Abstract
Background
Neurofibromatosis type 1 is an autosomal-dominant disease characterized by café-au-lait spots and neurofibromas, as well as various other symptoms in the bones, eyes, and nervous system. Due to its connection with vascular fragility, neurofibromatosis type 1 has been reported to be associated with vascular lesions, such as aneurysms. However, there have been few reports of abdominal visceral aneurysms associated with neurofibromatosis type 1. Furthermore, there have been no reports of robotic treatment of aneurysms associated with neurofibromatosis type 1. In this report, we describe the case of a patient with neurofibromatosis type 1 with a splenic artery aneurysm who was successfully treated with robotic surgery.
Case presentation
This report describes a 41-year-old Asian woman with a history of neurofibromatosis type 1 who was referred to our hospital for evaluation of a 28 mm splenic artery aneurysm observed on abdominal ultrasound. The aneurysm was in the splenic hilum, and transcatheter arterial embolization was attempted; however, this was difficult due to the tortuosity of the splenic artery. Thus, we suggested minimally invasive robotic surgery for treatment and resection of the splenic artery aneurysm with preservation of the spleen. The postoperative course was uneventful, and the patient was discharged on the eighth day after surgery. At 1 year of follow-up, the patient was doing well, with no evidence of recurrence.
Conclusion
We encountered a rare case of splenic artery aneurysm in a patient with neurofibromatosis type 1 who was successfully treated with robotic surgery. There is no consensus on treatment modalities for neurofibromatosis-related aneurysms, and endovascular treatment is considered safe and effective; however, surgery remains an important treatment modality. Especially in patients with stable hemodynamic status, robotic surgery may be considered as definitive treatment. To our knowledge, this is the first successfully treated case of a splenic artery aneurysm in a patient with neurofibromatosis type 1.
Similar content being viewed by others
Background
Neurofibromatosis type 1 (NF1), also known as von Recklinghausen’s disease, is an autosomal-dominant disease that causes various manifestations such as multiple flat, light-brown patches of skin pigment (café-au-lait spots), freckling of skinfolds, visible neurofibromas under the skin, and small nodules of the iris (Lisch nodules) [1, 2]. NF1 occurs in 1 in 3000–4000 people worldwide. Due to its connection to vascular fragility, NF1 has been reported to be associated with vasculopathy, with vascular lesions reported to complicate 3–9% of cases [3]. The spectrum of vasculopathy in NF1 includes aneurysms, stenosis, and arteriovenous malformations of medium- and large-sized vessels. Although there have been some reports of abdominal visceral arteries such as the liver artery, superior mesenteric artery (SMA), and inferior mesenteric artery (IMA) [4,5,6], there have been no reports of splenic artery aneurysms, and this is believed to be the first reported aneurysm of this type worldwide.
Aneurysms are often asymptomatic and may be found incidentally on imaging studies; however, they have the potential to be fatal due to life-threatening bleeding from ruptured aneurysms [7, 8]. Careful medical management, such as avoiding hypertension, is first necessary to avoid aneurysm rupture. Surgical intervention is guided by the age and comorbidities of the patient as well as the site and type of aneurysm. Open aneurysmectomy or endovascular treatment is often the treatment of choice, but there have been no reports of robotic treatment of aneurysms associated with NF1. In this report, we describe the case of a patient with NF1 with a splenic aneurysm who was successfully treated with robotic surgery.
