs would lead to enhanced osteogenic differentiation. And the osteogenic effect may attribute to well cell behaviour under the presence of MNC scaffolds and the externally applied EMFs. For instance, by using scanning electron microscopy, Moradian et al. [43] observed that the presence of EMFs significantly promoted cell attachment on the MNC scaffolds. Similarly, Meshkini et al. [179] identified that the combination of magnetic nanofibres and EMFs significantly promoted MSCs adhere to material surface, and the number of adhered cells increases in an MNC content-dependent manner, which implies that it is the interaction of MNC and external EMFs affect biological behaviour of the loaded cell. Additionally, a combination of Mg.ATP-modified magnetic nanofibres and EMFs seems to be a great candidate in BTE applications [179]. The introduction of magnesium (Mg) and adenosine 5'-triphosphate (ATP), both of which are beneficial for osteogenic differentiation, allows the magnetic nanofibres to work synergistically with EMFs on enhancing osteogenic differentiation of MSCs. Given the solid foundation provided by these studies, it can be concluded that EMFs’ stimulation is, besides static magnetic fields, a potential tool to provide magnetic cues for MNC-based BTE.
The combination of EMFs and MNC exploits interactions between MNPs and the externally applied field. The interactions between MNC-based scaffold and loaded cell under magnetic force have been reviewed by Filippi et al. [172], whereas distinction from the constant magnetic force provided by static magnetic fields, EMFs such as PEMF, offers oscillating magnetic force. It is postulated that the interactions of MNC and loaded cells induced by EMFs include the following aspects. First, oscillating magnetic force can induce mechanical vibration of each MNP in the MNC scaffold [172]. The vibration of MNPs at the interface of MNC scaffold provides mechanical stimulation to cells, which might subsequently influence the ion channels, and activate the mechanotransduction pathway [180]. The vibration of MNPs induced by EMFs can also be exploited to control drug release. Thus, it is feasible to remotely control biological factor delivery and release by EMFs’ application. For instance, it has been identified that vibration resulting from EMFs is capable of promoting BMP-2 to release from MNC microspheres, even though the BMP-2 proteins have a chemical link with these magnetic microspheres [139]. In addition to vibration, heat generated by MNPs under EMFs exposure can also affect drug release. Zhang et al. [181] incorporated MNPs and thermo-responsive agar into polyethylene glycol (PEG) hydrogel, and the added agar was expected to change its network due to the generated heat of MNPs. Therefore, under the vibration and generated heat of MNPs, the release of drug was significantly promoted when EMFs were applied [181]. Accumulating studies indicated that mild heat generated by external heat sources could contribute to osteogenic differentiation and mineralization of MSCs by inducing upregulation of heat shock protein (HSP) and further activating the downstream signalling pathways [182,183,184]. Consequently, the thermal effect of MNPs under EMFs can be employed to facilitate osteogenic differentiation and lead to accelerated bone regeneration. Cao et al. [185] identified that MNPs-incorporated chitosan hydrogel generated mild heat under high-frequency EMFs, and the generated mild heat promoted osteogenic differentiation ability of MSCs in vitro. Wang et al. [186] further demonstrated that mild heat generated by MNPs under high-frequency EMFs facilitated osteogenesis by inducing HSP90 accumulation and the subsequent activation of PI3K/Akt pathway. The interactions of MNC and cells in the presence of EMFs are summarized in Fig. 5.
Interactions of MNC and cells under EMFs’ exposure. (1) Vibrations of MNPs induced by the presence of EMF provide mechanical cues for cells load on MNC [180]. (2, 3) Vibrations and generated heat of MNPs in the presence of EMFs affect the release of biological factors such as BMP-2 which would subsequently exert biological effects on adjacent cells [139, 181]. (4) Mild heat generated by MNC under high-frequency EMFs contributes to enhanced osteogenesis [186]. MNC magnetic nanocomposites, EMFs electromagnetic fields, MNPs magnetic nanoparticles, BMP-2 bone morphogenetic protein-2, HSP 90 heat shock protein 90. This image was drawn by the authors. Created with BioRender.com
EMFs promote the benefits of tissue-engineered scaffold on loaded cells
Bone-tissue-engineered scaffold, the most essential part of BTE, evolved from the initial biological substance that simply provides mechanical support to the current biomaterials that are tailored to possess osteoinductive or osteoconductive characteristics [5]. In most studies that employed EMFs to assist BTE, biological effects of EMFs on the loaded cell were mainly focused; in contrast, the benefits of tissue-engineered scaffold were rarely discussed. Undoubtedly, tissue-engineered scaffold essentially benefits the bone regeneration within bone defects under the circumstance of harnessing EMFs to assist BTE. Owing to the lack of direct evidence for clarifying the benefits of tissue-engineered scaffold, we can only indirectly understand the real role of tissue-engineered scaffold in bone defects treatment using the triad of stem cells, materials, and EMFs exposure by biological changes of cells on different scaffold. The presence of scaffolds dramatically affects biological effects induced by EMFs is firmly justified based on studies reviewed above. For instance, Schwartz et al. demonstrated that PEMF promoted osteogenesis by stimulating osteoprotegerin (OPG) production in osteoblasts. And they found that under the PEMF exposure, osteoblasts cultured on calcium phosphates showed significantly higher OPG production than the osteoblasts cultured on tissue culture polystyrene plastic [131]. Thus, it is believed that EMFs exposure involved in and promoted the positively biological effects supported by tissue-engineered scaffold on loaded cell. A recent study further indicated that the mechanical microenvironments established by tissue-engineered scaffold elicited distinct mechanotransduction responses and ultimately influenced MSCs responses to EMFs’ stimulation. In other words, EMFs influenced the signalling pathways activated by mechanical microenvironments of scaffolds, and further augmented the biological effects [187].
Clinical applications of EMFs in bone defect treatment
A huge amount of in vitro studies builds a solid foundation for the clinical applications of EMFs in dealing with the skeletal disorder. Numerous clinical studies were carried on determining the clinical implications of PEMF, the FDA-approved electromagnetic field therapy. Ehnert et al. [37] summarized clinical studies on the effect of PEMF treatment on bone fracture non-unions, osteotomies, acute bone fractures, spinal fusion, osteoporosis, and osteoarthritis. And they concluded that EMFs presented a valuable adjunctive therapy for bone and osteochondral defects [37]. The clinical studies have indicated that PEMF presented a beneficial treatment in the management of delayed union and nonunion fractures, both of which are major complications in the treatment of skeletal defects [188]. For instance, in a prospective randomized controlled study that enrolled 58 patients, patients who received PEMF treatment for an average duration of 4.8 months had a union rate of 77.4%, whereas patients in the control group only had a successful union rate of 48.1% [189]. Spinal fusion surgery is usually performed when orthopaedic defects occur at load-bearing spine which is essential to the mobility of patients [5]. A randomized double-blind prospective study demonstrated that 92.2% of patients in PEMF treatment group had a successful fusion as determined by blinded radiographic evaluation, while the control group had a success rate of 67.9% [190]. Other clinical trials also advocated the application of PEMF for spinal fusion [191, 192]. Conclusively, PEMF proves its benefits in clinically treating bone defects. However, scant clinical data exist to demonstrate the efficiency and feasibility of a combination of EMFs and BTE in treating bone defects.
