Abstract
Introduction
Thrombotic events are more than twice as common in inflammatory bowel disease patients as in the general population. We report an interesting and rare case of portal vein thrombosis as a venous thromboembolic event in the context of extraintestinal manifestations of Crohn’s disease. We also conducted a literature review on portal vein thrombosis associated with inflammatory bowel disease, with the following concepts: inflammatory bowel diseases, ulcerative colitis, Crohn’s disease, portal vein, and thrombosis.
Case presentation
A 24-year-old Syrian female with active chronic Crohn’s disease was diagnosed 11 years ago and classified as A1L3B1P according to the Montreal classification. She had no prior surgical history. Her previous medications included azathioprine and prednisolone. Her Crohn’s disease activity index was 390 points. Gastroduodenoscopy revealed grade I esophageal varices, a complication of portal hypertension. Meanwhile, a colonoscopy revealed several deep ulcers in the sigmoid, rectum, and descending colon. An investigation of portal vein hypertension revealed portal vein thrombosis. We used corticosteroids to induce remission, followed by tapering; additionally she received ustekinumab to induce and maintain remission. She began on low-molecular-weight heparin for 1 week, warfarin for 3 months, and then apixaban, a novel oral anticoagulant, after excluding antiphospholipid syndrome. Primary prophylaxis for esophageal varices was not required. After 1 year, she achieved clinical, biochemical, and endoscopic remission. Despite 1 year of treatment, a computed tomography scan revealed no improvement in portal vein recanalization.
Conclusion
Portal vein thrombosis is a rare and poorly defined complication of inflammatory bowel disease. It is usually exacerbated by inflammatory bowel disease. The symptoms are nonspecific and may mimic a flare-up of inflammatory bowel disease, making the diagnosis difficult. Portal vein Doppler ultrasound for hospital-admitted inflammatory bowel disease patients may contribute to the diagnosis and management of this complication.
Similar content being viewed by others
Introduction
Extraintestinal manifestations can affect almost any organ system and have a negative impact on the patient’s functional status and quality of life. Extraintestinal manifestations are most commonly observed in the joints, skin, hepatobiliary tract, eyes, heart, pancreas, and vascular system. Portal vein thrombosis (PVT) is an obscure and poorly defined complication of many diseases, including cirrhosis, intraabdominal infection, intraabdominal surgery, pancreatitis, primary hematologic disorders, and inflammatory bowel disease (IBD) [1]. The prevalence of PVT in patients with IBD ranges from 0.17% to 1.7% [1], and may be associated with inherited or acquired hypercoagulability risk factors and has a benign outcome [1]. It can be difficult to diagnose PVT in patients with IBD because its extremely generic symptoms, such as abdominal discomfort, can frequently originate from any of its triggering events. Therefore, it should come as no surprise that the diagnosis is frequently made by accident when imaging is performed to check for one of these triggering processes, also the laboratory results are nonspecific [1]. We report an interesting and uncommon case of PVT associated with Crohn’s disease that was discovered when investigating the cause of esophageal varices related to portal vein hypertension. We also conducted a literature review on portal vein thrombosis associated with inflammatory bowel disease using the following concepts: inflammatory bowel disease, ulcerative colitis, Crohn’s disease, portal vein, and thrombosis.
