Abstract
Background
Superior rectal artery (SRA) aneurysms are rare. Although melena is the most common symptom, it has not been observed in cases of aneurysms located in the SRA trunk. Here, we report a case of a ruptured SRA trunk aneurysm successfully treated with coil embolization. Including our case, three of the four reported cases of SRA trunk aneurysms were related to neurofibromatosis type 1 (NF1).
Case presentation
A 52-year-old woman with NF1 was referred to our hospital for the investigation of an abdominal mass with back pain. She had previously undergone a blood transfusion at another hospital for anemia without melena. Computed tomography angiography revealed a ruptured SRA trunk aneurysm measuring 3 cm in diameter and surrounded by a retroperitoneal hematoma. The aneurysm was isolated by embolizing the SRA trunk distally and proximally. Distal embolization was performed retrogradely from the internal iliac artery (IIA) via the middle rectal artery (MRA)-SRA anastomosis because the antegrade approach from the inferior mesenteric artery (IMA) failed. To our knowledge, this is the first case of successful coil embolization of an IMA branch through the IIA.
Conclusion
SRA trunk aneurysms are rare; however, they are frequently associated with NF1. Antegrade distal embolization beyond the aneurysm is sometimes difficult to achieve. In such cases, a retrograde approach via MRA-SRA anastomosis can be the choice for isolating SRA trunk aneurysms.
Similar content being viewed by others
Background
Neurofibromatosis type 1 (NF1) is an autosomal dominant multisystem disorder characterized by multiple café-au-lait macules, intertriginous freckling, and cutaneous neurofibromas. Less common but potentially more serious manifestations include optic nerve and other central nervous system gliomas; malignant peripheral nerve sheath tumors; scoliosis; tibial dysplasia; and gastrointestinal, endocrine, or pulmonary disease (Friedman JM et al. 1998). Vasculopathy, including aneurysm formation, also occurs in medium to large arteries. According to a review on aneurysms caused by neurofibromatosis (Bargiela et al. 2018), NF1-related aneurysms were observed in the head and neck in 27 of 58 patients, subclavian and intercostal arteries in 14 patients, visceral arteries in 8 patients, and other uncommon regions in 9 patients. In addition, to the best of our knowledge, a superior rectal artery (SRA) trunk aneurysm related to NF1 has been reported in two patients (Makino et al. 2013; Yow et al. 2015). Herein, we report the third case of a ruptured SRA trunk aneurysm related to NF1, emphasizing the importance of isolating aneurysms to avoid re-bleeding after coil embolization.
Case presentation
A 52-year-old woman with NF1 was referred to our hospital for an abdominal mass with back pain and anemia. She had undergone mastectomy for left breast cancer and colectomy for colon cancer, 3 and 17 years ago, respectively. In the previous hospital, she had hypotension (blood pressure = ~70 mmHg) with loss of consciousness, and unenhanced computed tomography revealed a retroperitoneal mass suspected to be a neurogenic tumor related to NF1. At that time, her hemoglobin level was almost normal at 11.6 g/dL. However, it gradually decreased to 6.1 g/dL over the next 6 days. She had received a total blood transfusion of 16 packed red blood cell units before admission to our hospital. On admission, her blood pressure and heart rate were 119/96 mmHg and 96 beats per minute, respectively. Laboratory data showed a hemoglobin level of 10.0 g/dL, platelet count of 629,000/μL, prothrombin time of 13.9 s, and activated partial thromboplastin time of 48.8 s. Computed tomography angiography (CTA) revealed a saccular SRA trunk aneurysm that measured 3 cm in diameter and was surrounded by an increased large retroperitoneal hematoma (Fig. 1). In addition, two hypodense masses protruding from the left second and third sacral foramen were observed, which were indicative of neurofibromas related to NF1 (not shown). Therefore, we treated the ruptured SRA trunk aneurysm with transcatheter arterial embolization (TAE).