Conclusion and future perspectives
Conclusively, the excellent performance of EMFs in bone regeneration renders it a promising adjunctive therapy for BTE. EMFs represents a non-invasive, and non-contact treatment, it has been exploited for many biomedical applications. For instance, PEMF, the most widely applied and FDA-approved electromagnetic field, has been put into clinical treatment of fractures, osteoarthritis, and osteoporosis for many years, whereas PEMF has not been clinically applied for bone defects that required bone substitutes. For critical-sized or large bone defects, simple EMFs’ stimulation can hardly help for bone regeneration within defects, whereas, when combined with stem cell-loaded scaffolds, EMFs show the surprising ability of facilitating bone regeneration. Consequently, EMFs that provide biophysical stimuli can be regarded as a promising complement to the classic triad of cells, materials, and biochemical factors in BTE [193, 194]. We summarized some advantages of EMFs in assisting BTE including cell adhesion enhancement, stem cells osteogenic differentiation augment, angiogenesis improvement, and M2 macrophage induction. Therefore, by remotely affecting multiple cell types and their biological behaviour, EMFs seem perfectly suited for BTE applications. Additionally, the employment of EMFs does help for addressing some hurdles in BTE applications, such as the suboptimal osteointegration of hydroxyapatite and Ti implant. However, there still exist some limitations in EMFs’ application. As is known, the biological effects induced by EMFs may vary with many parameters including frequency, continuous exposure time, and even directionalities [195]. It implies that much work remains to be done before determining the best parameters capable of inducing the desired biological effects. Meanwhile, a poor understanding of the underlying mechanisms further damps the broad clinical application of EMFs [37]. To build a solid foundation for EMFs’ applications in BTE, future studies are required to clarify the safety, optimal EMFs parameter settings for human exposures as well as the molecular and cellular mechanisms of EMFs on the living organism.
EMFs have shown promises in combining diverse material types and their combinations, especially materials with piezoelectricity and materials with magnetic responsiveness. Piezoelectric materials under external electric fields generate structural deformation, which subsequently leads to the generation of electric activity. It is the actuated deformation and electric activity contribute to the strengthened performance of piezoelectric materials under EMFs exposure. Moreover, MNC and MNPs are attractive materials owing to their exceptional properties and excellent performance in biomedical applications. The vibration and induced heat of MNPs under EMFs exposure confer MNC with the capability of stimulating osteogenesis and remote controlling of biological factors release. These materials that can respond to external stimuli are called stimuli-responsive biomaterials. Stimuli-responsive biomaterials bring a great deal of attention to the field of BTE as they can partially recapitulate the dynamic environment of living tissue under external stimuli [196]. Externally applied EMFs emerging as a potential external stimulus. This is because EMFs can not only directly influence cells, but also interact with bone tissue engineering materials and subsequently promote osteogenesis. The combination of EMFs and stimuli-responsive biomaterials is promising in BTE application. Further studies are needed for deepening our knowledge of interactions between EMFs and BTE scaffolds, which in turn would help for better designing of scaffolds aimed at EMFs-assisted BTE. In summary, EMFs as an ideal adjunctive therapy hold great potential in combining with diverse biomaterials for BTE applications. That harnessing EMFs to assist successful performance of biomaterials would be an effective strategy for bone defects treatment.
Availability of data and materials
Data sharing is not applicable to this article as no new data were created or analysed in this study.
Abbreviations
- BTE:
-
Bone tissue engineering
- EMFs:
-
Electromagnetic fields
- MCs:
-
Mesenchymal stem cells
- PEMF:
-
Pulsed electromagnetic fields
- SEMF:
-
Sinusoidal electromagnetic field
- RGD:
-
Arginine-glycine-aspartic acid
- ECM:
-
Extracellular matrix
- ALP:
-
Alkaline phosphatase
- Runx2:
-
Runt-related transcription factor 2
- VEGF:
-
Vascular endothelial growth factor
- FGF:
-
Fibroblast growth factor
- PCL:
-
Poly(ε-caprolactone)
- BMSCs:
-
Bone marrow-derived mesenchymal stem cells
- iPSCs:
-
Induced pluripotent stem cells
- ADSCs:
-
Adipose-derived mesenchymal stem cells
- PLGA:
-
Poly(lactic-co-glycolic acid)
- HAp:
-
Hydroxyapatite
- Ti:
-
Titanium
- BMP-2:
-
Bone morphogenetic protein-2
- OCN:
-
Osteocalcin
- Col-1:
-
Collagen I
- OSX:
-
Osterix
- PLLA:
-
Poly(l-lactic acid)
- CMC:
-
Carboxymethyl chitosan
- PVDF:
-
Polyvinylidene fluoride
- PANI:
-
Polyaniline
- MNC:
-
Magnetic nanocomposites
- MNPs:
-
Magnetic nanoparticles
References
Atala A, Kasper FK, Mikos AG. Engineering complex tissues. Sci Transl Med. 2012;4(160):160rv112.
Panetta NJ, Gupta DM, Slater BJ, Kwan MD, Liu KJ, Longaker MT. Tissue engineering in cleft palate and other congenital malformations. Pediatr Res. 2008;63(5):545–51.
Loebel C, Burdick JA. Engineering stem and stromal cell therapies for musculoskeletal tissue repair. Cell Stem Cell. 2018;22(3):325–39.
McDermott AM, Herberg S, Mason DE, Collins JM, Pearson HB, Dawahare JH, Tang R, Patwa AN, Grinstaff MW, Kelly DJ, et al. Recapitulating bone development through engineered mesenchymal condensations and mechanical cues for tissue regeneration. Sci Transl Med. 2019;11(495):eaav7756.
Koons GL, Diba M, Mikos AG. Materials design for bone-tissue engineering. Nat Rev Mater. 2020;5(8):584–603.
Minkowitz RB, Bhadsavle S, Walsh M, Egol KA. Removal of painful orthopaedic implants after fracture union. J Bone Joint Surg Am. 2007;89(9):1906–12.
Bhumiratana S, Bernhard JC, Alfi DM, Yeager K, Eton RE, Bova J, Shah F, Gimble JM, Lopez MJ, Eisig SB, et al. Tissue-engineered autologous grafts for facial bone reconstruction. Sci Transl Med. 2016;8(343):343ra383.
Lee JW, Han HS, Han KJ, Park J, Jeon H, Ok MR, Seok HK, Ahn JP, Lee KE, Lee DH, et al. Long-term clinical study and multiscale analysis of in vivo biodegradation mechanism of Mg alloy. Proc Natl Acad Sci U S A. 2016;113(3):716–21.
Russell N, Oliver RA, Walsh WR. The effect of sterilization methods on the osteoconductivity of allograft bone in a critical-sized bilateral tibial defect model in rabbits. Biomaterials. 2013;34(33):8185–94.
Curry AS, Pensa NW, Barlow AM, Bellis SL. Taking cues from the extracellular matrix to design bone-mimetic regenerative scaffolds. Matrix Biol. 2016;52–54:397–412.
Kim BS, Mooney DJ. Development of biocompatible synthetic extracellular matrices for tissue engineering. Trends Biotechnol. 1998;16(5):224–30.
Dong L, Wang C. Harnessing the power of macrophages/monocytes for enhanced bone tissue engineering. Trends Biotechnol. 2013;31(6):342–6.
Meijer GJ, de Bruijn JD, Koole R, van Blitterswijk CA. Cell-based bone tissue engineering. PLoS Med. 2007;4(2): e9.
Grayson WL, Bunnell BA, Martin E, Frazier T, Hung BP, Gimble JM. Stromal cells and stem cells in clinical bone regeneration. Nat Rev Endocrinol. 2015;11(3):140–50.