Case report
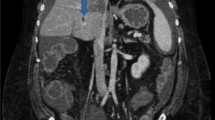
We evaluated a 24-year-old Syrian female with active chronic Crohn’s disease, diagnosed 11 years ago. She was classified as A1L3B1P according to the Montreal classification [2]. She had no prior surgical history; her past medications included azathioprine 2.5 mg/kg/day since diagnosis until now and prednisolone 1 mg/kg up to 40 mg during flares, then tapering [3]. Furthermore, she did not use oral contraceptive pills. Her weight was 50 kg, her height was 161 cm, and she had a body mass index of 19.29 kg/m2. She complained of watery, bloody diarrhea up to eight times a day, accompanied by abdominal pain in the prior month. Her Crohn’s disease activity index (CDAI) was 390 points. Initial blood tests confirmed leukocytosis, anemia, elevated fecal calprotectin (FC), and C-reactive protein (CRP) levels. Stool cultures, Clostridium difficile toxin, Escherichia coli, and Cryptosporidium, as well as microscopy for ova and parasites, all returned negative. The hypercoagulability work-up revealed negative results for anti-Beta-2 Glycoprotein-1 IgM antibodies, antinuclear antibodies (ANA), fibrinogen, protein S (activity), antithrombin III, and homocysteine, whereas lupus anticoagulant (LA1, LA2) was positive. Factor II mutation and factor V Leiden mutation were normal, whereas the methylenetetrahydrofolate reductase mutation was a homozygous mutant gene. The portal system and suprahepatic vein ultrasound revealed a thrombus that covered nearly half of the lumen of the portal vein and splenomegaly. Gogastroduodenoscopy showed grade I esophageal varices (less than 5 mm, without bleeding risk signs), which indicate portal vein hypertension owing to splenomegaly and esophageal varices. In light of the patient’s recent onset of abdominal pain and the absence of portosystemic collaterals on Doppler ultrasound, a recent PVT is a strong possibility [4]. The colonoscopy revealed several deep ulcers in the sigmoid, rectum, and descending colon Fig. 1. The biopsies were negative for Clostridium difficile, and immunohistochemical staining was negative for cytomegalovirus (CMV) [3, 5]. The median liver stiffness measured by FibroScan was 2.4 kPa, which suggests the absence of fibrosis. Protein electrophoresis was normal. The abdomen and pelvis contrast-enhanced computed tomography (CT) scan confirmed the PVT and displayed thickening in the descending colon (Fig. 2). Antiphospholipid syndrome was initially diagnosed on the basis of an antiphospholipid profile, a history of PVT (thrombotic event), and an association with Crohn’s disease [6]. She initially received corticosteroids to achieve disease remission, followed by ustekinumab to induce and maintain therapy (390 mg intravenous induction followed by 90 mg subcutaneous every 8 weeks) owing to moderate-to-severe Crohn’s disease unresponsive to azathioprine [7, 8]. She began on low-molecular-weight heparin (LMWH) for 1 week, and warfarin for 3 months with an international normalized ratio (INR) target of 2–3. The lupus anticoagulant (LA1, LA2) was retested after 12 weeks and returned to negative [6], so we switched to apixaban, a novel oral anticoagulant (NOAC) [4]. The 1-year reevaluation indicated clinical, biochemical, and endoscopic remission with CDAI of 150 points, normal lab test, and normal endoscopy. The patient’s tests are presented in Table 1. Despite 1 year of treatment, a CT scan revealed no improvement in portal vein recanalization. We continued 90 mg of subcutaneous (SC) ustekinumab every 8 weeks, while we stopped apixaban [3, 4].
Review of literature
Methods
To facilitate this literature review, we used a combination of keywords and database subject headings to search the MEDLINE (through PubMed) database on 1 July 2023 for the following concepts: Crohn’s disease, ulcerative colitis, IBD, portal vein, PVT, and thrombosis. We also manually searched the reference lists of the included papers. We returned the research on 7 April 2024, and no new findings were obtained.
Eligibility criteria
We searched for any case reports, case series, observational, or interventional studies that addressed portal vein thrombosis associated with inflammatory bowel disease. Table 2 summarizes the basic features and treatment outcomes of the reported cases.