Case presentation
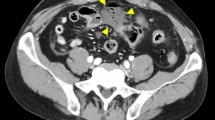
A 41-year-old Asian woman was referred to our hospital for evaluation of a splenic artery aneurysm noted on abdominal ultrasound. The patient had no subjective symptoms. The patient’s clinical history included neurofibromatosis type 1, Noonan syndrome, peptic gastric ulcer, endometriosis, and ventricular septal defects. At the age of 27 years, the patient underwent excision of a cutaneous nodule on the right thigh, the pathological diagnosis of which was plexiform neurofibroma. The patient had no relevant family history of vasculopathy, and no psychosocial history. She had no history of regular medications. The patient had no history of smoking or alcohol consumption. The patient was 151 cm tall and weighed 60 kg. On the initial medical examination, there were no relevant physical or neurological findings, her blood pressure was 125/77 mmHg, and her pulse rate was 85 beats per minute. The patient’s laboratory data were within normal limits. Contrast-enhanced computed tomography (CT) showed a 28-mm aneurysm in the splenic hilum (Fig. 1A, B). Angiography revealed similar findings (Fig. 1C). She was asymptomatic, but because her splenic artery aneurysm was > 20 mm in diameter, it had the potential to rupture, causing life-threatening complications. Therefore, we determined that it was indicated for treatment. Transcatheter arterial embolization (TAE) was attempted, but it was difficult due to the tortuosity of the splenic artery (Fig. 1D). Therefore, we suggested surgery, especially minimally invasive procedures, such as laparoscopic and robotic techniques. The patient underwent robotic resection of the splenic artery aneurysm with preservation of the spleen (Fig. 2A). The postoperative course was uneventful, and the patient was discharged on the eighth day after surgery. At 1 year of follow-up, the patient was doing well, with no evidence of recurrence.
A Robotic aneurysmectomy was performed, and the spleen could be preserved. B Resected specimen of splenic artery aneurysm. C A histopathological examination showed severe intimal thickening of the aneurysm, and the internal elastic lamina over half of the circumference had disappeared. D Neurofibroma attached to the adventitia were observed
Pathological findings
The gross examination revealed that the aneurysm was approximately 20 mm in size (Fig. 2B). A histopathological examination showed severe intimal thickening of the aneurysm, with the internal elastic lamina of over half of the circumference having disappeared (Fig. 2C). Neurofibromas attached to adventitia were also observed (Fig. 2D). The pathological diagnosis was a splenic artery aneurysm associated with neurofibromatosis.
Discussion
Neurofibromatosis type 1 (NF1), also known as von Recklinghausen’s disease, is an autosomal dominant disease that causes various manifestations such as multiple flat, light-brown patches of skin pigment (café-au-lait spots), freckling of the skinfolds, visible neurofibromas under the skin, and small nodules of the iris (Lisch nodules) [1, 2]. NF1 occurs in 1 in 3000–4000 people worldwide. Due to vascular fragility, NF1 has been reported to be associated with vascular lesions, such as stenosis, occlusion, aneurysm, arteriovenous malformation, and arteriovenous fistula, with vascular complications reported in 3–9% of cases [3]. The first site where vascular lesions are most likely to occur is the renal artery, followed by the carotid and cerebral arteries, and the subclavian and vertebral arteries [9]. Stenosis of the renal arteries results in renal vascular hypertension [10]. The vascular lesions are often asymptomatic; however, they have the potential to be fatal due to massive bleeding from ruptured aneurysms [7, 8] and are the second leading cause of death after soft tissue malignancies in NF1 patients [9]. Several hypotheses have been proposed to explain the mechanism by which NF1 causes aneurysms [11, 12]: (1) weakening of the vessel wall due to direct infiltration of the neurofibroma into the intima; (2) weakening of the artery due to ischemia caused by compression of the nutrient vessels by the neurofibroma; and (3) weakening of the endoelastic plate due to flattening of the tunica media support cells by proliferation of spindle-shaped cells in the intima.
Treatment options for aneurysms include surgical resection of the aneurysm with or without revascularization and endovascular procedures such as coil embolization and stent grafting [13]. Although there is no consensus on treatment modalities, a systematic review of endovascular treatment of neurofibromatosis-related aneurysms reported that endovascular treatment is safe and effective, regardless of age or hemodynamic status [14]. However, endovascular treatment is difficult to perform when there is vascular tortuosity or when there is a risk of impaired blood flow to the peripheral organs. In splenic artery aneurysms, endovascular treatment is often the first choice because of its superior perioperative mortality and short-term results; however, surgery is performed when endovascular treatment is considered difficult [15, 16].