Jiang F, Zhang W, Zhou M, Zhou Z, Shen M, Chen N, Jiang X. Human amniotic mesenchymal stromal cells promote bone regeneration via activating endogenous regeneration. Theranostics. 2020;10(14):6216–30.
Ma J, Both SK, Yang F, Cui FZ, Pan J, Meijer GJ, Jansen JA, van den Beucken JJ. Concise review: cell-based strategies in bone tissue engineering and regenerative medicine. Stem Cells Transl Med. 2014;3(1):98–107.
Carter CS, Huang SC, Searby CC, Cassaidy B, Miller MJ, Grzesik WJ, Piorczynski TB, Pak TK, Walsh SA, Acevedo M, et al. Exposure to static magnetic and electric fields treats type 2 diabetes. Cell Metab. 2020;32(6):1076.
Doglioni C, Pignatti J, Coleman M. Why did life develop on the surface of the Earth in the Cambrian? Geosci Front. 2016;7(6):865–73.
Honkonen I, Kuvshinov A, Rastätter L, Pulkkinen A. Predicting global ground geoelectric field with coupled geospace and three-dimensional geomagnetic induction models. Space Weather. 2018;16(8):1028–41.
Wiltschko W, Wiltschko R. Magnetic compass of European robins. Science. 1972;176(4030):62–4.
Saliev T, Mustapova Z, Kulsharova G, Bulanin D, Mikhalovsky S. Therapeutic potential of electromagnetic fields for tissue engineering and wound healing. Cell Prolif. 2014;47(6):485–93.
Hollenberg AM, Huber A, Smith CO, Eliseev RA. Electromagnetic stimulation increases mitochondrial function in osteogenic cells and promotes bone fracture repair. Sci Rep. 2021;11(1):19114.
Yang X, He H, Ye W, Perry TA, He C. Effects of pulsed electromagnetic field therapy on pain, stiffness, physical function, and quality of life in patients with osteoarthritis: a systematic review and meta-analysis of randomized placebo-controlled trials. Phys Ther. 2020;100(7):1118–31.
Elshiwi AM, Hamada HA, Mosaad D, Ragab IMA, Koura GM, Alrawaili SM. Effect of pulsed electromagnetic field on nonspecific low back pain patients: a randomized controlled trial. Braz J Phys Ther. 2019;23(3):244–9.
Zhai M, Yan X, Liu J, Long Z, Zhao S, Li W, Liu Y, Hai C. Electromagnetic fields ameliorate insulin resistance and hepatic steatosis by modulating redox homeostasis and SREBP-1c expression in db/db mice. Diabetes Metab Syndr Obes. 2021;14:1035–42.
Li W, Huang C, Ma T, Wang J, Liu W, Yan J, Sheng G, Zhang R, Wu H, Liu C. Low-frequency electromagnetic fields combined with tissue engineering techniques accelerate intervertebral fusion. Stem Cell Res Ther. 2021;12(1):143.
Gualdi G, Costantini E, Reale M, Amerio P. Wound repair and extremely low frequency-electromagnetic field: insight from in vitro study and potential clinical application. Int J Mol Sci. 2021;22(9):5037.
Armstrong JPK, Stevens MM. Using remote fields for complex tissue engineering. Trends Biotechnol. 2020;38(3):254–63.
Hao Z, Song Z, Huang J, Huang K, Panetta A, Gu Z, Wu J. The scaffold microenvironment for stem cell based bone tissue engineering. Biomater Sci. 2017;5(8):1382–92.
Bicer M, Sheard J, Iandolo D, Boateng SY, Cottrell GS, Widera D. Electrical stimulation of adipose-derived stem cells in 3D nanofibrillar cellulose increases their osteogenic potential. Biomolecules. 2020;10(12):1696.
Li W, Zhao S, He W, Zhang M, Li S, Xu Y. Static magnetic fields accelerate osteogenesis by regulating FLRT/BMP pathway. Biochem Biophys Res Commun. 2020;527(1):83–9.
Wu M, Fannin J, Rice KM, Wang B, Blough ER. Effect of aging on cellular mechanotransduction. Ageing Res Rev. 2011;10(1):1–15.
Santos LJ, Reis RL, Gomes ME. Harnessing magnetic-mechano actuation in regenerative medicine and tissue engineering. Trends Biotechnol. 2015;33(8):471–9.
Burger EH, Klein-Nulend J. Mechanotransduction in bone–role of the lacuno-canalicular network. FASEB J. 1999;13(Suppl):S101-112.
Goodman CA, Hornberger TA, Robling AG. Bone and skeletal muscle: key players in mechanotransduction and potential overlapping mechanisms. Bone. 2015;80:24–36.
Sikavitsas VI, Temenoff JS, Mikos AG. Biomaterials and bone mechanotransduction. Biomaterials. 2001;22(19):2581–93.
Ehnert S, Schröter S, Aspera-Werz RH, Eisler W, Falldorf K, Ronniger M, Nussler AK. Translational insights into extremely low frequency pulsed electromagnetic fields (ELF-PEMFs) for bone regeneration after trauma and orthopedic surgery. J Clin Med. 2019;8(12):2028.
Font LP, Cardonne MM, Kemps H, Meesen R, Salmon OF, González FG, Lambrichts I, Rigo JM, Brône B, Bronckaers A. Non-pulsed sinusoidal electromagnetic field rescues animals from severe ischemic stroke via NO activation. Front Neurosci. 2019;13:561.
Williams DF. On the mechanisms of biocompatibility. Biomaterials. 2008;29(20):2941–53.
Wagener V, Schilling A, Mainka A, Hennig D, Gerum R, Kelch ML, Keim S, Fabry B, Virtanen S. Cell adhesion on surface-functionalized magnesium. ACS Appl Mater Interfaces. 2016;8(19):11998–2006.
Chen Y, Aspera-Werz RH, Menger MM, Falldorf K, Ronniger M, Stacke C, Histing T, Nussler AK, Ehnert S. Exposure to 16 Hz pulsed electromagnetic fields protect the structural integrity of primary cilia and associated TGF-β signaling in osteoprogenitor cells harmed by cigarette smoke. Int J Mol Sci. 2021;22(13):7036.
Wang J, An Y, Li F, Li D, Jing D, Guo T, Luo E, Ma C. The effects of pulsed electromagnetic field on the functions of osteoblasts on implant surfaces with different topographies. Acta Biomater. 2014;10(2):975–85.
Moradian E, Rabiee SM, Haghighipour N, Salimi-Kenari H. Fabrication and physicochemical characterization of a novel magnetic nanocomposite scaffold: electromagnetic field effect on biological properties. Mater Sci Eng C Mater Biol Appl. 2020;116:111222.
Bueno VB, Takahashi SH, Catalani LH, de Torresi SI, Petri DF. Biocompatible xanthan/polypyrrole scaffolds for tissue engineering. Mater Sci Eng C Mater Biol Appl. 2015;52:121–8.
Power KA, Fitzgerald KT, Gallagher WM. Examination of cell-host-biomaterial interactions via high-throughput technologies: a re-appraisal. Biomaterials. 2010;31(26):6667–74.
Roach P, Farrar D, Perry CC. Interpretation of protein adsorption: surface-induced conformational changes. J Am Chem Soc. 2005;127(22):8168–73.
Dong Y, Suryani L, Zhou X, Muthukumaran P, Rakshit M, Yang F, Wen F, Hassanbhai AM, Parida K, Simon DT, et al. Synergistic effect of PVDF-coated PCL-TCP scaffolds and pulsed electromagnetic field on osteogenesis. Int J Mol Sci. 2021;22(12):7036.