Discussion
Crohn’s disease is linked to a variety of extraintestinal complications. Oral aphthous ulcers, peripheral arthritis, erythema nodosum, and episcleritis are frequently associated with active intestinal disease. Whereas uveitis and ankylosing spondylitis are usually unrelated to disease activity, pyoderma gangrenosum and primary sclerosing cholangitis have a questionable relationship to disease activity [9]. Venous thromboembolic events are fearsome manifestations that are related to disease activity and associated with significant morbidity and mortality [9]. Deep vein thrombosis (DVT) is the most prevalent thrombotic event, followed by pulmonary embolism (PE). The relative risk of thrombotic events in patients with inflammatory bowel disease was 2.03 [10]. Although inflammatory bowel disease treatment options have improved over the last three decades [11], thrombotic events among hospitalized individuals with inflammatory bowel disease continued to rise [12]. The overall thrombotic risk did not differ between sexes or between individuals who have ulcerative colitis or Crohn’s disease [13]. There have been very few reports of portal vein thrombosis in the context of inflammatory bowel disease. The presenting indications, symptoms, and laboratory data are all extremely nonspecific, and a PVT diagnosis is nearly always made by chance. It is important to note that PVT is related to disease activity, particularly IBD flare. We found that portal vein thrombosis affects both men and women, with a small male predominance. It is also more frequent in individuals with ulcerative colitis than in those with Crohn’s disease. It is a rare complication in Crohn’s disease, identified in only 14 cases. Hypercoagulability testing in a subset of patients (around half) revealed inherited or acquired hypercoagulability factors in some, with antiphospholipid antibodies and factor V Leiden mutation being the most common. Treatment for thrombosis in Crohn’s disease involves tailored anticoagulation (heparin, warfarin, DOACs) or even surgery, with outcomes ranging from successful resolution to bleeding or death. However, limitations include the use of case reports and retrospective studies, and the small number of CD cases, which hinder definitive conclusions. There are no recommendations for thrombophilia screening in cases of portal vein thrombosis; many reports, including ours, have included thrombophilia testing. Naymagon et al. suggested that thrombophilia testing is not required in cases of clearly triggered PVT, such as after recent surgery or in the setting of a recent or active intraabdominal infection or IBD-flare [1]; moreover, he suggested that thrombophilia testing should be undertaken if PVT is not induced, such as spontaneous PVT in an otherwise stable and inactive IBD patient, or patients with a history of previous venous thromboembolism or unexplained blood count abnormalities [1]. Furthermore, testing for antiphospholipid syndrome and paroxysmal nocturnal hemoglobinuria may affect management and should be considered in certain conditions, such as a history of autoimmune disease or arterial thrombosis for antiphospholipid syndrome and unexplained cytopenia or evidence of intravascular hemolysis for paroxysmal nocturnal hemoglobinuria. Other thrombophilia testing are often unnecessary because the results have little impact on therapy [1]. A mutation of JAK2 could be detected in splanchnic vein thrombosis and thus provide a marker of latent myeloproliferative neoplasms (MPNs), which are a major primary cause of abdominal vein thrombosis [14]. MPNs are made up of three key rare diseases: (1) polycythemia vera, which leads to an elevation in all blood cells, especially red blood cells; (2) essential thrombocythemia, which leads to an increase in platelets; and (3) primary myelofibrosis, a bone marrow disorder that leads to defects in blood cell production [14, 15]. MPNs were diagnosed through a variety of criteria, including the typical alterations in peripheral blood cells [4], as she had chronic active CD with possible previous CD-flare and a normal blood profile which excludes MPNs [1, 14, 15]. We screened for antiphospholipid syndrome antibodies because the patient was a young female with a significant thrombotic event without a clear relationship with a Crohn’s disease flare. Although the lupus anticoagulant (LA1, LA2) was initially positive, it was found to be negative 12 weeks later. The explanations for the false positive in our instance were anticoagulant treatment, including therapy with LWMH, which is indicated to every patient admitted to the hospital with inflammatory bowel disease, and later warfarin for the management of portal vein thrombosis [3, 6, 13]. For PVT management, literature was unclear concerning the selection of anticoagulants. Most patients who require anticoagulation are started on LMWH, or unfractionated heparin, and then switched to vitamin K antagonists (VKAs) to maintain a goal international normalization rate of 2–3. While VKAs can be substituted orally with direct oral anticoagulants (DOACs) or novel oral anticoagulants (NOACs). These medications do not require monitoring of the INR because of their speedier onset of action and lesser risk of bleeding. DOACs are just as effective as VKAs for treating deep vein thrombosis, pulmonary embolism, and stroke prevention in patients with atrial fibrillation, and may be considered owing to potentially less frequent monitoring needs and a fixed dosing regimen, which could enhance medication adherence. However, owing to unbalanced hemostasis, patients with cirrhosis have been excluded from most trials. Our case was portal hypertension without cirrhosis; therefore, DOACs or NOACs are not contraindicated after excluding antiphospholipid syndrome. For Crohn’s disease treatment, ustekinumab was more suitable than tumor necrosis factor inhibitors (anti-TNFα), as ustekinumab had low immunogenicity (generating antidrug antibodies), so it is feasible to avoid a combination of azathioprine and ustekinumab, in contrast to anti-TNF treatment, which necessitates such a combination [3, 6, 7, 9]. Ustekinumab helped to eliminate the drug interactions of azathioprine and warfarin, note that warfarin was the only therapeutic option owing to the initial diagnosis of antiphospholipid syndrome. In addition, ustekinumab had the lowest rate of serious infections among the biological treatments [7]. Esophageal varices primary prophylaxis is not required, as primary prophylaxis must be initiated upon the detection of high-risk varices, such as small varices with red signs, medium or large varices regardless of Child–Pugh classification, or small varices in patients classified as Child–Pugh C [16]. It is possible to discontinue anticoagulant treatment after a year, whether or not portal vein recanalization occurs, because a longer period of anticoagulant treatment is unlikely to enhance the probability of recanalization if it does not occur after a year [4].