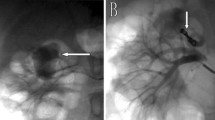
Under local anesthesia, employing the femoral artery approach, inferior mesenteric artery (IMA) angiography was performed using a 4-F shepherd hook-type catheter (Medikit Co. Ltd., Tokyo, Japan), and an SRA trunk aneurysm was confirmed (Fig. 2a). A 2.6-F high-flow-type microcatheter (Masters HF; ASAHI INTECC Co. Ltd., Aichi, Japan) was selectively advanced into the SRA over a 0.016-in micro guidewire (Meister; ASAHI INTECC Co. Ltd., Aichi, Japan). SRA angiography revealed the distal vessel of the aneurysm (Fig. 2b). To isolate the aneurysm, we attempted to embolize the distal vessel; however, microcatheter insertion failed via the antegrade approach. Meanwhile, the distal vessel disappeared on the aneurysmogram; this might have resulted from thrombogenesis or retrograde blood flow. Proximal embolization without isolation of the aneurysm was considered insufficient for hemostasis because re-bleeding due to retrograde blood flow could occur. Therefore, we attempted a retrograde approach to the distal vessel. A shepherd hook-type catheter was advanced into the right common iliac artery. Right internal iliac arteriography with a high-flow type microcatheter revealed the middle rectal artery (MRA) arising from the internal pudendal artery. The inferior pudendal arteriogram revealed anastomosis between the MRA and SRA (Fig. 2c). To pass through the narrow and tortuous anastomosis, a 1.6-F microcatheter (Marvel S; Tokai Medical Products Inc., Aichi, Japan) with a 0.014-in micro guidewire was inserted coaxially into the high-flow microcatheter. Using this double coaxial microcatheter system (Hongo et al. 2014), the high-flow microcatheter was advanced using the MRA-SRA anastomosis into the distal SRA trunk to be embolized with a coil (Interlock, 3 mm/12 cm, Boston Scientific, MA, USA) (Fig. 2d). Finally, the aneurysm was isolated with proximal embolization of the SRA trunk via the IMA using three coils (Interlock 2 mm/6 cm × 3, Boston Scientific, MA, USA). The opacification of the aneurysm disappeared on retrograde and antegrade angiography, confirming complete cessation of blood flow into the aneurysm (Fig. 2e, f). The post-procedural course was uneventful. Anemia improvement and absence of bowel ischemia were observed. On follow-up CTA performed 3 months later, the aneurysm was not opacified and the hematoma had decreased in size (Fig. 3).
a Inferior mesenteric arteriogram showing the superior rectal artery (SRA) trunk aneurysm (arrow). b Aneurysmogram showing the distal SRA trunk (arrows). c Internal pudendal arteriogram showing the middle rectal artery (MRA) (arrow) and MRA-SRA anastomosis (arrowheads). d A high-flow type microcatheter is advanced into the SRA trunk distal to the aneurysm (arrow) via MRA-SRA anastomosis using the coaxial microcatheter system. e Retrograde arteriogram after coil embolization (arrow) showing complete occlusion of distal SRA. f The final inferior mesenteric arteriogram after proximal coil embolization showing complete cessation of blood flow into the aneurysm. Note that the coils are deployed distally (arrow) and proximally to the aneurysm (arrowhead)
Coronal partial maximum intensity projection image reconstructed from computed tomography angiography obtained 3 months after transcatheter arterial embolization showing the lack of enhancement of the superior rectal artery trunk aneurysm. Note the coils deployed proximally and distally to the aneurysm (arrows) and decreased size of the retroperitoneal hematoma (arrowheads)
Discussion
Visceral arterial aneurysms are rare, with a prevalence of 0.1 to 2% (Tulsyan et al. 2007). SRA aneurysms are extremely rare and have been documented in only 14 case reports, including the present report (Table 1). The most common causes of SRA aneurysms were trauma and NF1 in three cases. All NF1-related SRA aneurysms were observed in the arterial trunk and had ruptured at the time of diagnosis. The vascular fragility of middle to large arteries in NF1 can be related to the location of these aneurysms. The lesions were found to be located away from the rectal mucosa; thus, no patient complained of melena, and CTA was required for diagnosis.