Ratner BD, Bryant SJ. Biomaterials: where we have been and where we are going. Annu Rev Biomed Eng. 2004;6:41–75.
Shin H, Jo S, Mikos AG. Biomimetic materials for tissue engineering. Biomaterials. 2003;24(24):4353–64.
Funk RH, Monsees TK. Effects of electromagnetic fields on cells: physiological and therapeutical approaches and molecular mechanisms of interaction. A review. Cells Tissues Organs. 2006;182(2):59–78.
Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK. Extracellular matrix structure. Adv Drug Deliv Rev. 2016;97:4–27.
Berrier AL, Yamada KM. Cell-matrix adhesion. J Cell Physiol. 2007;213(3):565–73.
Huegel J, Choi DS, Nuss CA, Minnig MCC, Tucker JJ, Kuntz AF, Waldorff EI, Zhang N, Ryaby JT, Soslowsky LJ. Effects of pulsed electromagnetic field therapy at different frequencies and durations on rotator cuff tendon-to-bone healing in a rat model. J Shoulder Elbow Surg. 2018;27(3):553–60.
Aaron RK, Boyan BD, Ciombor DM, Schwartz Z, Simon BJ. Stimulation of growth factor synthesis by electric and electromagnetic fields. Clin Orthop Relat Res. 2004;419:30–7.
Chalidis B, Sachinis N, Assiotis A, Maccauro G. Stimulation of bone formation and fracture healing with pulsed electromagnetic fields: biologic responses and clinical implications. Int J Immunopathol Pharmacol. 2011;24(1 Suppl 2):17–20.
Chen Y, Menger MM, Braun BJ, Schweizer S, Linnemann C, Falldorf K, Ronniger M, Wang H, Histing T, Nussler AK, et al. Modulation of macrophage activity by pulsed electromagnetic fields in the context of fracture healing. Bioengineering (Basel). 2021;8(11):167.
Lee Y-J, Jang W, Im H, Sung J-S. Extremely low frequency electromagnetic fields enhance neuronal differentiation of human mesenchymal stem cells on graphene-based substrates. Curr Appl Phys. 2015;15:S95–102.
Mao HY, Laurent S, Chen W, Akhavan O, Imani M, Ashkarran AA, Mahmoudi M. Graphene: promises, facts, opportunities, and challenges in nanomedicine. Chem Rev. 2013;113(5):3407–24.
Lim KT, Seonwoo H, Choi KS, Jin H, Jang KJ, Kim J, Kim JW, Kim SY, Choung PH, Chung JH. Pulsed-electromagnetic-field-assisted reduced graphene oxide substrates for multidifferentiation of human mesenchymal stem cells. Adv Healthc Mater. 2016;5(16):2069–79.
Hartig M, Joos U, Wiesmann HP. Capacitively coupled electric fields accelerate proliferation of osteoblast-like primary cells and increase bone extracellular matrix formation in vitro. Eur Biophys J. 2000;29(7):499–506.
Zhao C, Tan A, Pastorin G, Ho HK. Nanomaterial scaffolds for stem cell proliferation and differentiation in tissue engineering. Biotechnol Adv. 2013;31(5):654–68.
Bacakova L, Zarubova J, Travnickova M, Musilkova J, Pajorova J, Slepicka P, Kasalkova NS, Svorcik V, Kolska Z, Motarjemi H, et al. Stem cells: their source, potency and use in regenerative therapies with focus on adipose-derived stem cells—a review. Biotechnol Adv. 2018;36(4):1111–26.
Van Huizen AV, Morton JM, Kinsey LJ, Von Kannon DG, Saad MA, Birkholz TR, Czajka JM, Cyrus J, Barnes FS, Beane WS. Weak magnetic fields alter stem cell-mediated growth. Sci Adv. 2019;5(1):7201.
Tamrin SH, Majedi FS, Tondar M, Sanati-Nezhad A, Hasani-Sadrabadi MM. Electromagnetic fields and stem cell fate: when physics meets biology. Rev Physiol Biochem Pharmacol. 2016;171:63–97.
Maziarz A, Kocan B, Bester M, Budzik S, Cholewa M, Ochiya T, Banas A. How electromagnetic fields can influence adult stem cells: positive and negative impacts. Stem Cell Res Ther. 2016;7(1):54.
Sherrard RM, Morellini N, Jourdan N, El-Esawi M, Arthaut LD, Niessner C, Rouyer F, Klarsfeld A, Doulazmi M, Witczak J, et al. Low-intensity electromagnetic fields induce human cryptochrome to modulate intracellular reactive oxygen species. PLoS Biol. 2018;16(10): e2006229.
Pooam M, Jourdan N, El Esawi M, Sherrard RM, Ahmad M. HEK293 cell response to static magnetic fields via the radical pair mechanism may explain therapeutic effects of pulsed electromagnetic fields. PLoS ONE. 2020;15(12): e0243038.
Ehnert S, Fentz AK, Schreiner A, Birk J, Wilbrand B, Ziegler P, Reumann MK, Wang H, Falldorf K, Nussler AK. Extremely low frequency pulsed electromagnetic fields cause antioxidative defense mechanisms in human osteoblasts via induction of •O(2)(-) and H(2)O(2). Sci Rep. 2017;7(1):14544.
Varani K, Vincenzi F, Pasquini S, Blo I, Salati S, Cadossi M, De Mattei M. Pulsed electromagnetic field stimulation in osteogenesis and chondrogenesis: signaling pathways and therapeutic implications. Int J Mol Sci. 2021;22(2):809.
Caliogna L, Medetti M, Bina V, Brancato AM, Castelli A, Jannelli E, Ivone A, Gastaldi G, Annunziata S, Mosconi M, et al. Pulsed electromagnetic fields in bone healing: molecular pathways and clinical applications. Int J Mol Sci. 2021;22(14):7403.
Yang D, Zhao Z, Bai F, Wang S, Tomsia AP, Bai H. Promoting cell migration in tissue engineering scaffolds with graded channels. Adv Healthc Mater. 2017;6(18):1700472.
Zhang Y, Yan J, Xu H, Yang Y, Li W, Wu H, Liu C. Extremely low frequency electromagnetic fields promote mesenchymal stem cell migration by increasing intracellular Ca(2+) and activating the FAK/Rho GTPases signaling pathways in vitro. Stem Cell Res Ther. 2018;9(1):143.
Sun LY, Hsieh DK, Yu TC, Chiu HT, Lu SF, Luo GH, Kuo TK, Lee OK, Chiou TW. Effect of pulsed electromagnetic field on the proliferation and differentiation potential of human bone marrow mesenchymal stem cells. Bioelectromagnetics. 2009;30(4):251–60.
Bai WF, Zhang MS, Huang H, Zhu HX, Xu WC. Effects of 50 Hz electromagnetic fields on human epidermal stem cells cultured on collagen sponge scaffolds. Int J Radiat Biol. 2012;88(7):523–30.
Hamid HA, Sarmadi VH, Prasad V, Ramasamy R, Miskon A. Electromagnetic field exposure as a plausible approach to enhance the proliferation and differrentiation of mesenchymal stem cells in clinically relevant scenarios. J Zhejiang Univ Sci B. 2022;23(1):42–57.
Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25(10):2648–59.
Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE. 2008;3(4): e1886.
Fan W, Qian F, Ma Q, Zhang P, Chen T, Chen C, Zhang Y, Deng P, Zhou Z, Yu Z. 50 Hz electromagnetic field exposure promotes proliferation and cytokine production of bone marrow mesenchymal stem cells. Int J Clin Exp Med. 2015;8(5):7394–404.
