Abstract
Introduction
Tel Hashomer camptodactyly syndrome is a rare disease and only a few cases have been reported. Dermatoglyphics potentially provide relevant phenotypic biomarkers that were initially noted as a vital clinical feature of this disease. Dermatoglyphics possibly can indicate growth disturbances that took place during early fetal development at the time when epidermal ridges were being formed into discernable patterns. Consequently, these intrauterine effects might well have occurred in association with the expression of the Tel Hashomer camptodactyly syndrome. Therefore, this review was undertaken to provide, as far as we know, the first attempt to broadly assess dermatoglyphic features that are connected with the Tel Hashomer camptodactyly syndrome. If a developmental association between dermatoglyphics and Tel Hashomer camptodactyly can be firmly established, this would probably document that Tel Hashomer camptodactyly disease has its origins during the early fetal period.
Methods
A systematic literature search was conducted using articles from PubMed (Medline), POPLINE, Trip Database, Cochrane Library, and gray literature up to 31 March 2015. The review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.
Results
Fourteen relevant publications were included in the review. There were 23 cases of patients with Tel Hashomer camptodactyly syndrome that were described in these published articles. We reviewed the dermatoglyphics of 21 available cases out of all of the published and electronically available cases of Tel Hashomer camptodactyly. Eight cases reported whorls to be the most common digital pattern with an expected rise of ridge count. Two cases show significantly high frequencies of arch patterns. Further, there were increased numbers of palmar creases, along with abnormal flexion creases or other palmar dermatoglyphic abnormalities reported in all cases.
Conclusion
This review highlighted the desirability of thoroughly observing and recording dermatoglyphic features when reporting on future patients with Tel Hashomer camptodactyly syndrome, in conjunction with carrying out modern molecular methods.
Similar content being viewed by others
Introduction
Tel Hashomer camptodactyly (THC) syndrome is a rare disease first termed by Goodman et al. in 1976 after examining two sisters with camptodactyly [1]. Earlier in 1972, they reported two brother and sister pairs having similar clinical features [2]. Up to the present time, a literature search has found only 23 cases. THC is mainly characterized by the presence of camptodactyly with muscular hypoplasia and weakness, skeletal dysplasia, facial dysmorphism (facial asymmetry, small mouth, broad nasal bridge, long philtrum, and hypertelorism), and abnormal dermatoglyphics: Online Mendelian Inheritance in Man® (OMIM) #211960, The portal for rare diseases and orphan drugs (ORPHA) 3292 [3, 4]. In addition, mitral valve prolapse, spina bifida, scoliosis, inguinal hernia, winging scapulae, clubbed feet, syndactyly and clinodactyly were indicated as clinical features [3, 4]. THC is considered to be a disease with autosomal recessive inheritance [5]. Mochizuki et al. [6] recently reviewed the molecular characteristics of a patient described by Toriello et al. in 1990 [7] and suggested that at least several cases of THC may actually be Ehlers–Danlos syndrome.
Goodman et al. [1] stated the importance of dermatoglyphic biomarkers as clinical features when diagnosing THC. Dermatoglyphic characters that need to be present to diagnose THC are: (a) presence of seven or more whorls on digits (these whorls extend beyond the borders of the terminal phalanges), (b) low main line index, caused by the highly vertical orientation of the A to D radiants, and (c) numerous palmar creases that obliterate the normal structure of the ridges and openings of the sweat pores. We systematically analyzed all published cases of THC syndrome to describe the importance of dermatoglyphics in diagnosing this rare disease.
Methods
The review has been conducted and reported using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines [8].
Search strategy
We conducted a search of the literature for articles indexed in PubMed® (Medline), POPLINE, TRIP Database, and Cochrane Library database, from earliest dates to 31 March 2015. In addition, we searched the gray literature sources of Google Scholar, OpenGrey, and Google, from earliest date to 31 March 2015. The reference lists of the studies selected were manually searched for any relevant studies. We did not restrict the searches based on language or publication status. The following terms were used to search the literature: “Tel Hashomer camptodactyly”, “Tel Hashomer camptodactyly syndrome.”