In the general population, splenic aneurysms are the most common type of abdominal visceral aneurysms, accounting for approximately 60% [17], with an estimated prevalence of 0.8% [18]. In contrast, in a report examining 40 aneurysm lesions associated with NF1, there were only two cases involving the liver artery, one case involving the gastroduodenal artery, and one case involving the pancreaticoduodenal artery, indicating that abdominal visceral aneurysms are rare [9]. There have been several reports of abdominal visceral aneurysms associated with NF1, including liver, superior mesenteric, inferior mesenteric, celiac, gastroduodenal, and superior rectal arteries [4,5,6, 9, 19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34] (Table 1). However, to our knowledge, there are no reports of splenic aneurysms associated with NF1 in the relevant English literature, and we believe that this is the first report of such an aneurysm. The reason why which splenic aneurysms are more frequent in the general population but less frequent in the NF1 population is unclear, but the results are interesting.
In recent years, remarkable progress has been made in minimally invasive surgery, especially robotic surgery, and its indications are expanding. Robotic surgery is considered to enable more precise manipulation due to its features, such as a wide range of motion with multiple joints, a high-magnification 3D field of view, and sufficient traction without shaking. Rompianesi et al. compared robotic and laparoscopic pancreaticoduodenectomy with spleen preservation and reported a higher rate of spleen preservation with robotic surgery [35]. A systematic review of laparoscopic and robotic surgeries for splenic aneurysms reported that 3.6% of laparoscopic patients underwent distal pancreatectomy, while no patients underwent pancreatectomy in robotic surgery, and 29.8% of laparoscopic patients underwent splenectomy, while 9.1% underwent splenectomy in robotic surgery [36]. In splenic artery aneurysmectomy, robotic surgery may contribute to preservation of the pancreas and spleen because of its dexterity.
The present case was asymptomatic, and the aneurysm was discovered incidentally. Pathological findings showed a neurofibroma infiltrating the adventitia, and the ischemic effect due to pressure drainage was suspected to be the mechanism of pathogenesis. The first step of treatment would have been a minimally invasive endovascular embolization procedure; however, the vascular tortuosity was so severe that surgical treatment was necessary. As the patient had a stable hemodynamic status, we presented robotic surgical treatment, which is considered to be one of the less invasive techniques in surgery. As a result, the patient was able to benefit from minimally invasive surgery, with preservation of the pancreas and spleen and early discharge without perioperative complications.
Conclusions
We encountered a rare case of splenic artery aneurysm in a patient with NF1 who was successfully treated by robotic surgery. In the present case, robotic aneurysmectomy was accomplished with preservation of the pancreas and spleen, suggesting that robotic surgery may be useful for preserving the pancreas and spleen in splenic aneurysmectomy. The present case suggests that robotic surgery can be performed safely even in NF1 cases, which are considered to have vascular fragility. Especially in patients with stable hemodynamic status, robotic surgery may be considered as definitive treatment. To our knowledge, this is the first successfully treated case of a splenic artery aneurysm in a patient with NF1.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article.
Abbreviations
- CT:
-
Computed tomography
- IMA:
-
Inferior mesenteric artery
- NF1:
-
Neurofibromatosis type 1
- SMA:
-
Superior mesenteric artery
- TAE:
-
Transcatheter arterial embolization
References
Legius E, Messiaen L, Wolkenstein P, Pancza P, Avery RA, Berman Y, Blakeley J, Babovic-Vuksanovic D, Cunha KS, Ferner R, et al. Revised diagnostic criteria for neurofibromatosis type 1 and Legius syndrome: an international consensus recommendation. Genet Med. 2021;23(8):1506–13.
Tamura R. Current understanding of neurofibromatosis type 1, 2, and schwannomatosis. Int J Mol Sci. 2021;22(11):5850.
Das Gupta TK, Brasfield RD. Von Recklinghausen’s disease. CA Cancer J Clin. 1971;21(3):174–83.
Fukushima N, Aoki H, Takenaga S, Morikawa K, Ogawa M, Yanaga K. Ruptured visceral artery aneurysms in a patient of neurofibromatosis type 1 (NF-1) successfully treated by endovascular treatment. Surg Case Rep. 2020;6(1):18.
Morris ME, Jones RG, Walker SK, Yancey AE, Dwivedi AJ, Ross CB. Obstructive jaundice secondary to multiple hepatic artery aneurysms in a 14-year-old boy with neurofibromatosis type 1. Ann Vasc Surg. 2013;27(5):673.e671-674.