Li W, Liu W, Wang W, Wang J, Ma T, Chen J, Wu H, Liu C. Sinusoidal electromagnetic fields accelerate bone regeneration by boosting the multifunctionality of bone marrow mesenchymal stem cells. Stem Cell Res Ther. 2021;12(1):234.
De Mattei M, Grassilli S, Pellati A, Brugnoli F, De Marchi E, Contartese D, Bertagnolo V. Pulsed electromagnetic fields modulate miRNAs during osteogenic differentiation of bone mesenchymal stem cells: a possible role in the osteogenic-angiogenic coupling. Stem Cell Rev Rep. 2020;16(5):1005–12.
Filipowska J, Tomaszewski KA, Niedźwiedzki Ł, Walocha JA, Niedźwiedzki T. The role of vasculature in bone development, regeneration and proper systemic functioning. Angiogenesis. 2017;20(3):291–302.
Zhang Y, Xie Y, Hao Z, Zhou P, Wang P, Fang S, Li L, Xu S, Xia Y. Umbilical mesenchymal stem cell-derived exosome-encapsulated hydrogels accelerate bone repair by enhancing angiogenesis. ACS Appl Mater Interfaces. 2021;13(16):18472–87.
Li S, Song C, Yang S, Yu W, Zhang W, Zhang G, Xi Z, Lu E. Supercritical CO(2) foamed composite scaffolds incorporating bioactive lipids promote vascularized bone regeneration via Hif-1α upregulation and enhanced type H vessel formation. Acta Biomater. 2019;94:253–67.
Tang Y, Luo K, Chen Y, Chen Y, Zhou R, Chen C, Tan J, Deng M, Dai Q, Yu X, et al. Phosphorylation inhibition of protein-tyrosine phosphatase 1B tyrosine-152 induces bone regeneration coupled with angiogenesis for bone tissue engineering. Bioact Mater. 2021;6(7):2039–57.
Liu Y, Zhu Z, Pei X, Zhang X, Cheng X, Hu S, Gao X, Wang J, Chen J, Wan Q. ZIF-8-modified multifunctional bone-adhesive hydrogels promoting angiogenesis and osteogenesis for bone regeneration. ACS Appl Mater Interfaces. 2020;12(33):36978–95.
Ross CL, Ang DC, Almeida-Porada G. Targeting mesenchymal stromal cells/pericytes (MSCs) with pulsed electromagnetic field (PEMF) has the potential to treat rheumatoid ARTHRITIS. Front Immunol. 2019;10:266.
Yen-Patton GP, Patton WF, Beer DM, Jacobson BS. Endothelial cell response to pulsed electromagnetic fields: stimulation of growth rate and angiogenesis in vitro. J Cell Physiol. 1988;134(1):37–46.
Peng L, Fu C, Liang Z, Zhang Q, Xiong F, Chen L, He C, Wei Q. Pulsed electromagnetic fields increase angiogenesis and improve cardiac function after myocardial ischemia in mice. Circ J. 2020;84(2):186–93.
Tepper OM, Callaghan MJ, Chang EI, Galiano RD, Bhatt KA, Baharestani S, Gan J, Simon B, Hopper RA, Levine JP, et al. Electromagnetic fields increase in vitro and in vivo angiogenesis through endothelial release of FGF-2. FASEB J. 2004;18(11):1231–3.
Wang Q, Zhou J, Wang X, Xu Y, Liang Z, Gu X, He C. Coupling induction of osteogenesis and type H vessels by pulsed electromagnetic fields in ovariectomy-induced osteoporosis in mice. Bone. 2022;154:116211.
Kusumbe AP, Ramasamy SK, Adams RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507(7492):323–8.
Ramasamy SK, Kusumbe AP, Wang L, Adams RH. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature. 2014;507(7492):376–80.
Huang J, Yin H, Rao SS, Xie PL, Cao X, Rao T, Liu SY, Wang ZX, Cao J, Hu Y, et al. Harmine enhances type H vessel formation and prevents bone loss in ovariectomized mice. Theranostics. 2018;8(9):2435–46.
Chen J, Tu C, Tang X, Li H, Yan J, Ma Y, Wu H, Liu C. The combinatory effect of sinusoidal electromagnetic field and VEGF promotes osteogenesis and angiogenesis of mesenchymal stem cell-laden PCL/HA implants in a rat subcritical cranial defect. Stem Cell Res Ther. 2019;10(1):379.
Tu C, Chen J, Huang C, Xiao Y, Tang X, Li H, Ma Y, Yan J, Li W, Wu H, et al. Effects of electromagnetic fields treatment on rat critical-sized calvarial defects with a 3D-printed composite scaffold. Stem Cell Res Ther. 2020;11(1):433.
Michalski MN, McCauley LK. Macrophages and skeletal health. Pharmacol Ther. 2017;174:43–54.
Vishwakarma A, Bhise NS, Evangelista MB, Rouwkema J, Dokmeci MR, Ghaemmaghami AM, Vrana NE, Khademhosseini A. Engineering immunomodulatory biomaterials to tune the inflammatory response. Trends Biotechnol. 2016;34(6):470–82.
Bai L, Du Z, Du J, Yao W, Zhang J, Weng Z, Liu S, Zhao Y, Liu Y, Zhang X, et al. A multifaceted coating on titanium dictates osteoimmunomodulation and osteo/angio-genesis towards ameliorative osseointegration. Biomaterials. 2018;162:154–69.
Zhu Y, Liang H, Liu X, Wu J, Yang C, Wong TM, Kwan KYH, Cheung KMC, Wu S, Yeung KWK. Regulation of macrophage polarization through surface topography design to facilitate implant-to-bone osteointegration. Sci Adv. 2021;7(14):eabf6654.
Yu Y, Dai K, Gao Z, Tang W, Shen T, Yuan Y, Wang J, Liu C. Sulfated polysaccharide directs therapeutic angiogenesis via endogenous VEGF secretion of macrophages. Sci Adv. 2021;7(7):eabd8217.
Qiao W, Wong KHM, Shen J, Wang W, Wu J, Li J, Lin Z, Chen Z, Matinlinna JP, Zheng Y, et al. TRPM7 kinase-mediated immunomodulation in macrophage plays a central role in magnesium ion-induced bone regeneration. Nat Commun. 2021;12(1):2885.
Cockerill I, Su Y, Lee JH, Berman D, Young ML, Zheng Y, Zhu D. Micro-/nanotopography on bioresorbable zinc dictates cytocompatibility, bone cell differentiation, and macrophage polarization. Nano Lett. 2020;20(6):4594–602.
Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Investig. 2012;122(3):787–95.
Crawford L, Wyatt M, Bryers J, Ratner B. Biocompatibility evolves: phenomenology to toxicology to regeneration. Adv Healthc Mater. 2021;10(11):e2002153.
Shang W, Chen G, Li Y, Zhuo Y, Wang Y, Fang Z, Yu Y, Ren H. Static magnetic field accelerates diabetic wound healing by facilitating resolution of inflammation. J Diabetes Res. 2019;2019:5641271.
Dai X, Heng BC, Bai Y, You F, Sun X, Li Y, Tang Z, Xu M, Zhang X, Deng X. Restoration of electrical microenvironment enhances bone regeneration under diabetic conditions by modulating macrophage polarization. Bioact Mater. 2021;6(7):2029–38.
Vinhas A, Rodrigues MT, Gonçalves AI, Reis RL, Gomes ME. Magnetic responsive materials modulate the inflammatory profile of IL-1β conditioned tendon cells. Acta Biomater. 2020;117:235–45.