Conclusion
PVT symptoms are similar to the symptoms of an inflammatory bowel disease flare. Initial tests for antiphospholipid syndrome were falsely positive [17]. The wise choice of ustekinumab as the first-line biological treatment, which aided in weaning off azathioprine, led to avoiding azathioprine–warfarin interactions. Using DOACs or NOACs for the management of portal vein thrombosis in case of portal vein hypertension. Finally, the management of esophageal varices in the context of anticoagulant treatment. The use of portal vein Doppler ultrasound, particularly during flare-ups of inflammatory bowel disease, may contribute to the diagnosis and management of this uncommon complication.
Availability of data and materials
Not applicable.
Abbreviations
- PVT:
-
Portal vein thrombosis
- IBD:
-
Inflammatory bowel disease
- CD:
-
Crohn’s disease
- CRP:
-
C-reactive protein
- INR:
-
International normalized ratio
- VKAs:
-
K antagonists
- CT:
-
Computed tomography
- SC:
-
Subcutaneous
- APS:
-
Antiphospholipid syndrome
- DOAC:
-
Direct oral anticoagulants
- NOAC:
-
Novel oral anticoagulants
- LMWH:
-
Low molecular weight heparin
- HbsAg:
-
Hepatitis B surface antigen
- HbsAb:
-
Hepatitis B surface antibody
- HbcAb:
-
Hepatitis B core antibody
References
Naymagon L, Tremblay D, Zubizarreta N, Moshier E, Naymagon S, Mascarenhas J, Schiano T. The natural history, treatments, and outcomes of portal vein thrombosis in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2021. https://doi.org/10.1093/ibd/izaa053.
Satsangi J, Silverberg MS, Vermeire S, Colombel J. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749–53. https://doi.org/10.1136/gut.2005.082909.
Torres J, Bonovas S, Doherty G, Kucharzik T, Gisbert JP, Raine T, et al. ECCO Guidelines on therapeutics in Crohn’s disease: medical treatment. J Crohn Colitis. 2020;14:4–22. https://doi.org/10.1093/ecco-jcc/jjz180.
EASL Clinical Practice Guidelines. Vascular diseases of the liver. J Hepatol. 2016;64:179–202. https://doi.org/10.1016/j.jhep.2015.07.040.
Kucharzik T, Ellul P, Greuter T, Rahier JF, Verstockt B, Abreu C, Albuquerque A, Allocca M, Esteve M, Farraye FA, Gordon H. ECCO guidelines on the prevention, diagnosis, and management of infections in inflammatory bowel disease. J Crohns Colitis. 2021;15:879–913. https://doi.org/10.1093/ecco-jcc/jjab052.
Tektonidou MG, Andreoli L, Limper M, Amoura Z, Cervera R, Costedoat-Chalumeau N, et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis. 2019;78:1296–304. https://doi.org/10.1136/annrheumdis-2019-215213.
Sandborn WJ, Rebuck R, Wang Y, Zou B, Adedokun OJ, Gasink C, et al. Five-year efficacy and safety of ustekinumab treatment in Crohn’s disease: the IM-UNITI trial. Clin Gastroenterol Hepatol. 2022;20:578-590.e4. https://doi.org/10.1016/j.cgh.2021.02.025.