Sheth S, Kansakar N, Agarwal P, Singh R, Mishra A. Ruptured inferior mesenteric artery aneurysm in a patient with neurofibromatosis type I and its management. Int Surg J. 2018;5(2):732–4.
Miyamoto K, Nakamura M, Suzuki K, Katsuki S, Kaki Y, Inoue G, Ohno T, Sasaki J, Dohi K, Hayashi M. Diagnosis of neurofibromatosis type 1 after rupture of aneurysm and consequent fatal hemothorax. Am J Emerg Med. 2020;38(7):1543.e1543-1543.e1545.
Tatebe S, Asami F, Shinohara H, Okamoto T, Kuraoka S. Ruptured aneurysm of the subclavian artery in a patient with von Recklinghausen’s disease. Circ J. 2005;69(4):503–6.
Oderich GS, Sullivan TM, Bower TC, Gloviczki P, Miller DV, Babovic-Vuksanovic D, Macedo TA, Stanson A. Vascular abnormalities in patients with neurofibromatosis syndrome type I: clinical spectrum, management, and results. J Vasc Surg. 2007;46(3):475–84.
Criado E, Izquierdo L, Luján S, Puras E, del Mar EM. Abdominal aortic coarctation, renovascular, hypertension, and neurofibromatosis. Ann Vasc Surg. 2002;16(3):363–7.
Greene JF Jr, Fitzwater JE, Burgess J. Arterial lesions associated with neurofibromatosis. Am J Clin Pathol. 1974;62(4):481–7.
Leier CV, DeWan CJ, Anatasia LF. Fatal hemorrhage as a complication of neurofibromatosis. Vasc Surg. 1972;6(2):98–101.
Delis KT, Gloviczki P. Neurofibromatosis type 1: from presentation and diagnosis to vascular and endovascular therapy. Perspect Vasc Surg Endovasc Ther. 2006;18(3):226–37. https://doi.org/10.1177/1531003506296488.
Bargiela D, Verkerk MM, Wee I, Welman K, Ng E, Choong A. The endovascular management of neurofibromatosis-associated aneurysms: a systematic review. Eur J Radiol. 2018;100:66–75.
Salimi J, Foroutani L, MiratashiYazdi SA. Management of huge splenic artery aneurysm with new hybrid procedure including endovascular and open surgical approach: case series. Int J Surg Case Rep. 2021;89: 106585.
Hogendoorn W, Lavida A, Hunink MG, Moll FL, Geroulakos G, Muhs BE, Sumpio BE. Open repair, endovascular repair, and conservative management of true splenic artery aneurysms. J Vasc Surg. 2014;60(6):1667-1676.e1661.
Messina LM, Shanley CJ. Visceral artery aneurysms. Surg Clin N Am. 1997;77(2):425–42.
Madoff DC, Denys A, Wallace MJ, Murthy R, Gupta S, Pillsbury EP, Ahrar K, Bessoud B, Hicks ME. Splenic arterial interventions: anatomy, indications, technical considerations, and potential complications. Radiographics. 2005;25(Suppl 1):S191-211.
Huffman JL, Gahtan V, Bowers VD, Mills JL. Neurofibromatosis and arterial aneurysms. Am Surg. 1996;62(4):311–4.
Hassen-Khodja R, Declemy S, Batt M, Castanet J, Perrin C, Ortonne JP, Le Bas P. Visceral artery aneurysms in Von Recklinghausen’s neurofibromatosis. J Vasc Surg. 1997;25(3):572–5.
Serleth HJ, Cogbill TH, Gundersen SB 3rd. Ruptured pancreaticoduodenal artery aneurysms and pheochromocytoma in a pregnant patient with neurofibromatosis. Surgery. 1998;124(1):100–2.
Sacar M, Tulukoglu E, Ucak A, Guler A, Yilmaz AT. Inferior mesenteric artery aneurysm combined with renal artery stenosis in a patient with neurofibromatosis. Perspect Vasc Surg Endovasc Ther. 2006;18(3):217–20.
Rao V, Day CP, Manimaran N, Hurlow RA, Orme R. Spontaneous rupture of the hepatic artery in a patient with type 1 neurofibromatosis treated by embolization: a case report. Cardiovasc Intervent Radiol. 2007;30(1):124–5.