Vinhas A, Almeida AF, Gonçalves AI, Rodrigues MT, Gomes ME. Magnetic stimulation drives macrophage polarization in cell to-cell communication with IL-1β primed tendon cells. Int J Mol Sci. 2020;21(15):5441.
Fu J, Li Y, Zhang Y, Liang Y, Zheng Y, Li Z, Zhu S, Li C, Cui Z, Wu S. An engineered pseudo-macrophage for rapid treatment of bacteria-infected osteomyelitis via microwave-excited anti-infection and immunoregulation. Adv Mater. 2021;33(41):e2102926.
Nair LS, Laurencin CT. Biodegradable polymers as biomaterials. Prog Polym Sci. 2007;32(8–9):762–98.
Yadav A, Ghosh S, Samanta A, Pal J, Srivastava RK. Emulsion templated scaffolds of poly(ε-caprolactone)—a review. Chem Commun (Camb). 2022;58(10):1468–80.
Arjmand M, Ardeshirylajimi A, Maghsoudi H, Azadian E. Osteogenic differentiation potential of mesenchymal stem cells cultured on nanofibrous scaffold improved in the presence of pulsed electromagnetic field. J Cell Physiol. 2018;233(2):1061–70.
Ardeshirylajimi A, Khojasteh A. Synergism of electrospun nanofibers and pulsed electromagnetic field on osteogenic differentiation of induced pluripotent stem cells. ASAIO J. 2018;64(2):253–60.
Gentile P, Chiono V, Carmagnola I, Hatton PV. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int J Mol Sci. 2014;15(3):3640–59.
Washington MA, Swiner DJ, Bell KR, Fedorchak MV, Little SR, Meyer TY. The impact of monomer sequence and stereochemistry on the swelling and erosion of biodegradable poly(lactic-co-glycolic acid) matrices. Biomaterials. 2017;117:66–76.
Kirillova A, Kelly C, von Windheim N, Gall K. Bioinspired mineral-organic bioresorbable bone adhesive. Adv Healthc Mater. 2018;7(17):e1800467.
Pan Z, Ding J. Poly(lactide-co-glycolide) porous scaffolds for tissue engineering and regenerative medicine. Interface Focus. 2012;2(3):366–77.
Tsai MT, Chang WH, Chang K, Hou RJ, Wu TW. Pulsed electromagnetic fields affect osteoblast proliferation and differentiation in bone tissue engineering. Bioelectromagnetics. 2007;28(7):519–28.
Zhong C, Zhang X, Xu Z, He R. Effects of low-intensity electromagnetic fields on the proliferation and differentiation of cultured mouse bone marrow stromal cells. Phys Ther. 2012;92(9):1208–19.
Guo L, Liang Z, Yang L, Du W, Yu T, Tang H, Li C, Qiu H. The role of natural polymers in bone tissue engineering. J Control Release. 2021;338:571–82.
Muzzarelli R, Baldassarre V, Conti F, Ferrara P, Biagini G, Gazzanelli G, Vasi V. Biological activity of chitosan: ultrastructural study. Biomaterials. 1988;9(3):247–52.
Kim IY, Seo SJ, Moon HS, Yoo MK, Park IY, Kim BC, Cho CS. Chitosan and its derivatives for tissue engineering applications. Biotechnol Adv. 2008;26(1):1–21.
Zhang Y, Venugopal JR, El-Turki A, Ramakrishna S, Su B, Lim CT. Electrospun biomimetic nanocomposite nanofibers of hydroxyapatite/chitosan for bone tissue engineering. Biomaterials. 2008;29(32):4314–22.
Lin HY, Lu KH. Repairing large bone fractures with low frequency electromagnetic fields. J Orthop Res. 2010;28(2):265–70.
Shavandi A, Silva TH, Bekhit AA, Bekhit AEA. Keratin: dissolution, extraction and biomedical application. Biomater Sci. 2017;5(9):1699–735.
Feroz S, Muhammad N, Ranayake J, Dias G. Keratin-based materials for biomedical applications. Bioact Mater. 2020;5(3):496–509.
Bloise N, Patrucco A, Bruni G, Montagna G, Caringella R, Fassina L, Tonin C, Visai L. In vitro production of calcified bone matrix onto wool keratin scaffolds via osteogenic factors and electromagnetic stimulus. Materials (Basel). 2020;13(14):3052.
Tang Z, Li X, Tan Y, Fan H, Zhang X. The material and biological characteristics of osteoinductive calcium phosphate ceramics. Regen Biomater. 2018;5(1):43–59.
Chang K, Chang WH, Huang S, Huang S, Shih C. Pulsed electromagnetic fields stimulation affects osteoclast formation by modulation of osteoprotegerin, RANK ligand and macrophage colony-stimulating factor. J Orthop Res. 2005;23(6):1308–14.
Schwartz Z, Simon BJ, Duran MA, Barabino G, Chaudhri R, Boyan BD. Pulsed electromagnetic fields enhance BMP-2 dependent osteoblastic differentiation of human mesenchymal stem cells. J Orthop Res. 2008;26(9):1250–5.
Schwartz Z, Fisher M, Lohmann CH, Simon BJ, Boyan BD. Osteoprotegerin (OPG) production by cells in the osteoblast lineage is regulated by pulsed electromagnetic fields in cultures grown on calcium phosphate substrates. Ann Biomed Eng. 2009;37(3):437–44.
Farokhi M, Mottaghitalab F, Samani S, Shokrgozar MA, Kundu SC, Reis RL, Fatahi Y, Kaplan DL. Silk fibroin/hydroxyapatite composites for bone tissue engineering. Biotechnol Adv. 2018;36(1):68–91.
Zhou H, Lee J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011;7(7):2769–81.
Jurczak P, Witkowska J, Rodziewicz-Motowidło S, Lach S. Proteins, peptides and peptidomimetics as active agents in implant surface functionalization. Adv Colloid Interface Sci. 2020;276:102083.
Shimizu T, Zerwekh JE, Videman T, Gill K, Mooney V, Holmes RE, Hagler HK. Bone ingrowth into porous calcium phosphate ceramics: influence of pulsing electromagnetic field. J Orthop Res. 1988;6(2):248–58.
Fini M, Cadossi R, Canè V, Cavani F, Giavaresi G, Krajewski A, Martini L, Aldini NN, Ravaglioli A, Rimondini L, et al. The effect of pulsed electromagnetic fields on the osteointegration of hydroxyapatite implants in cancellous bone: a morphologic and microstructural in vivo study. J Orthop Res. 2002;20(4):756–63.
Prakash D, Behari J. Synergistic role of hydroxyapatite nanoparticles and pulsed electromagnetic field therapy to prevent bone loss in rats following exposure to simulated microgravity. Int J Nanomed. 2009;4:133–44.
Abdul Halim NA, Hussein MZ, Kandar MK. Nanomaterials-upconverted hydroxyapatite for bone tissue engineering and a platform for drug delivery. Int J Nanomed. 2021;16:6477–96.
Fernandes Patrício TM, Mumcuoglu D, Montesi M, Panseri S, Witte-Bouma J, Garcia SF, Sandri M, Tampieri A, Farrell E, Sprio S. Bio-inspired polymeric iron-doped hydroxyapatite microspheres as a tunable carrier of rhBMP-2. Mater Sci Eng C Mater Biol Appl. 2021;119:111410.