TAE is the first choice of treatment for visceral arterial aneurysms owing to shorter hospitalization time and fewer cardiovascular complications (Barrionuevo et al. 2019);
however, in cases that require distal revascularization or bypass, surgery is the only choice. Although massive bleeding during surgery and perioperative mortality due to vascular fragility have been reported in NF1 (Chew et al. 2001), TAE has been reported to provide favorable outcomes with fewer major complications (15%), local recurrence (9.1%), and persistence of symptoms (4.5%) (Bargiela et al. 2018).
In two previous case reports of ruptured SRA trunk aneurysms related to NF1, TAE succeeded in one of the two cases (Makino et al. 2013), but failed in another case (Yow et al. 2015), requiring surgical aneurysmal exclusion for re-bleeding. In the latter case, the SRA could not be catheterized with a microcatheter, and only proximal embolization of the IMA was performed using coils. The authors considered that retrograde reperfusion of the aneurysm sac via patent collaterals could result in recurrent hemorrhage. To prevent this, the aneurysm should be isolated by embolizing the SRA trunk proximal and distal to it. In our case, antegrade distal embolization failed because the aneurysmal cavity was so large that the microcatheter or guidewire could not be directed to the orifice of the distal SRA trunk. Therefore, the distal SRA was embolized retrogradely via MRA-SRA anastomosis from the right internal iliac artery (IIA). To our knowledge, this is the first case of successful coil embolization of an IMA branch through the IIA. The double coaxial microcatheter system enabled the passage of a narrow and tortuous anastomosis. The glue consisting of n-butyl cyanoacrylate and iodized oil can be an alternative embolic agent for the treatment of ruptured aneurysms or pseudoaneurysms when distal catheterization is difficult. It was used in 3 of the 14 reported cases of SRA aneurysms (Table 1) because these patients’ conditions were unstable due to massive bleeding. The glue embolization has the advantage of quick hemostasis. However, it should be noted that embolizing a proximal aneurysm with the glue as in our case increases the risk of post-procedural bowel ischemia.
Conclusion
SRA trunk aneurysms are rare, but frequently associated with NF1. CTA is recommended for an early diagnosis because the aneurysm can rupture and cause hemorrhagic shock without causing melena. Isolation of aneurysms using embolization coils is necessary to prevent recurrent hemorrhage. Antegrade distal embolization beyond the aneurysm is sometimes difficult to achieve. In such instances, a retrograde approach employing MRA-SRA anastomosis can be used.
Availability of data and materials
The datasets used during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CTA:
-
Computed tomography angiography
- IIA:
-
Internal iliac artery
- IMA:
-
Inferior mesenteric artery
- MRA:
-
Middle rectal artery
- NF1:
-
Neurofibromatosis type 1
- SRA:
-
Superior rectal artery
- TAE:
-
Transcatheter arterial embolization
References
Baig MK, Lewis M, Stebbing JF, Marks CG (2003) Multiple microaneurysms of the superior hemorrhoidal artery: unusual recurrent massive rectal bleeding: report of a case. Dis Colon Rectum 46:978–980
Bargiela D, Verkerk MM, Wee I, Welman K, Ng E, Choong AMTL (2018) The endovascular management of neurofibromatosis-associated aneurysms: a systematic review. Eur J Radiol 100:66–75
Barrionuevo P, Malas MB, Nejim B, Haddad A, Morrow A, Ponce O, Hasan B, Seisa M, Chaer R, Murad MH (2019) A systematic review and meta-analysis of the management of visceral artery aneurysms. J Vasc Surg 70:1694–1699
Chew DKW, Muto PM, Gordon JK, Straceski AJ, Donaldson MC (2001) Spontaneous aortic dissection and rupture in a patient with neurofibromatosis. J Vasc Surg 34:364–366
Curfman KR, Shuman MP, Gorman KM, Schrock WB, Meade PG (2020) Post-traumatic retroperitoneal hematoma caused by superior rectal artery pseudoaneurysm. Am J Case Rep 21:e924529
Friedman JM (1998) Neurofibromatosis 1. In: GeneReviews. University of Washington, Seattle https://www.ncbi.nlm.nih.gov/books/NBK1109/, Accessed 27 May 2022
Hongo N, Kiyosue H, Shuto R, Kamei N, Miyamoto S, Tanoue S, Mori H (2014) Double coaxial microcatheter technique for transarterial aneurysm sac embolization of type II endoleaks after endovascular abdominal aortic repair. J Vasc Interv Radiol 25:709–716
Iqbal J, Kaman L, Parkash M (2011) Traumatic pseudoaneurysm of superior rectal artery - an unusual cause of massive lower gastrointestinal bleed: a case report. Gastroenterology Res 4:36–38
Janmohamed A, Noronha L, Saini A, Elton C (2011) An unusual cause of lower gastrointestinal haemorrhage. BMJ Case Rep. https://doi.org/10.1136/bcr.11.2011.5102.