Mendonça CT, Weingartner J, de Carvalho CA, Costa DS. Endovascular treatment of contained rupture of a superior mesenteric artery aneurysm resulting from neurofibromatosis type I. J Vasc Surg. 2010;51(2):461–4.
Kerger L, Tomescot A, Chafai N. Ruptured inferior mesenteric artery aneurysm in a patient with a type 1 neurofibromatosis. Ann Vasc Surg. 2012;26(6):858.e851-852.
Makino K, Kurita N, Kanai M, Kirita M. Spontaneous rupture of a dissecting aneurysm in the superior rectal artery of a patient with neurofibromatosis type 1: a case report. J Med Case Rep. 2013;7:249.
Im KS, Kim S, Lim JU, Jeon JW, Shin HP, Cha JM, Joo KR, Lee JI, Park JJ. Life-threatening duodenal ulcer bleeding from a ruptured gastroduodenal artery aneurysm in a patient with neurofibromatosis type 1. Korean J Gastroenterol. 2015;66(3):164–7.
Yow KH, Bennett J, Baptiste P, Giordano P. Successful combined management for ruptured superior rectal artery aneurysm in neurofibromatosis type 1. Ann Vasc Surg. 2015;29(6):1317.e1313-1316.
Lim JY, Choi YH, Lee SH. Unusual presentation and treatment of isolated spontaneous gastric artery dissection. Clin Exp Emerg Med. 2016;3(2):112–5.
Drucker NA, Blaibel MF, Nagaraju S, Wang SK, Goggins W, Fajardo A. Renal autotransplant and celiac artery bypass for aneurysmal degeneration related to neurofibromatosis type 1. Vasc Endovascular Surg. 2019;53(6):497–500.
Moro K, Kameyama H, Abe K, Tsuchida J, Tajima Y, Ichikawa H, Nakano M, Ikarashi M, Nagahashi M, Shimada Y, et al. Left colic artery aneurysm rupture after stent placement for abdominal aortic aneurysm associated with neurofibromatosis type 1. Surg Case Rep. 2019;5(1):12.
Nemoto H, Mori K, Takei Y, Kikuchi S, Hoshiai S, Yamamoto Y, Nakajima T. Treatment of ruptured rectal artery aneurysm in a patient with neurofibromatosis. CVIR Endovasc. 2022;5(1):37.
Takata Y, Katayama K, Shimizu H, Inoue R, Takasaki T, Takahashi S. Treatment of celiac artery rupture with a hybrid procedure involving aortic stent grafting and open surgery in a patient with neurofibromatosis type 1. J Vasc Surg Cases Innov Tech. 2022;8(4):625–8.
Rajahram D, Satchithanantham V, Veerasingam S, Tharmalingam T. Rare cause of fatal acute abdomen-celiac artery aneurysm. Int J Surg Case Rep. 2023;109: 108546.
Rompianesi G, Montalti R, Ambrosio L, Troisi RI. Robotic versus Laparoscopic Surgery for Spleen-Preserving Distal Pancreatectomies: systematic review and meta-analysis. J Pers Med. 2021;11(6):552. https://doi.org/10.3390/jpm11060552.
Ossola P, Mascioli F, Coletta D. Laparoscopic and Robotic Surgery for Splenic Artery Aneurysm: a systematic review. Ann Vasc Surg. 2020;68:527–35. https://doi.org/10.1016/j.avsg.2020.05.037.
Acknowledgements
We would like to thank Dr. Hideo Hattori for the pathological diagnosis. We would like to thank Japan Medical Communication (www.japan-mc.co.jp) for English language editing.
Funding
None.
Author information
Authors and Affiliations
Contributions
All authors read and approved the final manuscript. AU designed and wrote the paper. KS, RO, and HT analyzed and interpreted the patient’s data. HM, TK, and HI collected the patient’s data. KS and MM treated and followed the patient. YM and ST supervised this case report.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ueda, A., Saito, K., Murase, H. et al. Robotic resection for splenic artery aneurysm associated with neurofibromatosis type 1: a case report. J Med Case Reports 18, 104 (2024). https://doi.org/10.1186/s13256-024-04440-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13256-024-04440-3