Feagan BG, Sandborn WJ, Gasink C, Jacobstein D, Lang Y, Friedman JR, et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2016;375:1946–60. https://doi.org/10.1056/NEJMoa1602773.
Harbord M, Annese V, Vavricka SR, Allez M, Barreiro-de Acosta M, Boberg KM, et al. The first European evidence-based consensus on extra-intestinal manifestations in inflammatory bowel disease. J Crohn Colitis. 2016;10:239–54. https://doi.org/10.1093/ecco-jcc/jjv213.
Arvanitakis KD, Arvanitaki AD, Karkos CD, Zintzaras EΑ, Germanidis GS. The risk of venous thromboembolic events in patients with inflammatory bowel disease: a systematic review and meta-analysis. Ann Gastroenterol. 2021;34:680. https://doi.org/10.20524/aog.2021.0631.
Bernstein CN, Nugent Z, Singh H. Persistently high rate of venous thromboembolic disease in inflammatory bowel disease: a population-based study. Am J Gastroenterol. 2021. https://doi.org/10.14309/ajg.0000000000001237.
Faye AS, Lee KE, Dodson J, Chodosh J, Hudesman D, Remzi F, et al. Increasing rates of venous thromboembolism among hospitalised patients with inflammatory bowel disease: a nationwide analysis. Alimentary Pharmacol Ther. 2022. https://doi.org/10.1111/apt.17162.
Gordon H, Burisch J, Ellul P, Karmiris K, Katsanos K, Allocca M, et al. ECCO guidelines on extraintestinal manifestations in inflammatory bowel disease. J Crohn Colitis. 2023. https://doi.org/10.1093/ecco-jcc/jjad108.
Passamonti F, Maffioli M, Caramazza D, Cazzola M. Myeloproliferative neoplasms: From JAK2 mutations discovery to JAK2 inhibitor therapies. Oncotarget. 2011;2:485–90.
Tefferi A, Vardiman JW. Classification and diagnosis of myeloproliferative neoplasms: the 2008 World Health Organization criteria and point-of-care diagnostic algorithms. Leukemia. 2008;22:14–22. https://doi.org/10.1038/sj.leu.2404955.
Angeli P, Bernardi M, Villanueva C, Francoz C, Mookerjee RP, Trebicka J, et al. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406–60. https://doi.org/10.1016/j.jhep.2018.03.024.
Mallhi RS, Kushwaha N, Chatterjee T, Philip J. Antiphospholipid syndrome: a diagnostic challenge. Med J Armed Forces India. 2016;72:S31. https://doi.org/10.1016/j.mjafi.2016.05.001.
Nguyen MHN, Sieloff EM, Tariq T, Bannon SF. Portal vein thrombosis as an initial presentation of Crohn’s disease. Clin J Gastroenterol. 2021;14:581–3. https://doi.org/10.1007/s12328-020-01307-0.
Ma ASC, Ewing I, Murray CD, Hamilton MI. Hepatic portal venous gas and portal venous thrombosis following colonoscopy in a patient with terminal ileal Crohn’s disease. Case Rep. 2015;2015:bcr2014206854. https://doi.org/10.1136/bcr-2014-206854.
Mian HS, Lawlor R. Venous thrombosis in inflammatory bowel disease. CMAJ. 2015;187:55–55. https://doi.org/10.1503/cmaj.140251.
Maconi G, Bolzacchini E, Dell’Era A, Russo U, Ardizzone S, de Franchis R. Portal vein thrombosis in inflammatory bowel diseases: a single-center case series☆. J Crohn Colitis. 2012;6:362–7. https://doi.org/10.1016/j.crohns.2011.10.003.
Georgescu E, Dumitrescu D, Ionescu R. Portal cavernoma in a patient with Crohn’s disease associated with factor V Leiden mutation and antiphospholipid syndrome. J Gastrointestin Liver Dis. 2010;19(4):449–52.
McCabe JM, Mahadevan U, Vidyarthi A. An obscure harbinger. Difficult diagnosis of Crohn’s disease. Am J Med. 2009;122:516–8. https://doi.org/10.1016/j.amjmed.2009.02.003.
Di Fabio F, Obrand D, Satin R, Gordon PH. Successful treatment of extensive splanchnic arterial and portal vein thrombosis associated with ulcerative colitis. Colorect Dis. 2009. https://doi.org/10.1111/j.1463-1318.2008.01704.x.