Liang H, Liu X, Pi Y, Yu Q, Yin Y, Li X, Yang Y, Tian J. 3D-printed β-tricalcium phosphate scaffold combined with a pulse electromagnetic field promotes the repair of skull defects in rats. ACS Biomater Sci Eng. 2019;5(10):5359–67.
Habib M, Horne DA, Hussein K, Coughlin D, Waldorff EI, Zhang N, Ryaby JT, Lotz JC. Magnetic nanoparticles synergize with pulsed magnetic fields to stimulate osteogenesis in vitro. Tissue Eng Part A. 2021;27(5–6):402–12.
Ahn TK, Lee DH, Kim TS, Jang GC, Choi S, Oh JB, Ye G, Lee S. Modification of titanium implant and titanium dioxide for bone tissue engineering. Adv Exp Med Biol. 2018;1077:355–68.
Zuo W, Yu L, Lin J, Yang Y, Fei Q. Properties improvement of titanium alloys scaffolds in bone tissue engineering: a literature review. Ann Transl Med. 2021;9(15):1259.
Stich T, Alagboso F, Křenek T, Kovářík T, Alt V, Docheva D. Implant-bone-interface: reviewing the impact of titanium surface modifications on osteogenic processes in vitro and in vivo. Bioeng Transl Med. 2022;7(1):e10239.
Barak S, Neuman M, Iezzi G, Piattelli A, Perrotti V, Gabet Y. A new device for improving dental implants anchorage: a histological and micro-computed tomography study in the rabbit. Clin Oral Implants Res. 2016;27(8):935–42.
Nunes CMM, Ferreira CL, Bernardo DV, Lopes CCR, Collino L, da Silva Lima VC, de Camargo Reis Mello D, de Vasconcellos LMR, Jardini MAN. Evaluation of pulsed electromagnetic field protocols in implant osseointegration: in vivo and in vitro study. Clin Oral Investig. 2021;25(5):2925–37.
Ulrich SD, Seyler TM, Bennett D, Delanois RE, Saleh KJ, Thongtrangan I, Kuskowski M, Cheng EY, Sharkey PF, Parvizi J, et al. Total hip arthroplasties: what are the reasons for revision? Int Orthop. 2008;32(5):597–604.
Veronesi F, Fini M, Sartori M, Parrilli A, Martini L, Tschon M. Pulsed electromagnetic fields and platelet rich plasma alone and combined for the treatment of wear-mediated periprosthetic osteolysis: an in vivo study. Acta Biomater. 2018;77:106–15.
Santos L, Silva M, Gonçalves AI, Pesqueira T, Rodrigues MT, Gomes ME. In vitro and in vivo assessment of magnetically actuated biomaterials and prospects in tendon healing. Nanomedicine (Lond). 2016;11(9):1107–22.
Javed F, Romanos GE. Impact of diabetes mellitus and glycemic control on the osseointegration of dental implants: a systematic literature review. J Periodontol. 2009;80(11):1719–30.
de Oliveira P, Bonfante EA, Bergamo ETP, de Souza SLS, Riella L, Torroni A, Benalcazar Jalkh EB, Witek L, Lopez CD, Zambuzzi WF, et al. Obesity/metabolic syndrome and diabetes mellitus on peri-implantitis. Trends Endocrinol Metab. 2020;31(8):596–610.
Cai J, Li W, Sun T, Li X, Luo E, Jing D. Pulsed electromagnetic fields preserve bone architecture and mechanical properties and stimulate porous implant osseointegration by promoting bone anabolism in type 1 diabetic rabbits. Osteoporos Int. 2018;29(5):1177–91.
Cai J, Shao X, Yang Q, Yang Y, Yan Z, Luo E, Feng X, Jing D. Pulsed electromagnetic fields modify the adverse effects of glucocorticoids on bone architecture, bone strength and porous implant osseointegration by rescuing bone-anabolic actions. Bone. 2020;133:115266.
Bloise N, Petecchia L, Ceccarelli G, Fassina L, Usai C, Bertoglio F, Balli M, Vassalli M, Cusella De Angelis MG, Gavazzo P, et al. The effect of pulsed electromagnetic field exposure on osteoinduction of human mesenchymal stem cells cultured on nano-TiO2 surfaces. PLoS ONE. 2018;13(6):e0199046.
Woo KM, Seo J, Zhang R, Ma PX. Suppression of apoptosis by enhanced protein adsorption on polymer/hydroxyapatite composite scaffolds. Biomaterials. 2007;28(16):2622–30.
Sharifi F, Atyabi SM, Norouzian D, Zandi M, Irani S, Bakhshi H. Polycaprolactone/carboxymethyl chitosan nanofibrous scaffolds for bone tissue engineering application. Int J Biol Macromol. 2018;115:243–8.
Shapourzadeh A, Atyabi SM, Irani S, Bakhshi H. Osteoinductivity of polycaprolactone nanofibers grafted functionalized with carboxymethyl chitosan: synergic effect of β-carotene and electromagnetic field. Int J Biol Macromol. 2020;150:152–60.
Gelse K, Pöschl E, Aigner T. Collagens–structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55(12):1531–46.
Yang C, Hillas PJ, Báez JA, Nokelainen M, Balan J, Tang J, Spiro R, Polarek JW. The application of recombinant human collagen in tissue engineering. BioDrugs. 2004;18(2):103–19.
Sarker B, Hum J, Nazhat SN, Boccaccini AR. Combining collagen and bioactive glasses for bone tissue engineering: a review. Adv Healthc Mater. 2015;4(2):176–94.
Li Q, Lei X, Wang X, Cai Z, Lyu P, Zhang G. Hydroxyapatite/collagen three-dimensional printed scaffolds and their osteogenic effects on human bone marrow-derived mesenchymal stem cells. Tissue Eng Part A. 2019;25(17–18):1261–71.
Wang H, Tang X, Li W, Chen J, Li H, Yan J, Yuan X, Wu H, Liu C. Enhanced osteogenesis of bone marrow stem cells cultured on hydroxyapatite/collagen I scaffold in the presence of low-frequency magnetic field. J Mater Sci Mater Med. 2019;30(8):89.
Heydari Asl S, Hosseinpoor H, Parivar K, Hayati Roodbari N, Hanaee-Ahvaz H. Physical stimulation and scaffold composition efficiently support osteogenic differentiation of mesenchymal stem cells. Tissue Cell. 2018;50:1–7.
Khare D, Basu B, Dubey AK. Electrical stimulation and piezoelectric biomaterials for bone tissue engineering applications. Biomaterials. 2020;258: 120280.
Rajabi AH, Jaffe M, Arinzeh TL. Piezoelectric materials for tissue regeneration: a review. Acta Biomater. 2015;24:12–23.
Tandon B, Blaker JJ, Cartmell SH. Piezoelectric materials as stimulatory biomedical materials and scaffolds for bone repair. Acta Biomater. 2018;73:1–20.
Li Y, Liao C, Tjong SC. Electrospun polyvinylidene fluoride-based fibrous scaffolds with piezoelectric characteristics for bone and neural tissue engineering. Nanomaterials (Basel). 2019;9(7):952.
Mirzaei A, Saburi E, Enderami SE, Barati Bagherabad M, Enderami SE, Chokami M, Shapouri Moghadam A, Salarinia R, Ardeshirylajimi A, Mansouri V, et al. Synergistic effects of polyaniline and pulsed electromagnetic field to stem cells osteogenic differentiation on polyvinylidene fluoride scaffold. Artif Cells Nanomed Biotechnol. 2019;47(1):3058–66.