Eligibility criteria and data extraction
All studies that had diagnosed and reported THC syndrome were selected. From each, the following details were extracted: disease diagnosis, demographic details (age, sex, consanguinity, ancestry/lineage, country of case reported), and dermatoglyphic features. Initially, the full texts and abstract were screened and extracted by BTBW, and later SBA and RJM independently reviewed these studies for accuracy.
Results
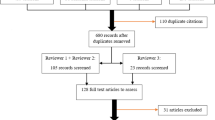
The search of electronic databases yielded 14 publications. In addition, three publications were obtained from gray literature sources and hand searching the reference lists (Fig. 1). Full texts are available for 13 publications [1, 2, 5–7, 9–16], only an abstract was available for two publications [17, 18], and an abstract or full text was unavailable for another two [19, 20]. Out of all 17 studies, only 14 publications were reviewed due to the unavailability of records for two publications [19, 20] and one publication reanalyzed a patient whose dermatoglyphics had been described previously [6].
There were 23 cases of THC described in the reviewed publications [6]. Six cases reported from Israel [1, 2, 5], three from Brazil [9, 12], three from India [14, 16], two from Italy [13, 18], three from Poland [10], one from the UK [11], two from the USA [7], two from Russia [17], and one from Hungary [15]. There were 11 females and nine males, and for three patients their sex was not reported in the available abstract [17, 18]. All reported cases were among siblings or first-degree relatives. Eleven cases were born to consanguineous parents [1, 9, 10, 13, 16] while nine were born to non- consanguineous parents [2, 5, 11, 12, 14, 15]. Two cases did not report the consanguinity of their parents [7] and in three cases the consanguinity was not reported in the available abstract [17, 18]. The dermatoglyphics were not reported in one case [16] and for another case dermatoglyphics were not reported in the abstract [18].
Key findings on dermatoglyphic features of the cases of THC are summarized in Table 1.
Discussion
Of the 21 cases that could be evaluated, eight reported whorls to be the most common digital pattern [1, 2, 7, 12, 15]. Of particular interest, four of these were females with THC syndrome who had at least eight whorls [1, 2, 7, 12]. Conversely, normal males tend to have higher frequencies of whorl patterns [21]. As expected, there were also high average ridge counts, since whorls usually do have more ridges than loops and, of course, arches have zero ridge counts. It is also of interest to note that two of the cases, involving a sister/brother pair, had high frequencies of digital arch patterns with the brother having nine arches [9]. Usually, normal females tend to have more arches than males [21]. Furthermore, there frequently were an increased number of palmar creases than would normally be observed, along with abnormal flexion creases or other palmar dermatoglyphic abnormalities reported in all cases.
The fact that these cases appear to show some unusual results, for instance, in terms of digital patterns from unexpectedly high whorl frequency, especially in females with THC, to a very high number of arches, notably in males with THC, might indicate that there could have been some growth disturbances that took place during early fetal development at the time when epidermal ridges were being formed. In addition, unusual findings with respect to palmar dermatoglyphic features and flexion creases might well be indicative of abnormal developmental conditions. Of course, this intrauterine effect might well have occurred in association with the camptodactyly syndrome. Accordingly, it seems apparent that dermatoglyphic biomarkers may provide important clues when applying differential diagnoses, in conjunction with current molecular testing.
Therefore, it is important to thoroughly observe and record dermatoglyphic features when reporting future patients with THC syndrome, in addition to carrying out modern molecular methods. A highly beneficial consequence of this practice is that possible associations of dermatoglyphic biomarkers in genetically confirmed cases of THC could then be used as a relevant diagnostic aid in countries that have limited medical diagnostic resources.
Abbreviations
- THC:
-
Tel Hashomer camptodactyly
References
Goodman RM, Katznelson MB, Hertz M, Katznelson A. Camptodactyly, with muscular hypoplasia, skeletal dysplasia, and abnormal palmar creases: Tel Hashomer camptodactyly syndrome. J Med Genet. 1976;13:136–41.
Goodman RM, Katznelson MB, Manor E. Camptodactyly: occurrence in two new genetic syndromes and its relationship to other syndromes. J Med Genet. 1972;9:203–12.
OMIM® Online Mendelian Inheritance in Man® [http://www.omim.org]. Accessed 12 May 2015.