Racine A, Nahon S, Jouannaud V, Caugant H, Lesgourgues B. Portal vein thrombosis in a patient with quiescent Crohn’s disease associated with hyperhomocysteinemia and antiphospholipid antibody syndrome 1-yr after an ileocecal resection. Am J Gastroenterol. 2008;103:499–501. https://doi.org/10.1111/j.1572-0241.2007.01646_18.x.
Babyatsky MW, Keroack MD, Blake MA, Rosenberg ES, Mino-Kenudson M. Case records of the Massachusetts General Hospital. Case 35–2007. A 30-year-old man with inflammatory bowel disease and recent onset of fever and bloody diarrhea. N Engl J Med. 2007;357:2068–76. https://doi.org/10.1056/NEJMcpc079029.
Palkovits J, Häfner M, Rand T, Vogelsang H, Kutilek M, Gangl A, et al. Portal vein thrombosis in ulcerative colitis complicated by bleeding from gastric varices. Inflamm Bowel Dis. 2007. https://doi.org/10.1002/ibd.20034.
Brueck M, Runde T, Rauber K, Kramer W. Fibrinogengesteuerte Urokinaselyse einer Portal- und Mesenterialvenenthrombose bei akutem Schub einer Colitis ulcerosa. Dtsch Med Wochenschr. 2006;131:84–8. https://doi.org/10.1055/s-2006-924929.
Shaked G, Czeiger D, Rozental A. Acute portal vein occlusion in a patient with Crohn’s disease. J Gastroenterol Hepatol. 2005;20:1472–3. https://doi.org/10.1111/j.1440-1746.2005.03981.x.
Guglielmi A, Fior F, Halmos O, Veraldi GF, Rossaro L, Ruzzenente A, et al. Transhepatic fibrinolysis of mesenteric and portal vein thrombosis in a patient with ulcerative colitis: a case report. World J Gastroenterol. 2005;11:2035–8. https://doi.org/10.3748/wjg.v11.i13.2035.
Tomita J, Okada H, Mizuno M, Nasu J, Nishimura M, Nakamura S, et al. A case of ulcerative colitis successfully treated with low-dose warfarin for portal vein thrombosis after colectomy. Nihon Shokakibyo Gakkai Zasshi. 2005;102:25–30.
Valera JM, Nei LuC, Smok G, Fernández M, Regonesi C, Brahm J. Hepatic veno-occlusive disease, idiopathic ulcerative colitis and portal thrombosis. Report of one case. Rev Med Chil. 2004;132:1091–5. https://doi.org/10.4067/s0034-98872004000900010.
Verna EC, Larghi A, Faddoul SG, Stein JA, Worman HJ. Portal vein thrombosis associated with Fusobacterium nucleatum septicemia in a patient with ulcerative colitis. J Clin Gastroenterol. 2004;38:611–2. https://doi.org/10.1097/00004836-200408000-00014.
Mijnhout GS, Klinkenberg EC, Lycklama G, Linskens R, Meuwissen SGM. Sepsis and elevated liver enzymes in a patient with inflammatory bowel disease: think of portal vein thrombosis. Dig Liver Dis. 2004;36:296–300. https://doi.org/10.1016/j.dld.2003.10.018.
Kluge S, Hahn KE, Lund CH, Gocht A, Kreymann G. Pylephlebitis with air in the portal vein system. An unusual focus in a patient with sepsis. Dtsch Med Wochenschr. 2003;128:1391–4. https://doi.org/10.1055/s-2003-40107.
Junge U, Wienke J, Schüler A. Acute Budd-Chiari syndrome, portal and splenic vein thrombosis in a patient with ulcerative colitis associated with antiphospholipid antibodies and protein C deficiency. Z Gastroenterol. 2001;39:845–52. https://doi.org/10.1055/s-2001-17864.
Hagimoto T, Seo M, Okada M, Shirotani T, Tanaka K, Tomita A, et al. Portal vein thrombosis successfully treated with a colectomy in active ulcerative colitis: report of a case. Dis Colon Rectum. 2001;44:587–90. https://doi.org/10.1007/BF02234334.