Mourdikoudis S, Kostopoulou A, LaGrow AP. Magnetic nanoparticle composites: synergistic effects and applications. Adv Sci (Weinh). 2021;8(12):2004951.
Xia Y, Guo Y, Yang Z, Chen H, Ren K, Weir MD, Chow LC, Reynolds MA, Zhang F, Gu N, et al. Iron oxide nanoparticle-calcium phosphate cement enhanced the osteogenic activities of stem cells through WNT/β-catenin signaling. Mater Sci Eng C Mater Biol Appl. 2019;104:109955.
Behrens S, Appel I. Magnetic nanocomposites. Curr Opin Biotechnol. 2016;39:89–96.
Filippi M, Garello F, Yasa O, Kasamkattil J, Scherberich A, Katzschmann RK. Engineered magnetic nanocomposites to modulate cellular function. Small. 2021;18:e2104079.
Xia Y, Sun J, Zhao L, Zhang F, Liang XJ, Guo Y, Weir MD, Reynolds MA, Gu N, Xu HHK. Magnetic field and nano-scaffolds with stem cells to enhance bone regeneration. Biomaterials. 2018;183:151–70.
Meng J, Xiao B, Zhang Y, Liu J, Xue H, Lei J, Kong H, Huang Y, Jin Z, Gu N, et al. Super-paramagnetic responsive nanofibrous scaffolds under static magnetic field enhance osteogenesis for bone repair in vivo. Sci Rep. 2013;3:2655.
Russo A, Bianchi M, Sartori M, Parrilli A, Panseri S, Ortolani A, Sandri M, Boi M, Salter DM, Maltarello MC, et al. Magnetic forces and magnetized biomaterials provide dynamic flux information during bone regeneration. J Mater Sci Mater Med. 2016;27(3):51.
Hao L, Li L, Wang P, Wang Z, Shi X, Guo M, Zhang P. Synergistic osteogenesis promoted by magnetically actuated nano-mechanical stimuli. Nanoscale. 2019;11(48):23423–37.
Huang J, Wang D, Chen J, Liu W, Duan L, You W, Zhu W, Xiong J, Wang D. Osteogenic differentiation of bone marrow mesenchymal stem cells by magnetic nanoparticle composite scaffolds under a pulsed electromagnetic field. Saudi Pharm J. 2017;25(4):575–9.
Zeng XB, Hu H, Xie LQ, Lan F, Jiang W, Wu Y, Gu ZW. Magnetic responsive hydroxyapatite composite scaffolds construction for bone defect reparation. Int J Nanomed. 2012;7:3365–78.
Meshkini A, Sistanipour E, Izadi A. Mg.ATP-decorated ultrafine magnetic nanofibers: a bone scaffold with high osteogenic and antibacterial properties in the presence of an electromagnetic field. Colloids Surf B Biointerfaces. 2022;210:112256.
Gil S, Mano JF. Magnetic composite biomaterials for tissue engineering. Biomater Sci. 2014;2(6):812–8.
Zhang L, Zuo X, Li S, Sun M, Xie H, Zhang K, Zhou J, Che L, Ma J, Jia Z, et al. Synergistic therapy of magnetism-responsive hydrogel for soft tissue injuries. Bioact Mater. 2019;4:160–6.
Shui C, Scutt A. Mild heat shock induces proliferation, alkaline phosphatase activity, and mineralization in human bone marrow stromal cells and Mg-63 cells in vitro. J Bone Miner Res. 2001;16(4):731–41.
Chen J, Shi ZD, Ji X, Morales J, Zhang J, Kaur N, Wang S. Enhanced osteogenesis of human mesenchymal stem cells by periodic heat shock in self-assembling peptide hydrogel. Tissue Eng Part A. 2013;19(5–6):716–28.
Tong L, Liao Q, Zhao Y, Huang H, Gao A, Zhang W, Gao X, Wei W, Guan M, Chu PK, et al. Near-infrared light control of bone regeneration with biodegradable photothermal osteoimplant. Biomaterials. 2019;193:1–11.
Cao Z, Wang D, Li Y, Xie W, Wang X, Tao L, Wei Y, Wang X, Zhao L. Effect of nanoheat stimulation mediated by magnetic nanocomposite hydrogel on the osteogenic differentiation of mesenchymal stem cells. Sci China Life Sci. 2018;61(4):448–56.
Wang L, Hu P, Jiang H, Zhao J, Tang J, Jiang D, Wang J, Shi J, Jia W. Mild hyperthermia-mediated osteogenesis and angiogenesis play a critical role in magnetothermal composite-induced bone regeneration. Nano Today. 2022;43:101401.
Celik C, Franco-Obregón A, Lee EH, Hui JH, Yang Z. Directionalities of magnetic fields and topographic scaffolds synergise to enhance MSC chondrogenesis. Acta Biomater. 2021;119:169–83.
Murray HB, Pethica BA. A follow-up study of the in-practice results of pulsed electromagnetic field therapy in the management of nonunion fractures. Orthop Res Rev. 2016;8:67–72.
Shi HF, Xiong J, Chen YX, Wang JF, Qiu XS, Wang YH, Qiu Y. Early application of pulsed electromagnetic field in the treatment of postoperative delayed union of long-bone fractures: a prospective randomized controlled study. BMC Musculoskelet Disord. 2013;14:35.
Mooney V. A randomized double-blind prospective study of the efficacy of pulsed electromagnetic fields for interbody lumbar fusions. Spine (Phila Pa 1976). 1990;15(7):708–12.
Foley KT, Mroz TE, Arnold PM, Chandler HC Jr, Dixon RA, Girasole GJ, Renkens KL Jr, Riew KD, Sasso RC, Smith RC, et al. Randomized, prospective, and controlled clinical trial of pulsed electromagnetic field stimulation for cervical fusion. Spine J. 2008;8(3):436–42.
Risso Neto MI, Zuiani GR, Cavali PTM, Veiga IG, Pasqualini W, Amato Filho ACS, Cliquet JÚNior A, Landim E, Miranda JBDE,. Effect of pulsed electromagnetic field on the consolidation of posterolateral arthrodeses in the lumbosacral spine: a prospective, double-blind. Randomized Study Coluna/Columna. 2017;16(3):206–12.
Hao Z, Xu Z, Wang X, Wang Y, Li H, Chen T, Hu Y, Chen R, Huang K, Chen C, et al. Biophysical stimuli as the fourth pillar of bone tissue engineering. Front Cell Dev Biol. 2021;9:790050.
Kazimierczak P, Przekora A. Bioengineered living bone grafts-a concise review on bioreactors and production techniques in vitro. Int J Mol Sci. 2022;23(3):1765.
Wong CJK, Tai YK, Yap JLY, Fong CHH, Loo LSW, Kukumberg M, Fröhlich J, Zhang S, Li JZ, Wang JW, et al. Brief exposure to directionally-specific pulsed electromagnetic fields stimulates extracellular vesicle release and is antagonized by streptomycin: a potential regenerative medicine and food industry paradigm. Biomaterials. 2022;287:121658.
Gelmi A, Schutt CE. Stimuli-responsive biomaterials: scaffolds for stem cell control. Adv Healthc Mater. 2021;10(1):e2001125.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 51877097). The funding body played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the design and concept of this article. HQZ drafted the manuscript. CXL, YL, QD, TQW, HL, TM, and HW critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhao, H., Liu, C., Liu, Y. et al. Harnessing electromagnetic fields to assist bone tissue engineering. Stem Cell Res Ther 14, 7 (2023). https://doi.org/10.1186/s13287-022-03217-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-022-03217-z