The portal for rare diseases and orphan drugs [http://www.orpha.net/consor/cgi-bin/index.php]. Accessed 12 May 2015.
Smolkin T, Blazer S, Gershoni-Baruch R, Makhoul IR. Tel Hashomer camptodactyly syndrome in identical twin infants. Clin Dysmorphol. 2011;20:214–6.
Mochizuki A, Hyland J, Brown T, Slavin TP. Is Tel Hashomer camptodactyly a distinct clinical entity? Am J Med Genet Part A. 2015;167:255–8.
Toriello HV, Higgins JV, Malvitz T, Waterman DF. Two siblings with Tel Hashomer camptodactyly and mitral valve prolapse. Am J Med Genet. 1990;36:398–403.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
Gollop TR, Colletto GM. The Tel Hashomer camptodactyly syndrome in a consanguineous Brazilian family. Am J Med Genet. 1984;17:399–406.
Tylki-Szymanska A. Three new cases of Tel Hashomer camptodactyly syndrome in one Arabic family. Am J Med Genet. 1986;23:759–63.
Patton MA, McDermot KD, Lake BD, Baraitser M. Tel Hashomer camptodactyly syndrome: report of a case with myopathic features. J Med Genet. 1986;23:268–71.
Pagnan NA, Gollop TR, Lederman H. The Tel Hashomer camptodactyly syndrome: report of a new case and review of the literature. Am J Med Genet. 1988;29:411–7.
Franceschini P, Vardeu MP, Signorile F, Testa A, Guala A, Franceschini D, Dalforno L. Inguinal Hernia and Atrial Septal Defect in Tel Hashomer Camptodactyly Syndrome: Report of a New Case Expanding the Phenotypic Spectrum of the Disease. Am J Med Genet. 1993;46:341–4.
Patel ZM, Adhia RA. Tel-Hashomer camptodactyly syndrome with hirsuitism in an Indian family. J Assoc Physicians India. 2004;52(Oct):837–8.
Melegh B, Hollódy K, Aszmann M, Méhes K. Tel Hashomer camptodactyly syndrome: 12-Year follow-up of a Hungarian patient and review. Am J Med Genet. 2005;135(A):320–3.
Shah K, Sreekanth R, Thomas B, Danda S. Tel Hashomer Camptodactyly Syndrome. West Indian Med J. 2013;62:81–3.
Rogovina EG, Aver’ianov IN, Nechkina NP, Logunova LV. The Tel Hashomer Camptodactyly Syndrome. Zh Nevrol Psikhiatr Im S S Korsakova. 1995;95:83–6.
Scarano G, Della Monica M, Lonardo F, Police MA, D’vanzo MG. Sindrome di Tel Hashomer. Riv Ital Pediatr. 1994;20:572–5.
Verellen-Dumoulin C, De Meyer R, Brucher JM, Gengoux P, Lapiere CM, Kulakowski KS. Camptodactyly with muscular hypoplasia, skeletal dysplasia and abnormal palmar creases: a clinical genetic, morphological and dermatological study. In: Sixth Int Congr Hum Genet. Jerusalem. USA: A R Liss; 1981:258.
Gollop T, Dal Colletto GM, Ferraretto I, Grimaldi A. New Manifestations Observed in the Tel Hashomer Camptodactyly Syndrome. Prog Clin Biol Res. 1982;104:269–77.
Cummins H, Midlo C. Finger Prints, Palms and Soles: An Introduction to Dermatoglyphics. New York: Dover Publications; 1961.
Acknowledgements
We acknowledge Dr Kosala Weerakoon for his support in retrieving the full text of some of the articles.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Authors’ contributions
BTBW conceived the idea. BTBW, RJM, and SBA were involved in study design, data analysis, drafting the article or revising it critically for important intellectual content, and all authors approved the final version.
Competing interests
All authors disclaim any financial or commercial involvement or other conflicts of interest.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Wijerathne, B.T.B., Meier, R.J. & Agampodi, S.B. The status of dermatoglyphics as a biomarker of Tel Hashomer camptodactyly syndrome: a review of the literature. J Med Case Reports 10, 258 (2016). https://doi.org/10.1186/s13256-016-1048-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13256-016-1048-7