Kim M, Song HJ, Kim S, Cho Y-K, Kim HU, Song B-C, Chang WY, Kim SH (2012) Massive life-threatening lower gastrointestinal hemorrhage caused by an internal hemorrhoid in a patient receiving antiplatelet therapy: a case report. Korean J Gastroenterol 60:253–257
Li C-C, Tsai H-L, Huang C-W, Yeh Y-S, Tsai T-H, Wang J-Y (2018) Iatrogenic pseudoaneurysm after bevacizumab therapy in patients with metastatic colorectal cancer: two case reports. Mol Clin Oncol 9:499–503
Liu H-P, Chew M-H, Ho K-S, Tang C-L (2013) Ischemic colitis due to a dissecting aneurysm of the superior rectal artery. Tech Coloproctol 17:331–333
Makino K, Kurita N, Kanai M, Kirita M (2013) Spontaneous rupture of a dissecting aneurysm in the superior rectal artery of a patient with neurofibromatosis type 1: a case report. J Med Case Rep 7:249–252
Marusca G, Yeddi A, Kiwan W, Masalmeh NA, Newberger S, Kakos R, Ehrinpreis M (2020) A unique case of severe hematochezia: ruptured pseudoaneurysm of the superior rectal artery. ACG Case Rep J 7:e00468
Nguyen C, Baliss M, Tayyem O, Merwat S (2020) Lower gastrointestinal hemorrhage caused by superior rectal artery pseudoaneurysm. ACG Case Rep J 7:e00387
Pond GD, Ovitt TW, Witte CL, Farrell K (1977) Aneurysm of the superior hemorrhoidal artery: an unusual cause of massive rectal bleeding. J Can Assoc Radiol 28:146–147
Tulsyan N, Kashyap VS, Greenberg RK, Sarac TP, Clair DG, Pierce G, Ouriel K (2007) The endovascular management of visceral artery aneurysms and pseudoaneurysms. J Vasc Surg 45:276–283
Yow KH, Bennett J, Baptiste P, Giordano P (2015) Successful combined management for ruptured superior rectal artery aneurysm in neurofibromatosis type 1. Ann Vasc Surg 29:P1317.E13–P1317.E16
Zakeri N, Cheah SO (2012) A case of massive lower gastrointestinal bleeding: superior rectal artery pseudoaneurysm. Ann Acad Med Singap 41:529–531
Acknowledgements
We would like to thank Editage [http://www.editage.com] for editing and reviewing this manuscript for English language.
Funding
Management expense grants from the University of Tsukuba
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. HN, KM, YT, SK, and SH performed the transarterial embolization. HN, YY, and KM obtained written informed consent for publication from the patient. KM and TN obtained institutional review board approval for publication. HN and KM were a major contributor in writing the manuscript. All authors revised the initial version and approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The University of Tsukuba hospital's clinical research ethics review board approved publishing the present case report (R04-042). Patient consent to participate is available.
Consent for publication
Patient consent for publication is available.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nemoto, H., Mori, K., Takei, Y. et al. Treatment of ruptured rectal artery aneurysm in a patient with neurofibromatosis. CVIR Endovasc 5, 37 (2022). https://doi.org/10.1186/s42155-022-00317-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42155-022-00317-y