Schäfer C, Zundler J, Bode CJ. Thrombolytic therapy in patients with portal vein thrombosis: case report and review of the literature. Eur J Gastroenterol Hepatol. 2000. https://doi.org/10.1097/00042737-200012100-00012.
Farkas LM, Nelson RL, Abcarian H. A case of portal venous system thrombosis in ulcerative colitis. J Am Coll Surg. 2000;190:94. https://doi.org/10.1016/s1072-7515(99)00236-7.
Baddley JW, Singh D, Correa P, Persich NJ. Crohn’s disease presenting as septic thrombophlebitis of the portal vein (pylephlebitis): case report and review of the literature. Am J Gastroenterol. 1999. https://doi.org/10.1111/j.1572-0241.1999.00959.x.
Yada S, Hizawa K, Aoyagi K, Hashizume M, Matsumoto T, Koga H, et al. Portal hypertensive gastropathy due to chronic portal vein occlusion in Crohn’s disease. Am J Gastroenterol. 1998. https://doi.org/10.1111/j.1572-0241.1998.424_e.x.
Zoepf T, Mayer D, Merckle E, Adler G, Beckh K. Portal vein thrombosis and multiple liver abscesses in Crohn’s disease—an example for successful conservative treatment. Z Gastroenterol. 1997;35:627–30.
Tung JY, Johnson JL, Liacouras CA. Portal-mesenteric pylephlebitis with hepatic abscesses in a patient with Crohn’s disease treated successfully with anticoagulation and antibiotics. J Pediatr Gastroenterol Nutr. 1996;23:474.
Tsujikawa T, Ihara T, Sasaki M, Inoue H, Fujiyama Y, Bamba T. Effectiveness of combined anticoagulant therapy for extending portal vein thrombosis in Crohn’s disease. Report of a case. Dis Colon Rectum. 1996. https://doi.org/10.1007/BF02054451.
Diehl SJ, Lehmann KJ, Manthe S. Georgi M [Septic portal vein thrombosis as a rare complication of Crohn disease with retroperitoneal abscess]. Aktuelle Radiol. 1996;6:99–101.
Miyazaki Y, Shinomura Y, Kitamura S, Hiraoka S, Tomoda K, Nezu R, et al. Portal vein thrombosis associated with active ulcerative colitis: percutaneous transhepatic recanalization. Am J Gastroenterol. 1995;90(9):1533–4.
Mathieu E, Fain O, Trinchet JC, Aurousseau MH, Stérin D, Thomas M. Portal vein thrombosis: a rare complication of Crohn disease. La Revue de Medecine Interne. 1994. https://doi.org/10.1016/s0248-8663(05)82504-4.
Crowe A, Taffinder N, Layer GT, Irvine A, Nicholls RJ. Portal vein thrombosis in a complicated case of Crohn’s disease. Postgrad Med J. 1992;68:291–3. https://doi.org/10.1136/pgmj.68.798.291.
Brinberg DE, Stefansson TB, Greicius FA, Kahlam SS, Molin C. Portal vein thrombosis in Crohn’s disease. Gastrointest Radiol. 1991;16:245–7. https://doi.org/10.1007/BF01887357.
Reh TE, Srivisal S, Schmidt EH. Portal venous thrombosis in ulcerative colitis: CT diagnosis with angiographic correlation. J Comput Assist Tomogr. 1980;4:545–7. https://doi.org/10.1097/00004728-198008000-00029.
Capron JP, Remond A, Lebrec D, Delamarre J, Dupas JL, Lorriaux A. Gastrointestinal bleeding due to chronic portal vein thrombosis in ulcerative colitis. Digest Dis Sci. 1979. https://doi.org/10.1007/BF01308436.
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
MA contributed to the design and implementation, writing, reading, interpreting, and drafting the research. All authors contributed, revised, and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The institutional review board and research ethics committee of Damascus hospital had approved this case report with the Ethics Approval number: EAN:2023-17.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Alhalabi, M., Nasri, D. & Aji, W. Portal vein thrombosis as extraintestinal complications of Crohn’s disease: a case report and review of literature. J Med Case Reports 18, 246 (2024). https://doi.org/10.1186/s13256-024-04560-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13256-024-04560-w